文章信息
- 房立霞, 曹英秀, 宋浩.
- FANG Li xia, CAO Ying xiu, SONG Hao.
- 工程大肠杆菌合成游离脂肪酸的研究进展
- Engineering Escherichia coli to Synthesize Free Fatty Acids: A Recent Progress
- 中国生物工程杂志, 2016, 36(11): 90-97
- China Biotechnology, 2016, 36(11): 90-97
- http://dx.doi.org/DOI:10.13523/j.cb.20161113
-
文章历史
- 收稿日期: 2016-03-31
- 修回日期: 2016-04-25
作为一种平台化合物,脂肪酸被广泛应用于多个领域。其衍生的烷/烯烃和脂肪酸酯比短链醇具有更高的能量密度,并且与汽油不存在10%~20%的混合壁垒,被认为是更有潜力的替代燃料[1]。而衍生的脂肪醇和三酰甘油等产品因具有优良的亲疏水性,也是洗涤剂、润滑剂、化妆品和药物等多种化学工业产品的主要原料[2]。但现阶段脂肪酸主要从石油或动植物中提取,存在可持续性差、与粮食争地等问题,因此利用工程微生物高效地合成可再生脂肪酸成为了近年来的研究热点[3-4]。
因为遗传背景清晰,大肠杆菌的脂肪酸代谢和调控被最早研究[5-6]。值得注意的是,除了合成细胞必需的脂质和细胞膜,大肠杆菌自身几乎不积累游离脂肪酸。但是通过表达合成路径中的关键基因[7]、敲除旁路代谢途径[8]、上下游代谢模块的适配[9]等代谢工程改造,大肠杆菌中脂肪酸的产量已经达到约9g/L[9-10],展示了大肠杆菌巨大的工业脂肪酸合成潜力。另外,随着合成生物学技术的成熟与运用,大肠杆菌脂肪酸合成速率和产品种类也得到了更快的发展。实时反馈调控[11-12]、体外重构代谢路径指导体内改造[13]等新思路,减少了无义工程菌株的构建数量。β氧化循环的逆转[14-15]和多物种合成路径的整合[16-17],也赋予大肠杆菌更高效地合成多种脂肪酸产品的能力。这些合成生物学技术极大地拓宽了大肠杆菌脂肪酸的合成方式和应用范围。本综述从代谢工程和合成生物学的角度对重组大肠杆菌合成脂肪酸的研究进展进行详细介绍并展望其发展前景。
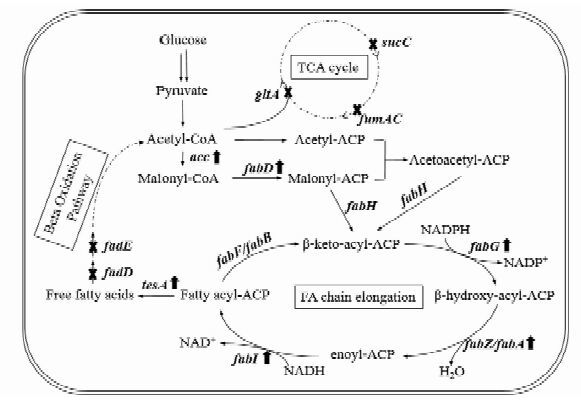
1 代谢工程手段提高大肠杆菌脂肪酸产量的代谢工程手段主要是通过调控脂肪酸代谢路径中基因的表达水平实现的,包括敲除脂肪酸降解路径和前体旁路代谢路径的基因、过表达脂肪酸合成路径的基因及组合优化,如图 1所示。
|
|
图 1
大肠杆菌脂肪酸代谢途径及其工程改造
Figure 1
Engineering the metabolic pathway of fatty acids of fatty acids in Escherichia coli
Notes: × gene deletion; |
提高脂肪酸的积累首先需要阻断脂肪酸的降解路径或其合成前体的旁路代谢途径。β氧化是脂肪酸降解的主要路径,脂酰辅酶A(coenzyme A,CoA)合酶(fadD)和脂酰CoA脱氢酶(fadE)分别催化β氧化的第一、二步反应,敲除fadD或fadE可以阻断脂肪酸的分解代谢[7-8]。另外,敲除三羧酸(tricarboxylicacid,TCA)循环中的若干基因可以减少乙酰CoA的旁路代谢分流,其中敲除琥珀酰CoA脱氢酶基因sucC可显著提高脂肪酸产量[18-19]。
过表达脂肪酸合成路径中的各基因是提高大肠杆菌脂肪酸合成能力的另一个有效手段。acc编码的乙酰CoA羧化酶(acetyl CoA carboxylase,ACC)催化乙酰CoA生成丙二酰CoA是脂肪酸合成过程的限速步骤[7, 20-22],丙二酰CoA生成丙二酰-酰基载体蛋白(acyl carrier protein,ACP)由fabD编码的酰基转移酶(acyltransferase,FabD)催化完成[23-24];fabI、fabZ和fabG编码的烯脂酰-ACP还原酶(enoyl-ACP reductase,FabI)、羟脂酰-ACP脱水酶(hydroxyacyl-ACP dehydratase,FabZ)和酮脂酰-ACP还原酶(ketoacyl-ACP reductase,FabG)催化脂肪酸合成路径的链延长反应[13, 18-19],过表达这些基因可以不同程度地提高脂肪酸的产量。脂酰-ACP的积累对脂肪酸合成途径中的很多酶都会产生反馈抑制作用[25-27],硫酯酶可以将脂肪酸从酰基运载蛋白上水解下来解除这种反馈抑制。过表达大肠杆菌本身的硫酯酶基因tesA′和tesB可大量合成C14脂肪酸[8, 10, 28];过表达不同植物和其他菌种来源的硫酯酶会专一性地大量合成某一种或几种特定长度的游离脂肪酸,如香樟樟脑(Cinnamomum camphorum,CcTE,C14,16)[13, 22]、加州月桂(Umbellularia californica,UcTE,C12)[7, 28]、蓖麻(Ricinus communis,RcTE,C14,16)[29]、麻疯树(Jatropha curcas,JcTE,C14,16)[29]、不动杆菌(Acinetobacter baylyi,AbTE,C8-16)[30]、化脓链球菌(Streptococcus pyogenes,SpTE,C12,14)[31]等,大大地丰富了产物的多样性。
在上述提及的单独敲除或过表达基因的手段中,过表达硫酯酶对大肠杆菌脂肪酸合成的促进效果最为明显,脂肪酸产量提高幅度可以达到几倍至几十倍[7, 30]。另外,综合应用上述策略可更大程度地提高脂肪酸产量(表 1)。例如,在敲除fadD的菌株中过表达tesA′和CcTE可将脂肪酸的产量提高近20倍[22, 30];在敲除fadD的菌株中,过表达fabZ和RcTE并敲除sucC可产得5.7g/L的C14,16脂肪酸[19]。
| E.coli | Deletion | Overexpression | Culture type | Titer(g/L) | Yield(% m/m) | Ref | |
| Others | Thioesterase | ||||||
| DH1 | fadD | - | TesA′ | Batch | 0.7 | 3.5 | [8] |
