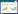文章信息
- 堵晶晶, 李强, 程霄, 沈林園, 李学伟, 张顺华, 朱砺.
- DU Jing-jing, LI Qiang, CHENG Xiao, SHEN Lin-yuan, LI Xue-wei, ZHANG Shun-hua, ZHU Li.
- CRISPR/Cas系统的研究进展及其在畜禽遗传改良中的应用前景
- Research Progress in CRISPR/Cas System and the Prospect in Animal Genetic Improvement
- 中国生物工程杂志, 2016, 36(7): 92-103
- CHINA BIOTECHNOLOGY, 2016, 36(7): 92-103
- http://dx.doi.org/DOI:10.13523/j.cb.20160713
-
文章历史
- 收稿日期: 2016-01-12
- 修回日期: 2016-02-28
2. 四川省畜牧总站 成都 610041
2. Sichuan Province General Station of Animal Husbandry, Chengdu 610041, China
运用基因敲除、过表达、诱变等手段对基因组特定位点进行修饰是研究基因生物学功能、治疗人类遗传性疾病、改变生物遗传性状的重要手段。与将目的基因随机导入、整合到基因组,或利用同源重组介导的打靶方式敲除目的基因而得到转基因机体的传统基因组编辑技术相比,近几年迅速发展起来的人工核酸酶介导的基因组编辑技术则具有编辑效率高、精确度高、成本低廉、易于操作等优点[1-2]。它是以一类可识别特定序列的核酸酶在DNA上切割形成双链DNA断裂(DNA double stranded break, DSB),之后借助细胞自身的修复系统,如非同源末端链接(non-homologous end joining, NHRJ)或同源重组(homology directed repair, HDR)修复DNA断裂,并对DNA断点附近的序列进行编辑,从而在不同生物或细胞中实现有效的基因定点编辑的新型技术,其作用机制如图 1所示[3]。
|
| 图 1 核酸酶介导基因编辑[3] Figure 1 Nuclease-induced genome editing Nuclease-induced double strand breaks(DSBs) can be repaired by nonhomologous end joining(NHEJ) or homology-directed repair(HDR) pathways. Imprecise NHEJ-mediated repair can produce insertion and/or deletion mutations of variable length at the site of the DBS. HDR-mediated repair can introduce precise point mutations or insertions from a single-stranded or double strand DNA donor template |
目前,已广泛运用于基因组定点编辑的人工核酸酶主要有3种:锌指内切核酸酶(zinc-finger nuclease, ZFN)[4-6]、类转录激活因子效应物核酸酶(transcripition activator-like effector nuclease, TALEN)[7-8]和CRISPR/Cas系统[3, 9-13]。其中,CRISPR/Cas系统是细菌及古细菌中的一种适应性免疫保护机制[14-15]。CRISPR/Cas系统可通过介导外源DNA降解实现抵抗病毒和外源DNA入侵[16]。同时,作为应用潜力最大的基因定点编辑方法,CRISPR/Cas系统在人源细胞染色体修饰上的成功应用更是极大地简化了当前的基因组编辑技术,该技术在2013年被Science杂志评为年度十大突破技术之首(http://news.sciencemag.org/breakthrough-of-the-year-2013"。)
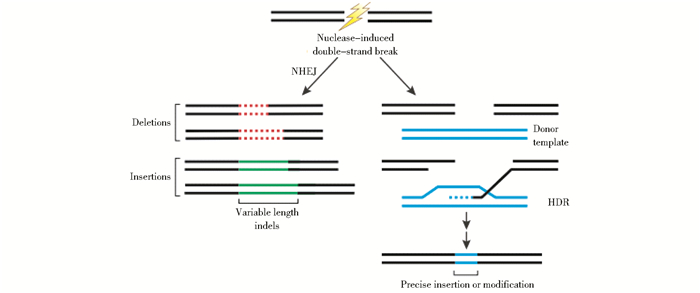
1 CRISPR/Cas系统的基本结构天然存在的CRISPR/Cas系统由CRISPR序列元件和Cas基因家族组成,其基本组成如图 2所示[17]。CRISPR(clustered regularly interspaced short palindromic repeat sequences)是广泛存在于原核生物基因中成簇的被不同间隔序列规律间隔的短回文重复序列[18],结构较为稳定。其中,源于外源基因片段(主要源于质粒与噬菌体)[19]、高度可变的间隔序列长度为21~72bp[20],同一个CRISPR位点基本不会出现相同或较相似的间隔序列,不同CRISPR基因间的间隔序列数目也存在较大差异;而长度为23~50bp,包含部分回文结构、二级结构稳定的重复序列虽整体不严格保守[21-23],但序列为GTTT/g的5′端和序列为GAAAC的3′端部分却保守。此外,CRISPR位点还存在一个类似于启动子、富含A/T、长度为300~500bp的前导区[24-25],该前导区位于CRISPR位点上游,具有种内保守、种间差异显著的特点[22]。研究发现,新的同向重复-间隔序列总是插入前导序列与其原来邻近的重复序列之间,这表明前导序列可为新插入的序列提供识别位点[26]。
