文章信息
- 徐丽, 吉彩霞, 刘晓骅, 喻婷婷, 罗进勇.
- XU Li, JI Cai-xia, LIU Xiao-hua, YU Ting-ting, LUO Jin-yong.
- DLX1对BMP9诱导的间充质干细胞C3H10T1/2成骨分化的影响
- Effects of DLX1 on BMP9-induced Osteogenic Differentiation of C3H10T1/2 Mesenchymal Stem Cells
- 中国生物工程杂志, 2017, 37(10): 8-15
- China Biotechnology, 2017, 37(10): 8-15
- http://dx.doi.org/DOI:10.13523/j.cb.20171002
-
文章历史
- 收稿日期: 2016-12-18
- 修回日期: 2017-04-25
间充质干细胞(mesenchymal stem cells, MSCs)是一类具有自我更新和多向分化潜能的成体干细胞,在骨髓中含量尤为丰富,在适宜条件下可向骨、软骨、肌肉等组织分化[1]。目前MSCs已成为骨组织工程重要的种子细胞。骨形态发生蛋白(bone morphogenetic proteins, BMPs)属于转化生长因子β(transforming growth factors β, TGFβ)超家族成员,因其具有诱导骨形成的能力而得名[2],在众多BMPs中,除BMP2,BMP4,BMP6和BMP7外,BMP9也被证实具有极强的诱导MSCs成骨分化的作用[3]。但其相关的分子机制还不甚清楚。
DLX(Distal-less Homeobox)属于同源异形盒基因家族,在脊椎动物中广泛存在并具有高度保守性,DLX家族包括至少6个成员即DLX1~DLX6[4]。DLX家族成员在胚胎和器官发育、细胞分化、肿瘤发生等领域均发挥着重要的调控作用[5-8]。在DLX家族中,DLX5是公认的成骨分化的关键因子[9],也是BMP9诱导成骨分化时的重要调节分子[10]。而DLX2和DLX3等也证实与骨形成关系密切[11-12]。然而,作为DLX家族成员,DLX1是否也与骨形成和成骨分化有关,目前知之甚少。因此,本研究旨在观察DLX1对BMP9诱导的MSCs成骨分化的影响,并初步探讨潜在分子机制。
1 材料与方法 1.1 材料BMP9腺病毒(Ad-BMP9)、和对照腺病毒(Ad-GFP)由芝加哥大学分子肿瘤实验室何通川教授惠赠;DLX1过表达腺病毒(Ad-DLX1)、DLX1的RNAi腺病毒(Ad-siDLX1),荧光素酶报告质粒p12xSBE由重庆医科大学临床检验诊断学实验室保存;小鼠间充质干细胞株C3H10T1/2购自美国典型菌种保藏中心(ATCC);碱性磷酸酶(ALP)定量检测试剂盒购自Clontech公司;napthol AS-MX phosphate、维生素C和β-磷酸甘油和茜素红S购自Sigma公司;RNA提取试剂Trizol购自Invitrogen公司;MMLV逆转录酶购自TaKaRa公司;PCR引物由TaKaRa公司合成;DMEM高糖培养基和优质胎牛血清购自Hyclone公司。其它试剂均为进口分装或国产分析纯。
实验动物选用3~4周龄的健康雌性免疫缺陷型BALB/c裸鼠,购自重庆医科大学实验动物中心,室温喂养于无菌层流动物房内,空气湿度为60%左右,饲料和水消毒后自由进食。
1.2 方法 1.2.1 细胞培养C3H10T1/2细胞用DMEM完全培养基,在37℃、5%CO2细胞培养箱中进行培养,待细胞融合度达80%左右时,用胰蛋白酶消化,进行传代。
1.2.2 细胞总RNA的提取和RT-PCR将C3H10T1/2细胞接种至T-25细胞培养瓶中,待细胞密度为30%左右时,分别加入Ad-BMP9和Ad-GFP腺病毒感染(感染率30%),48h观察到荧光表达后,Tirizol法提取细胞总RNA(按操作说明书进行)。随后进行RT-PCR检测DLX1的表达,引物序列如下:DLX1 Fwd Primer:TGTCTCCTTCTCCCATGTCC,DLX1 Rev Primer: GAACTGATGTAGGGGCTGGA。
1.2.3 ALP活性测定C3H10T1/2细胞按2×104/孔的数量接种至24孔板,待细胞融合度达30%左右时加入相应腺病毒(感染率30%),8h后换液,随后连续培养7d后进行ALP染色(按说明书进行)。
1.2.4 钙盐沉积实验C3H10T1/2细胞按2×104/孔的数量接种至24孔板,待细胞融合度达30%左右时加入相应腺病毒(感染率30%),8h后换液,随即连续培养14d后,进行茜素红染色,方法如下:弃去培养基,用PBS洗3次,每孔加入0.05%戊二醛溶液300μl固定10min,吸去戊二醛并用ddH2O洗3次,然后加入0.04%的茜素红S,在显微镜下观察,待出现红色颗粒物质堆积时,倒掉染液,加ddH2O终止反应,再用ddH2O清洗一遍,显微镜下观察并成像。