| DH1 | fadE | - | TesA′ | Batch | 1.1 | 6.0 | [8] |
| MG1655 | fadD/sucC | - | RcTE | Batch | 1.3 | 11 | [18] |
| BL21 | - | TesA′ | Fed-batch | 5.1 | 4.1 | [10] | |
| BL21 | - | - | AbTE | Fed-batch | 3.6 | <6.1 | [30] |
| MG1655 | - | - | SpTE | Batch | 0.34 | <3.4 | [31] |
| MG1655 | fadD | - | RcTE | Batch | 2.2 | <15 | [29] |
| MG1655 | fadD | - | JcTE | Batch | 2.1 | <15 | [29] |
| W3110 | fadD | - | TesA′ | Batch | 0.31 | <3.1 | [28] |
| W3110 | fadD | - | TesB | Batch | 0.18 | <1.8 | [28] |
| W3110 | fadD | - | UcTE | Batch | 0.12 | <1.2 | [28] |
| MG1655 | fadD | - | UcTE | Batch | 0.77 | <15 | [7] |
| MG1655 | fadD | ACC | UcTE | Batch | 0.81 | <16 | [7] |
| BL21 | fadD | ACC | TesA′/CcTE | Batch | 0.38 | - | [22] |
| BL21 | fadD | ACC | TesA′/CcTE | Fed-batch | 2.5 | 4.8 | [22] |
| MG1655 | fadD | FabD | RcTE | Batch | 1.3 | <16 | [23] |
| MG1655 | - | ACC/FabD | - | Batch | 0.25 | - | [24] |
| MG1655 | fadD | FabZ | RcTE | Batch | 1.7 | 14 | [18] |
| MG1655 | fadD/sucC | FabZ | RcTE | Batch | 5.7 | <38 | [19] |
| BL21 | fadE | FabZ/FabG/FabI | TesA′/CcTE | Batch | 0.65 | - | [13] |
1.2 上下游代谢模块适配
在多基因代谢路径中,利用代谢工程手段可以提高限速步骤等的反应速率,但由此积累或消耗的前体物和中间体并不能完全协调下游路径,甚至会降低细胞活力和路径产率。Xu等[9]基于乙酰CoA和丙二酰CoA这两个关键中间体将大肠杆菌脂肪酸合成路径划分为各自独立却又相互联系的三个模块: 上游乙酰CoA 形成模块、中游乙酰CoA活化模块和下游脂肪酸合酶模块。通过改变质粒的拷贝数来组合优化这三个模块的转录水平,使脂肪酸中间体(乙酰CoA和丙二酰CoA/ACP)的供需得以平衡。而且,通过定制上游和下游模块的核糖体结合位点来改善翻译效率,进而提高脂肪酸产量。将工程改造的菌株进行分批补料培养最终得到8.6g/L的脂肪酸(约22%理论产量)。
2 合成生物学策略与代谢工程的研究方法不同,合成生物学更侧重于从最基本的基因元件开始一步步构建零部件和回路,从而实现人工生物系统的目标功能[4]。利用合成生物学策略可以对脂肪酸代谢路径中的关键组分进行建模、表征和微调,从而优化代谢回路、指导理性工程改造,不仅提高了脂肪酸产量,而且丰富了终产物的多样性。
2.1 动态调控回路代谢工程手段对基因表达的调控主要是静态水平的,如拷贝数[32]、基因间隔[33]、启动子强度[34-35]、核糖体结合位点[36],但天然存在的代谢路径都是通过反馈机制动态地调控代谢物浓度,以维持细胞生长、增加能源利用率。在大肠杆菌脂肪酸合成路径中过表达acc可以促进关键中间体丙二酰CoA的合成,但会对细胞产生一定的毒性[20-21],所以有些学者在大肠杆菌中构建了感应并动态调控丙二酰CoA的回路[11-12, 37]。
FapR是枯草芽孢杆菌(Bacillus subtilis)中天然存在的脂肪酸合成路径的转录调控因子,可专一性地识别并结合启动子上游特定的DNA片段,抑制启动子下游基因的转录,若丙二酰CoA与FapR结合则会改变FapR的构象,使其从结合着的DNA上解离,从而基因转录得以开启[38-39]。特别地,FapR结合到大肠杆菌内源启动子PGAP的上游活化序列会开启PGAP下游基因的转录。基于FapR-丙二酰CoA对DNA转录的抑制/开启特性,Liu等[11]和Xu等[12]在大肠杆菌中分别构建了丙二酰CoA的动态调控回路FapR-PFapR-LacI-PT7-ACC和FapR-(PT7-ACC/PGAP-FAS),如图 2所示。由此实现了对丙二酰CoA的动态调控,平衡了细胞的生长和产物的形成,脂肪酸的效价分别提高了34%和15.7倍。
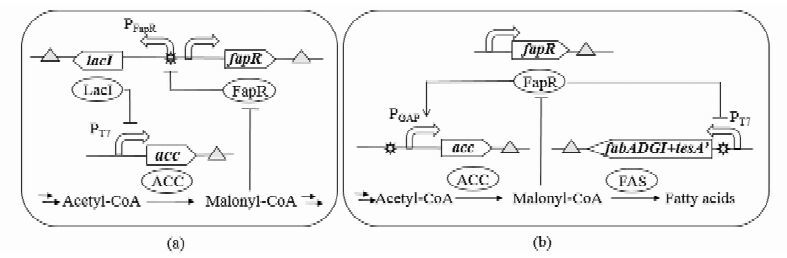
|
| 图 2 大肠杆菌丙二酰CoA的动态调控回路 Figure 2 Dynamic control pathway of malonyl-CoA in E.coli (a) The binding of FapR to PFapR represses the expression of LacI,activating PT7 to turn on the expression of ACC,which catalyses acetyl CoA to malonyl CoA; The accumulated malonyl CoA binds to FapR,making PFapR free,then the expressed LacI makes PT7 turn off the expression of ACC,which in turn decreases the content of malonyl CoA (b) When the concentration of malonyl CoA is very low,FapR turns on the expression of ACC and turns off the expression of FAS,leading to the accumulation of malonyl CoA; the enhanced malonyl CoA binds to FapR,making PFapR and PT7 free,which turns off the expression of ACC and turns on the expression of FAS and the transformation of malonyl CoA to fatty acids Represents the specific DNA sequence that FapR identifies and combines |
基于实验经验设计出的调控基因表达的手段理论上可以提高脂肪酸产量,但其实际结果不可预知,有时甚至与预期相反,而且实验周期较长。利用体外生物合成系统可以定量分析脂肪酸合成路径的调控,快速找出代谢路径的关键点,指导代谢工程手段的理性设计,从而减少了无义菌株的构建,加快了工程改造的速率。
体外研究最初是将培养的大肠杆菌细胞破碎弃去膜和核糖体制得无细胞提取液。通过在此无细胞培养液中添加脂肪酸合成路径中的关键成分(ACC、ACP、TesA),Liu等[40]证实脂肪酸的合成速率很大程度上取决于中间体丙二酰CoA的浓度。另外,与Keating等[41]的研究结果一致,当TesA和ACP的浓度很高时,脂肪酸的合成速率迅速下降。后期Yu等[13]将脂肪酸合酶(fatty acid synthase,FAS)和TesA共10个组分全部纯化后构建成体外重组体系。利用此体外体系进行动力学分析和滴定实验,发现脂肪酸的合成速率与FabI和FabZ的浓度呈双曲线的关系。将此研究结果应用于指导体内相应基因表达水平的调控,大肠杆菌脂肪酸的产量从0.45g/L提高到0.65g/L。在最佳反应条件下,体外脂肪酸合成系统产出的主要是C16和C18的饱和及单不饱和脂肪酸,单不饱和脂肪酸的相对丰度会随着羟脂酰硫酯脱水酶FabA浓度的增大或酮脂酰ACP合酶FabB浓度的减小而降低[42],由此可指导体内饱和脂肪酸的合成。
2.3 功能逆转的β氧化循环Dellomonaco等[15]和Clomburg等[14]成功地逆转大肠杆菌自身的β氧化途径,并以此逆转途径进行羧酸合成。在功能逆转的β氧化循环中,脂酰基链的延长不需要乙酰CoA到丙二酰CoA的耗能的转化,而是直接利用乙酰CoA,由此将碳源和能量最大化地用于目标产物的合成。
在没有脂肪酸的情况下,β氧化路径中的基因不能正常表达。在脂肪酸代谢的转录调控因子FadR和ato操纵子的反应调节子AtoC中引入突变[43]、用cAMP不依赖型的突变体(crp*)替代内源全局转录调控因子crp[44]、敲除反应调节子ArcA[45],综合应用上述策略实现只有葡萄糖作为碳源时β氧化路径中基因的组成型表达。在此基础上,敲除代谢旁路、过表达硫解酶基因fabA、羟脂酰CoA脱氢酶/烯脂酰CoA合酶基因fadB和脂酰CoA硫酯酶基因fadM,在大肠杆菌中成功构建出功能逆转的β氧化循环[15]。用矿物盐培养基进行分批发酵,胞外C10~18脂肪酸产量约为7.0g/L,产率为0.28g/g(约80%理论产率)。为了明确路径基因的组成及作用,Clomburg等[14]将多种硫解酶、3-羟酰基CoA脱氢酶、烯酯酰CoA水合酶、烯酯酰CoA还原酶进行重组纯化和体外动力学表征,最终选定硫解酶AtoB及FadA、羟脂酰CoA脱氢酶/烯脂酰CoA合酶FadB和眼虫(Euglena gracilis)来源的烯脂酰CoA还原酶egTER用于后期整合。将AtoB、FadBA和egTER的编码基因整合到宿主菌(本身带有硫酯酶终止路径)中,进行多轮功能逆转的β氧化循环成功产出265mg/L链长C6~12的羧酸。在功能逆转的β氧化循环中选择不同的终止路径可以高效合成不同碳链长度的脂肪酸[15, 46]及其衍生物,如ω-羟基酸、二羧酸[47]和脂肪醇[48]等。
2.4 异源合成路径的整合大肠杆菌内源碳链延长反应每个循环只能增加两个碳原子,因此限制其只能合成偶数链的脂肪酸。但在实际的燃料和化工领域应用中,为满足多种功能,需要其产品在链长上具有更大的多样性(不同长度的奇偶链、支直链)。为了打破大肠杆菌产物的局限性,Wu和San[16-17]利用4种不同来源的β-酮脂酰-ACP合成酶Ⅲ(β-ketoacyl-ACP synthase,FabH)来增加碳链延伸起始阶段的底物选择性,让丙酰CoA代替乙酰CoA进行缩合反应,从而得到奇数链长的游离脂肪酸。当采用枯草芽孢杆菌(Bacillus subtilis)中的FabH时,奇数链脂肪酸占总脂肪酸的比例可以达到83.2%[16]。
在大肠杆菌中异源表达脂肪酸合酶除了能够增加脂肪酸的产品种类外,还能够拓展脂肪酸类产品的合成方式。大肠杆菌中脂肪酸衍生物的合成都是基于脂酰CoA中间体的转化,但这些都涉及游离脂肪酸的释放/活化反过程的循环,Haushalter等[49]将棒状杆菌(Corynebacterium glutamicum)的I型脂肪酸合酶整合到大肠杆菌中,成功构建了无需硫酯水解/再生反过程的脂酰CoA合成体系。
3 发酵过程优化研究表明,对生产菌株进行分批补料培养可以得到2.5~7g/L脂肪酸,远高于分批培养[15, 22, 40]。在分批补料发酵系统中同时应用产物提取的单元操作可以减弱培养基中高浓度脂肪酸对细胞的抑制作用,产出约9g/L的脂肪酸[10]。在连续培养过程中细胞可以保持在最佳条件和最适生长期,对大肠杆菌生产菌株(用UcTE替换fadD、fadE和fadAB)进行连续培养,脂肪酸的产率比分批培养高15%[50]。
有关脂肪酸生产的研究多以碳源作为培养基配方中的限制性营养素,因此限制了细胞的潜能[50]。通过限制碳源、氮源、磷酸盐来培养生产菌株,其中限制磷酸盐的结果最佳:碳源(葡萄糖)合成脂肪酸的转化率为0.1,最高特定生物质产率为0.068g脂肪酸每克细胞干重每小时,与限制碳源相比分别提高了约45%和300%[51]。