1.2.5 Western blotC3H10T1/2细胞接种于直径100mm的培养皿中,待细胞贴壁后加入相应腺病毒(感染率30%),48h后裂解细胞并提取蛋白,经SDS-PAGE电泳、转膜、封闭液封闭、一抗(1:500稀释)4℃孵育过夜、二抗(1:5 000稀释)37℃孵育1h等过程后,显影成像保存。
1.2.6 免疫细胞化学C3H10T1/2细胞按2×104/孔接种于24孔板,待细胞融合度达30%左右时加入相应腺病毒(感染率30%),7d后进行免疫细胞化学染色。步骤如下:吸弃培养基,PBS洗两次,4%多聚甲醛固定10min,PBS洗两次;0.25%TriTonX-100室温孵育10min,PBS洗两次;3%H2O2室温孵育10min,PBS洗三次,每次3min;1:20稀释后的OCN一抗在4℃孵育过夜,PBS洗3次,每次3min;1:50稀释后的二抗37℃孵育10~15min,PBS洗3次,每次3min;DAB显色,室温5min后终止,ddH2O冲洗2次,苏木精复染3min,ddH2O冲洗2次,显微镜下观察。
1.2.7 荧光素酶报告基因实验C3H10T1/2细胞接种于T-25细胞培养瓶中,细胞融合度约达30%时,将3μg荧光素酶报告质粒p-12xSBE与15μl脂质体混合,加入到250μl无血清无双抗DMEM培养基中,孵育15min后转染C3H10T1/2细胞。转染6h后,更换培养液,继续培养细胞过夜。随后铺入24孔板中,待细胞贴壁后加入相应腺病毒(感染率30%)。12h或24h后裂解细胞离心,取上清与荧光素酶底物反应,定量检测。
1.2.8 动物实验和组织化学染色C3H10T1/2细胞接种至100mm培养皿中,待细胞融合度约为40%时,加入适量相应腺病毒感染(感染率30%,动物实验共分为3组,分别为:BMP9组,BMP+DLX1组、BMP9+siDLX1组),感染后继续培养24h,弃去培养基收集取细胞,用含100unit/ml青霉素和100mg/L链霉素的PBS重悬细胞,并接种于BALB/c裸鼠背部,每周观察裸鼠皮下成骨包块的大小。细胞接种4周后,断颈处死裸鼠,取皮下包块观察大小并成像。10%甲醛固定裸鼠皮下骨组织包块,并用Micro-CT (VivaCT 40, 瑞士)扫描,相应软件(Micro-CT 516.1)进行三维重建和数据分析。皮下包块经脱钙,石蜡包埋切片,H & E染色观察包块内的成骨情况,成像并保存。
1.2.9 统计分析计量资料以均数±标准差(x±s)表示,组间比较采用单因素方差分析,两组间比较采用t检验,统计学分析用SSPS17软件包处理。
2 结果 2.1 BMP9促进C3H10T1/2细胞中DLX1的表达RT-PCR检测BMP9腺病毒感染(感染率30%)C3H10T1/2细胞后DLX1的基因表达水平,结果显示:BMP9可以上调C3H10T1/2细胞中DLX1基因的表达(图 1a,图 1b)。Western blot检测BMP9腺病毒感染后,C3H10T1/2细胞中DLX1的蛋白表达水平,结果显示,BMP9也可增强C3H10T1/2细胞中DLX1蛋白的表达(图 1c,图 1d)。因此,BMP9可促进C3H10T1/2细胞中DLX1的表达。

|
| 图 1 BMP9上调C3H10T1/2细胞中DLX1的表达 Figure 1 BMP9 enhances the expression level of DLX1 in C3H10T1/2 cells (a) and (b) the gene expression level of DLX1 was detected by RT-PCR after exogenous Ad-BMP9 infection for 48 h; (c) and (d) the protein expression level of DLX1 was detected by Western blot after exogenous Ad-BMP9 infection for 72 h. GAPDH and β-actin were used as loading control, separately. ** P < 0.01 compared with Blank and Ad-GFP |
首先,Western blot验证DLX1过表达腺病毒可在C3H10T1/2细胞高表达DLX1蛋白(图 2a)。随后,利用DLX1,BMP9,BMP9+DLX1分别处理C3H10T1/2细胞,染色检测早期成骨分化指标ALP活性发现:虽然DLX1单独处理并不会影响C3H10T1/2细胞的ALP活性,但是DLX1却可以进一步促进由BMP9诱导的ALP活性(图 2b)。利用茜素红染色和免疫细胞化学分别检测晚期成骨指标钙盐沉积和OCN表达,结果显示:DLX1可以增强由BMP9诱导的钙盐沉积(图 2c)和OCN表达(图 2d)。因此,过表达DLX1可促进BMP9诱导的C3H10T1/2细胞成骨分化。