4 结论和展望利用脂肪酸代谢途径将可再生原料转化为高能量密度的燃料和高价值油脂化学品已成为近年来研究的热点。产油微生物(如酵母等)体内会积累大量的脂类,但其生长速率较慢并且脂肪酸调控机制过于复杂。通过敲除脂酰CoA合酶基因(faa1、faa2、faa4和fat1)、ABC转运蛋白基因pxa1和脂酰CoA氧化酶基因pox1并过表达三酰甘油酰基转移酶基因DGA1和三酰甘油脂酶基因TGL3,酿酒酵母(Saccharomyces cerevisiae)细胞最高可以产出2.2g/L游离脂肪酸[52];在解脂酵母(Yarrowia lipolytica)细胞中过表达加州月桂来源的硫酯酶基因UcTE,可以实现1.2g/L的C16脂肪酸的合成[53]。相比之下,大肠杆菌生长速率快且其脂肪酸代谢调控的机制也更为清晰,因此工程大肠杆菌合成游离脂肪酸及其衍生物的研究已取得重大进展。应用代谢工程手段来优化脂肪酸代谢路径,大肠杆菌脂肪酸的产量可以达到9g/L[9-10];联合应用合成生物学策略,不仅进一步提高了脂肪酸的产量,还加快了工程菌株的构建,丰富了产物的多样性。
大肠杆菌传统的基因敲除和过表达手段耗时长、效率低,所以应用新技术实现高通量基因组靶向基因的筛选和多重改造是构建脂肪酸高产菌株的一个重要研究方向。CIRSPR/Cas、CRISPR干扰(CRISPR interference,CRISPRi)[54]、CRISPR激活(CRISPR activation,CRISPRa)、sRNA[55]和RNA干扰(RNA interference,RNAi)[56]是近年来被广泛开发的基因组改造手段,具有高通量基因组基因扰动的应用潜力,通过特异性抑制和增强靶向基因的表达,高通量地筛选基因组中对脂肪酸合成产生影响的一系列基因。目前,本课题组利用CRISPRi技术对大肠杆菌脂肪酸代谢路径中的120个相关基因进行抑制,在2周内完成全部菌株的构建,对其中的15个基因进行单一抑制后脂肪酸产量提高20%以上,最高达1.6倍。后期我们拟采取不同强度的基因扰动,并将多位点、多强度扰动进行组合,以期大幅度提高游离脂肪酸产量。
脂肪酸的积累会影响膜的稳定和细胞活力,特别是当脂肪酸积累到一定程度时,其毒性作用不容忽视[3]。因此,产物毒性的减弱及解除也是未来研究的一个重要方向。首先,基于现有的毒性作用机制,如膜组分的改变或细胞酸化,通过工程改造提高大肠杆菌膜上饱和脂肪酸的比例来增强膜对脂肪酸的耐受性;构建脂肪酸的流出系统并阻断胞外脂肪酸的再吸收以减弱细胞酸性。其次,以脂肪酸作为选择压力,对大肠杆菌进行定向进化,筛选脂肪酸耐受性菌株。最后,利用转录组学和蛋白质组学等分析手段深入研究不同碳链长度脂肪酸的毒性作用机制,为工程改造耐酸性大肠杆菌菌株提供方向。
通过基因组的多重改造和产物毒性的解除,必将大幅度提高脂肪酸的产量。当工程改造的菌株能够产出脂肪酸接近理论产率(0.3~0.4g/g葡萄糖)时,结合发酵过程放大优化工艺有可能取代现有的石油化工技术,实现脂肪酸及其衍生物生产的可持续性和环境友好型。
| [1] | Janßen H J, Steinbüchel A. Fatty acid synthesis in Escherichia coli and its applications towards the production of fatty acid based biofuels. Biotechnology for Biofuels , 2014, 7 (1) : 7–32. DOI:10.1186/1754-6834-7-7 |
| [2] | Handke P, Lynch S A, Gill R T. Application and engineering of fatty acid biosynthesis in Escherichia coli for advanced fuels and chemicals. Metabolic Engineering , 2011, 13 (1) : 28–37. DOI:10.1016/j.ymben.2010.10.007 |
| [3] | Lennen R M, Pfleger B F. Engineering Escherichia coli to synthesize free fatty acids. Trends in Biotechnology , 2012, 30 (12) : 659–667. DOI:10.1016/j.tibtech.2012.09.006 |
| [4] | Tee T W, Chowdhury A, Maranas C D, et al. Systems metabolic engineering design:fatty acid production as an emerging case study. Biotechnology and Bioengineering , 2014, 111 (5) : 849–857. DOI:10.1002/bit.25205 |
| [5] | Cronan J E, Jr Weisberg L J, Allen R G. Regulation of membrane lipid synthesis in Escherichia coli. accumulation of free fatty acids of abnormal length during inhibition of phospholipid synthesis. The Journal of Biological Chemistry , 1975, 250 (15) : 5835–5840. |
| [6] | Magnuson K, Jackowski S, Rock C O, et al. Regulation of fatty acid biosynthesis in Escherichia coli. Microbiological Reviews , 1993, 57 (3) : 522–542. |
| [7] | Lennen R M, Braden D J, West R A, et al. A process for microbial hydrocarbon synthesis:overproduction of fatty acids in Escherichia coli and catalytic conversion to alkanes. Biotechnology and Bioengineering , 2010, 106 (2) : 193–202. DOI:10.1002/bit.v106:2 |
| [8] | Steen E J, Kang Y, Bokinsky G, et al. Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature , 2010, 463 (7280) : 559–562. DOI:10.1038/nature08721 |
| [9] | Xu P, Gu Q, Wang W, et al. Modular optimization of multi-gene pathways for fatty acids production in E. coli. Nature Communications , 2013, 4 : 1409–1416. DOI:10.1038/ncomms2425 |
| [10] | Liu H, Yu C, Feng D, et al. Production of extracellular fatty acid using engineered Escherichia coli. Microbial Cell Factories , 2012, 11 (1) : 1. DOI:10.1186/1475-2859-11-1 |
| [11] | Liu D, Xiao Y, Evans B S, et al. Negative feedback regulation of fatty acid production based on a malonyl-CoA sensor-actuator. ACS Synthetic Biology , 2014, 4 (2) : 132–140. |
| [12] | Xu P, Li L, Zhang F, et al. Improving fatty acids production by engineering dynamic pathway regulation and metabolic control. Proc Natl Acad Sci USA , 2014, 111 (31) : 11299–11304. DOI:10.1073/pnas.1406401111 |
| [13] | Yu X, Liu T, Zhu F, et al. In vitro reconstitution and steady-state analysis of the fatty acid synthase from Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America , 2011, 108 (46) : 18643–18648. DOI:10.1073/pnas.1110852108 |
| [14] | Clomburg J M, Vick J E, Blankschien M D, et al. A synthetic biology approach to engineer a functional reversal of the β-oxidation cycle. ACS Synthetic Biology , 2012, 1 (11) : 541–554. DOI:10.1021/sb3000782 |
| [15] | Dellomonaco C, Clomburg J M, Miller E N, et al. Engineered reversal of the beta-oxidation cycle for the synthesis of fuels and chemicals. Nature , 2011, 476 (7360) : 355–359. DOI:10.1038/nature10333 |
| [16] | Wu H, San K Y. Efficient odd straight medium chain free fatty acid production by metabolically engineered Escherichia coli. Biotechnology and Bioengineering , 2014, 111 (11) : 2209–2219. DOI:10.1002/bit.25296 |
| [17] | Wu H, San K Y. Engineering Escherichia coli for odd straight medium chain free fatty acid production. Applied Microbiology and Biotechnology , 2014, 98 (19) : 8145–8154. DOI:10.1007/s00253-014-5882-5 |
| [18] | Ranganathan S, Tee T W, Chowdhury A, et al. An integrated computational and experimental study for overproducing fatty acids in Escherichia coli. Metabolic Engineering , 2012, 14 (6) : 687–704. DOI:10.1016/j.ymben.2012.08.008 |
| [19] | San K Y, Li M, Zhang X. Bacteria and Method for Synthesizing Fatty Acids:US, 20140093921 A1. 2014-04-03. |
| [20] | Davis M S, Solbiati J, Cronan J E Jr. Overproduction of acetyl-CoA carboxylase activity increases the rate of fatty acid biosynthesis in Escherichia coli. The Journal of Biological Chemistry , 2000, 275 (37) : 28593–28598. DOI:10.1074/jbc.M004756200 |