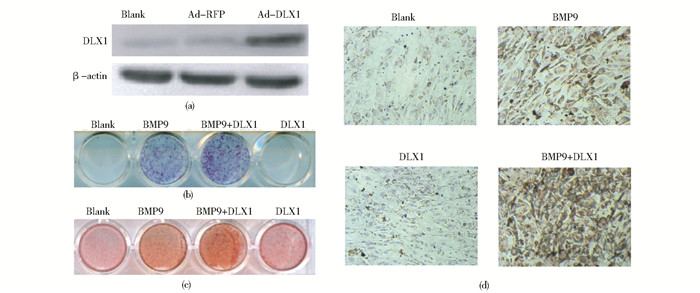
|
| 图 2 DLX1增强BMP9诱导C3H10T1/2细胞成骨分化 Figure 2 DLX1 promotes BMP9-induced osteogenic differentiation in C3H10T1/T cells (a) The expression of DLX1 was detected by Western blot after exogenous Ad-DLX1 adenovirus infection for 72 h in C3H10T1/2 cells (b) Overexpression of DLX1 promoted BMP9-induced ALP activity in C3H10T1/2 cells (ALP staining assay) (c) Overexpression of DLX1 promoted BMP9-induced calcium deposition in C3H10T1/2 cells (Alizarin Red S staining) (d) Overexpression of DLX1 promoted BMP9-induced OCN expression in C3H10T1/2 cells (Immunocytochemistry assay, x150) |
首先,Western blot验证DLX1的RNAi腺病毒(Ad-siDLX1)可抑制C3H10T1/2细胞中DLX1的表达(图 3a)。随后ALP染色结果显示:RNAi抑制DLX1表达可导致BMP9诱导的C3H10T1/2细胞ALP活性降低(图 3b)。随后利用茜素红染色和免疫细胞化学分别检测晚期成骨指标钙盐沉积和OCN表达,结果显示:RNAi抑制DLX1表达后,由BMP9诱导的C3H10T1/2细胞钙盐沉积(图 3c)和OCN表达(图 3d)相应减弱。因此,抑制DLX1表达,可减弱BMP9诱导的C3H10T1/2细胞成骨分化。
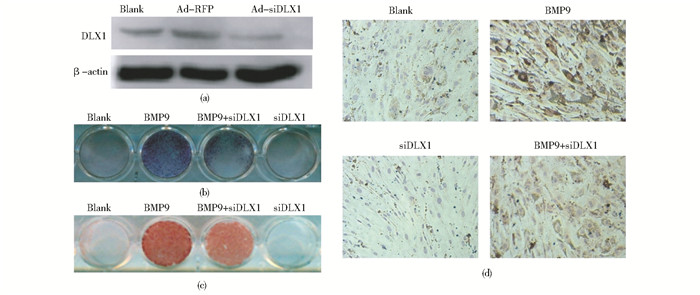
|
| 图 3 RNAi抑制DLX1表达可减弱BMP9诱导C3H10T1/2细胞成骨分化 Figure 3 Inhibition of DLX1 by RNAi decreases BMP9-induced osteogenic differentiation in C3H10T1/T cells (a) The expression of DLX1 was detected by Western blot after exogenous Ad-siDLX1 adenovirus infection for 72 h in C3H10T1/2 cells (b) Inhibition of DLX1 by RNAi decreased BMP9-induced ALP activity in C3H10T1/2 cells (ALP staining assay) (c) Inhibition of DLX1 by RNAi inhibited BMP9-induced calcium deposition in C3H10T1/2 cells (Alizarin Red S staining) (d) Inhibition of DLX1 by RNAi reduced BMP9-induced OCN expression in C3H10T1/2 cells (Immunocytochemistry assay, x150) |
最后,通过裸鼠皮下异位成骨进一步分析DLX1对BMP9诱导的C3H10T1/2细胞成骨分化的影响。首先,发现与BMP9组相比,BMP9+siDLX1的皮下包块明显较小,而BMP9+DLX1组皮下包块则进一步增大(图 4a)。Micro-CT分析发现,与BMP9组相比,BMP9+siDLX1组的包块体积(TV)、骨体积(BV)以及骨体积与总体积中的比值(BV/TV)相应下降(图 4b,图 4c),而BMP9+DLX1组皮下包块的TV,BV及BV/TV均进一步增加(图 4b,图 4c)。H&E染色显示:BMP9+siDLX1组中骨小梁的数量减少,而BMP9+DLX1组中骨小梁的数量进一步增加。因此,过表达DLX1可使BMP9诱导的C3H10T1/2细胞在裸鼠皮下异位成骨进一步增强,而RNAi抑制DLX1表达后,可使BMP9诱导的C3H10T1/2细胞在裸鼠皮下异位成骨减弱。