| [21] | Zha W, Rubin-Pitel S B, Shao Z, et al. Improving cellular malonyl-CoA level in Escherichia coli via metabolic engineering. Metabolic Engineering , 2009, 11 (3) : 192–198. DOI:10.1016/j.ymben.2009.01.005 |
| [22] | Lu X, Vora H, Khosla C. Overproduction of free fatty acids in E. coli:implications for biodiesel production. Metabolic Engineering , 2008, 10 (6) : 333–339. DOI:10.1016/j.ymben.2008.08.006 |
| [23] | Zhang X, Agrawal A, San K Y. Improving fatty acid production in Escherichia coli through the overexpression of malonyl coA-Acyl carrier protein transacylase. Biotechnology Progress , 2012, 28 (1) : 60–65. DOI:10.1002/btpr.716 |
| [24] | Lee S, Jeon E, Yun H S, et al. Improvement of fatty acid biosynthesis by engineered recombinant Escherichia coli. Biotechnology and Bioprocess Engineering , 2011, 16 (4) : 706–713. DOI:10.1007/s12257-011-0034-6 |
| [25] | Heath R J, Rock C O. Regulation of fatty acid elongation and initiation by Acyl-Acyl carrier protein in Escherichia coli. The Journal of Biological Chemistry , 1996, 271 (4) : 1833–1836. DOI:10.1074/jbc.271.4.1833 |
| [26] | Jiang P, Cronan J E, J r. Inhibition of fatty acid synthesis in Escherichia coli in the absence of phospholipid synthesis and release of inhibition by thioesterase action. Journal of Bacteriology , 1994, 176 (10) : 2814–2821. DOI:10.1128/jb.176.10.2814-2821.1994 |
| [27] | Cho H, Cronan J E Jr. Defective export of a periplasmic enzyme disrupts regulation of fatty acid synthesis. The Journal of Biological Chemistry , 1995, 270 (9) : 4216–4219. DOI:10.1074/jbc.270.9.4216 |
| [28] | Choi Y J, Lee S Y. Microbial production of short-chain alkanes. Nature , 2013, 502 (7472) : 571–574. DOI:10.1038/nature12536 |
| [29] | Zhang X, Li M, Agrawal A, et al. Efficient free fatty acid production in Escherichia coli using plant acyl-ACP thioesterases. Metabolic Engineering , 2011, 13 (6) : 713–722. DOI:10.1016/j.ymben.2011.09.007 |
| [30] | Zheng Y, Li L, Liu Q, et al. Boosting the free fatty acid synthesis of Escherichia coli by expression of a cytosolic acinetobacter baylyi thioesterase. Biotechnology for Biofuels , 2012, 5 (1) : 76–87. DOI:10.1186/1754-6834-5-76 |
| [31] | Lee S, Park S, Lee J. Improvement of free fatty acid production in Escherichia coli using codon-optimized Streptococcus pyogenes acyl-ACP thioesterase. Bioprocess and Biosystems Engineering , 2013, 36 (10) : 1519–1525. DOI:10.1007/s00449-012-0882-2 |
| [32] | Tyo K E, Ajikumar P K, Stephanopoulos G. Stabilized gene duplication enables long-term selection-free heterologous pathway expression. Nature Biotechnology , 2009, 27 (8) : 760–765. DOI:10.1038/nbt.1555 |
| [33] | Pfleger B F, Pitera D J, Smolke C D, et al. Combinatorial engineering of intergenic regions in operons tunes expression of multiple genes. Nature Biotechnology , 2006, 24 (8) : 1027–1032. DOI:10.1038/nbt1226 |
| [34] | De Mey M, Maertens J, Lequeux G J, et al. Construction and model-based analysis of a promoter library for E. coli:an indispensable tool for metabolic engineering. BMC Biotechnology , 2007, 7 : 34–47. DOI:10.1186/1472-6750-7-34 |
| [35] | Alper H, Fischer C, Nevoigt E, et al. Tuning genetic control through promoter engineering. Proceedings of the National Academy of Sciences of the United States of America , 2005, 102 (36) : 12678–12683. DOI:10.1073/pnas.0504604102 |
| [36] | Salis H M, Mirsky E A, Voigt C A. Automated design of synthetic ribosome binding sites to control protein expression. Nature Biotechnology , 2009, 27 (10) : 946–950. DOI:10.1038/nbt.1568 |
| [37] | Xu P, Wang W, Li L, et al. Design and kinetic analysis of a hybrid promoter-regulator system for malonyl-CoA sensing in Escherichia coli. ACS Chemical Biology , 2013, 9 (2) : 451–458. |
| [38] | Schujman G E, Guerin M, Buschiazzo A, et al. Structural basis of lipid biosynthesis regulation in Gram-positive bacteria. The EMBO Journal , 2006, 25 (17) : 4074–4083. DOI:10.1038/sj.emboj.7601284 |
| [39] | Schujman G E, Paoletti L, Grossman A D, et al. FapR, a bacterial transcription factor involved in global regulation of membrane lipid biosynthesis. Developmental Cell , 2003, 4 (5) : 663–672. DOI:10.1016/S1534-5807(03)00123-0 |
| [40] | Liu T, Vora H, Khosla C. Quantitative analysis and engineering of fatty acid biosynthesis in E. coli. Metabolic Engineering , 2010, 12 (4) : 378–386. DOI:10.1016/j.ymben.2010.02.003 |
| [41] | Keating D H, Carey M R, Cronan J E. The unmodified (apo) form of Escherichia coli acyl carrier protein is a potent inhibitor of cell growth. Journal of Biological Chemistry , 1995, 270 (38) : 22229–22235. DOI:10.1074/jbc.270.38.22229 |
| [42] | Xiao X, Yu X, Khosla C. Metabolic flux between unsaturated and saturated fatty acids is controlled by the FabA:FabB ratio in the fully reconstituted fatty acid biosynthetic pathway of Escherichia coli. Biochemistry , 2013, 52 (46) : 8304–8312. DOI:10.1021/bi401116n |
| [43] | Dellomonaco C, Rivera C, Campbell P, et al. Engineered respiro-fermentative metabolism for the production of biofuels and biochemicals from fatty acid-rich feedstocks. Applied and Environmental Microbiology , 2010, 76 (15) : 5067–5078. DOI:10.1128/AEM.00046-10 |
| [44] | Eppler T, Boos W. Glycerol-3-phosphate-mediated repression of malT in Escherichia coli does not require metabolism, depends on enzyme ⅡAGlc and is mediated by cAMP levels. Molecular Microbiology , 1999, 33 (6) : 1221–1231. |