|
| 图 4 DLX1对BMP9诱导的C3H10T1/2细胞裸鼠皮下异位成骨的影响 Figure 4 The effects of DLX1 on BMP9-induced entopic bone formation of C3H10T1/2 cells (a) Gross image of retrieved sample (b) 3D reconstruction of bone masses by Micro-CT analysis (c) Total volume (TV), bone volume (BV) and BV/TV of retrieved sample by Micro-CT analysis (d)H&E staining of retrieved sample (x150, BT: Bone Trabecula) * P < 0.05 compared with BMP9 ** P < 0.01 compare with BMP9 |
BMP9可以通过经典的Smad1/5/8信号发挥诱导成骨分化活性。因此,利用荧光素酶报告基因实验确认DLX1是否影响BMP9诱导的Smad1/5/8信号的活化,结果显示:过表达DLX1可促进BMP9诱导的Smad1/5/8的转录调控活性,使得荧光素酶活性增加;反之,RNAi抑制DLX1后,BMP9诱导的Smad1/5/8的转录调控活性降低(图 5)。故DLX1可影响BMP9诱导的Smad1/5/8信号的活化。然而,通过Western blot检测却发现:无论过表达DLX1和RNAi抑制DLX1,BMP9诱导的Smad1/5/8磷酸化并未发生改变。
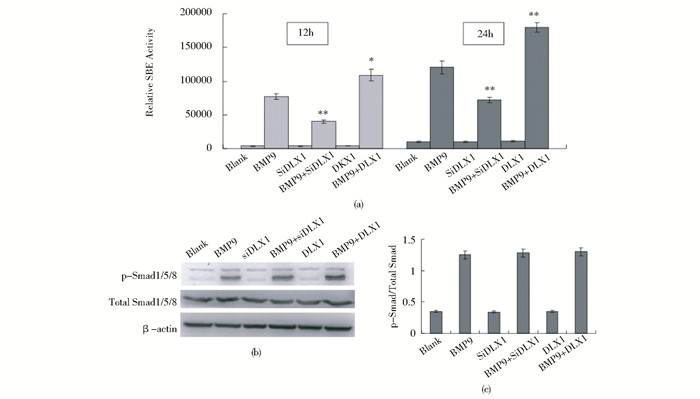
|
| 图 5 DLX1对BMP9激活的Smad信号的影响 Figure 5 The effects of DLX1 on BMP9-induced Smad activation (a) The effects of DLX1 on BMP9-induced Smad1/5/8 transcriptional activity (luciferase reporter assay) (b) and (c) The effects of DLX1 on BMP9-induced Smad1/5/8 phosphorylation (Western blot) * P < 0.05 compared with BMP9, ** P < 0.01 compare with BMP9 |
定向诱导种子细胞如MSCs等成骨分化,一直是骨组织工程中的一个核心问题。BMPs家族中的BMP2、BMP4、BMP6和BMP7等均可诱导MSCs成骨分化。近来研究表明,BMPs家族中的另一成员BMP9具有更为强效的促进MSCs成骨分化的能力,甚至超过已在临床使用的BMP2和BMP7[3]。在相关机制研究中发现:BMP9可活化Smad1/5/8、MAPK、CREB/PKA等信号途径[13-14],而Runx2、Id、CTGF等转录因子是BMP9诱导MSCs成骨分化时重要的调控分子[13-14]。尽管如此,由于对BMP9诱导成骨的研究起步相对较晚,目前对于BMP9诱导MSCs成骨分化分子机制的了解还不够深入。
在骨发育的晚期阶段,所有DLX家族成员在分化的骨组织中都有表达,表明了DLX家族在骨形成中的潜在重要作用[5, 15]。DLX家族中DLX5是成骨分化的关键调控因子,基因敲除DLX5可以导致骨形成障碍[16],DLX5也是BMP9诱导成骨分化的重要靶分子[10]。DLX2、DLX3、DLX4均已被证实与成骨分化相关[17]。DLX1是同源异形盒DLX家族的重要成员,DLX1在发育和细胞分化方面的研究,主要集中于神经分化领域[18-20],但在成骨分化研究中报道极少。在本研究中发现,BMP9可以促进间充质干细胞C3H10T1/2中DLX1的表达,因此,DLX1极可能也有调节BMP9诱导成骨分化的作用。随后的体外和体内实验均证实,DLX1可调控BMP9诱导的C3H10T1/2细胞成骨分化,上调DLX1表达可以促进BMP9诱导的C3H10T1/2细胞成骨分化,下调DLX1表达可抑制BMP9诱导的C3H10T1/2细胞成骨分化。在裸鼠皮下异位成骨实验中,发现过表达的DLX1可以促进BMP9诱导的裸鼠皮下异位成骨,成骨数量和质量明显高于BMP9单独处理组,H & E染色可见骨小梁明显增多;而RNAi抑制DLX1的表达则导致BMP9诱导的成骨数量和质量下降,使得骨小梁生成减少。