| [45] | Cho B K, Knight E M, Palsson B O. Transcriptional regulation of the fad regulon genes of Escherichia coli by ArcA. Microbiology , 2006, 152 (8) : 2207–2219. DOI:10.1099/mic.0.28912-0 |
| [46] | Zhuang Q, Wang Q, Liang Q, et al. Synthesis of polyhydroxyalkanoates from glucose that contain medium-chain-length monomers via the reversed fatty acid β-oxidation cycle in Escherichia coli. Metabolic Engineering , 2014, 24 : 78–86. DOI:10.1016/j.ymben.2014.05.004 |
| [47] | Clomburg J M, Blankschien M D, Vick J E, et al. Integrated engineering of β-oxidation reversal and ω-oxidation pathways for the synthesis of medium chain ω-functionalized carboxylic acids. Metabolic Engineering , 2015, 28 : 202–212. DOI:10.1016/j.ymben.2015.01.007 |
| [48] | Kim S, Clomburg J M, Gonzalez R. Synthesis of medium-chain length (C6~C10) fuels and chemicals via β-oxidation reversal in Escherichia coli. Journal of Industrial Microbiology & Biotechnology , 2015, 42 (3) : 465–475. |
| [49] | Haushalter R W, Groff D, Deutsch S, et al. Development of an orthogonal fatty acid biosynthesis system in E. coli for oleochemical production. Metabolic Engineering , 2015, 30 : 1–6. DOI:10.1016/j.ymben.2015.04.003 |
| [50] | Youngquist J T, Lennen R M, Ranatunga D R, et al. Kinetic modeling of free fatty acid production in Escherichia coli based on continuous cultivation of a plasmid free strain. Biotechnology and Bioengineering , 2012, 109 (6) : 1518–1527. DOI:10.1002/bit.24420 |
| [51] | Youngquist J T, Rose J P, Pfleger B F. Free fatty acid production in Escherichia coli under phosphate-limited conditions. Applied Microbiology and Biotechnology , 2013, 97 (11) : 5149–5159. DOI:10.1007/s00253-013-4911-0 |
| [52] | Leber C, Polson B, Fernandez-Moya R, et al. Overproduction and secretion of free fatty acids through disrupted neutral lipid recycle in Saccharomyces cerevisiae. Metabolic Engineering , 2015, 28 : 54–62. DOI:10.1016/j.ymben.2014.11.006 |
| [53] | Rutter C D, Zhang S, Rao C V. Engineering Yarrowia lipolytica for production of medium-chain fatty acids. Applied Microbiology and Biotechnology , 2015, 99 (17) : 7359–7368. DOI:10.1007/s00253-015-6764-1 |
| [54] | Wu J, Du G, Chen J, et al. Enhancing flavonoid production by systematically tuning the central metabolic pathways based on a CRISPR interference system in Escherichia coli. Scientific Reports , 2015, 5 : 13477–13490. DOI:10.1038/srep13477 |
| [55] | Na D, Yoo S M, Chung H, et al. Metabolic engineering of Escherichia coli using synthetic small regulatory RNAs. Nature Biotechnology , 2013, 31 (2) : 170–174. DOI:10.1038/nbt.2461 |
| [56] | Si T, Luo Y, Bao Z, et al. RNAi-assisted genome evolution in Saccharomyces cerevisiae for complex phenotype engineering. ACS Synthetic Biology , 2014, 4 (3) : 283–291. |
 2016, Vol. 36
2016, Vol. 36