Smad1/5/8信号是BMP9诱导成骨分化的重要信号途径之一[14],那么,DLX1是否会影响Smad1/5/8信号的活化呢。结果显示:DLX1可以影响BMP9诱导的Smad1/5/8的转录调控活性,但是对Smad1/5/8的磷酸化(Smad1/5/8的活化形式)并无明显影响。由此推测,DLX1可能以辅助因子(Co-factor)的方式促进Smad1/5/8的转录调控活性,而非以直接改变Smad1/5/8磷酸化来发挥作用。
总之,以上结果表明DLX1可调控BMP9诱导的MSCs成骨分化,其调控作用可至少通过影响Smad1/5/8信号途径来实现。而实际上,BMP9调控MSCs成骨分化的信号转导网络涉及众多信号途径和下游的多种转录因子,具有高度复杂性。在后期的实验工作中将进一步分析DLX1与该网络中的其它信号途径(如MAPK等)的交互对话,及与其它成骨关键转录因子(如Runx2等)的相互作用。
| [1] |
Pittenger M F, Mackay A M, Beck S C, et al. Multilineage potential of adult human mesenchymal stem cells. Science, 1999, 284(5411): 143-147. DOI:10.1126/science.284.5411.143 |
| [2] |
Urist M R. Bone:formation by autoinduction. Science, 1965, 150(3698): 893-839. DOI:10.1126/science.150.3698.893 |
| [3] |
Kang Q, Sun M H, Cheng H, et al. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther, 2004, 11(17): 1312-1320. DOI:10.1038/sj.gt.3302298 |
| [4] |
Panganiban G, Rubenstein J L. Developmental functions of the Distal-less/Dlx homeobox genes. Development, 2002, 129(19): 4371-4386. |
| [5] |
Merlo G R, Zerega B, Paleari L, et al. Multiple functions of Dlx genes. Int J Dev Biol, 2000, 44(6): 619-626. |
| [6] |
Zhang L, Yang M, Gan L, et al. DLX4 upregulates TWIST and enhances tumor migration, invasion and metastasis. Int J Biol Sci, 2012, 8(8): 1178-1187. DOI:10.7150/ijbs.4458 |
| [7] |
Dai X, Iwasaki H, Watanabe M, et al. Dlx1 transcription factor regulates dendritic growth and postsynaptic differentiation through inhibition of neuropilin-2 and PAK3 expression. Eur J Neurosci, 2014, 39(4): 531-547. DOI:10.1111/ejn.2014.39.issue-4 |
| [8] |
McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell, 1992, 68(2): 283-302. DOI:10.1016/0092-8674(92)90471-N |
| [9] |
Nishimura R, Hata K, Matsubara T, et al. Regulation of bone and cartilage development by network between BMP signalling and transcription factors. J Biochem, 2012, 151(3): 247-254. DOI:10.1093/jb/mvs004 |
| [10] |
Liu C, Weng Y, Yuan T, et al. CXCL12/CXCR4 signal axis plays an important role in mediating bone morphogenetic protein 9-induced osteogenic differentiation of mesenchymal stem cells. Int J Med Sci, 2013, 10(9): 1181-1192. DOI:10.7150/ijms.6657 |
| [11] |
Sun H, Liu Z, Li B, et al. Effects of DLX2 overexpression on the osteogenic differentiation of MC3T3-E1 cells. Exp Ther Med, 2015, 9(6): 2173-2179. DOI:10.3892/etm.2015.2378 |
| [12] |
Hassan M Q, Javed A, Morasso M I, et al. Dlx3 transcriptional regulation of osteoblast differentiation:temporal recruitment of Msx2, Dlx3, and Dlx5 homeodomain proteins to chromatin of the osteocalcin gene. Mol Cell Biol, 2004, 24(20): 9248-9261. DOI:10.1128/MCB.24.20.9248-9261.2004 |
| [13] |
罗进勇. 骨形态发生蛋白9促成骨的分子机制. 重庆医学, 2016, 45(9): 1153-1162. Luo J Y. The molecular mechanism of BMP9-induced osteogenesis. Chongqing Medicine, 2016, 45(9): 1153-1162. |
| [14] |
Lamplot J D, Qin J, Nan G, et al. BMP9 signaling in stem cell differentiation and osteogenesis. Am J Stem Cells, 2013, 8;2(1): 1-21. |
| [15] |
Simeone A, Acampora D, Pannese M, et al. Cloning and characterization of two members of the vertebrate Dlx gene family. Proc Nat Acad Sci USA, 1994, 15;91(6): 2250-2254. |
| [16] |
Acampora D, Merlo G R, Paleari L, et al. Craniofacial, vestibular and bone defects in mice lacking the Distal-less-related gene Dlx5. Development, 1999, 126(17): 3795-3809. |
| [17] |
Levi G, Gitton Y. Dlx genes and the maintenance of bone homeostasis and skeletal integrity. Cell Death Differ, 2014, 21(9): 1345-1346. DOI:10.1038/cdd.2014.94 |
| [18] |
Dai X, Iwasaki H, Watanabe M, et al. Dlx1 transcription factor regulates dendritic growth and postsynaptic differentiation through inhibition of neuropilin-2 and PAK3 expression. Eur J Neurosci, 2014, 39(4): 531-547. DOI:10.1111/ejn.2014.39.issue-4 |
| [19] |
Jones D L, Howard M A, Stanco A, et al. Deletion of Dlx1 results in reduced glutamatergic input to hippocampal interneurons. J Neurophysiol, 2011, 105(5): 1984-1991. DOI:10.1152/jn.00056.2011 |
| [20] |
Cobos I, Calcagnotto M E, Vilaythong A J, et al. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci, 2005, 8(8): 1059-1068. DOI:10.1038/nn1499 |
 2017, Vol. 37
2017, Vol. 37