|
| 图 2 CRISPR结构图[18] Figure 2 Features of CRISPR loci Typically, clustered, regularly interspaced short palindromic repeats (CRISPRs, white boxes) are preceded by a leader sequence (black box) that is AT-rich but otherwise not conserved. The number of repeats can vary substantially, from a minimum of two to a few hundred. Repeat length, however, is restricted to 23 to 50 nucleotides. Repeats are separated by similarly sized, non-repetitive spacers (coloured boxes) that share sequence identity with fragments of plasmids and bacteriophage genomes and specify the targets of CRISPR interference. A set of CRISPR-associated (cas) genes immediately precedes or follows the repeats. These genes are conserved, can be classified into different families and subtypes, and encode the protein machinery responsible for CRISPR activity |
Cas(CRISPR-associated gene, Cas gene)作为存在于CRISPR位点一端的几组编码蛋白质的基因序列。其编码的蛋白质称为Cas蛋白,是CRISPR免疫防御途径中的基本组成成分。研究发现,Cas是一类与CRISPR重复序列相连且与CRISPR相关、数目多达45个[27]的多态性家族蛋白,通常位于CRISPR位点下游或分散于基因组的其他位置(表 1)。尽管目前对Cas蛋白的分类并不统一,但研究证明Cas对CRISPR功能的行使起到了重要作用[28],如Cas3蛋白可扮演解旋酶、核酸酶的角色[30],在靶基因剪切过程中发挥重要作用。而Cas1蛋白和Cas2蛋白则主要在获得新的重复序列过程中发挥作用[30]。
| Core proteins | Example locus | Specific | ||
| HMM | COG | Putative function/Family | ||
| Cas1 | AF1878 | TIGR00287 | COG1518 | Putative novel nuclease |
| Cas2 | AF1876 | TIGR01573 | COG1343, COG3512 | — |
| CT1918 | TIGR01873 | COG1343 | — | |
| Cas3 | AF1874 | TIGR01587 | COG1203 | Helicase (PF00271) |
| AF1875 | TIGR01596 | COG2254 | Nuclease (PF01966) | |
| YPO2467 | TIGR02562 | COG1203 | Helicase (PF00271) | |
| Cas4 | AF1877 | TIGR00372 | COG1468 | RecB-family exonuclease |
| Cas5 | AF1872 | TIGR02593 | — | — |
| Cas6 | AF1859 | TIGR01877 | COG1583 | — |
| Notes: HMM, Hidden Markov models; COG, Clusters of orthologous groups | ||||
2 CRISPR/Cas系统作用原理
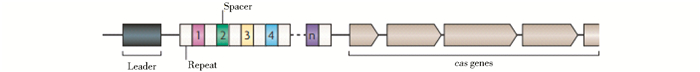
基因组编辑技术的核心是将DNA双链定点切开,造成双链断裂缺口。尽管锌指蛋白内切核酸酶、类转录激活因子效应物核酸酶、CRISPR/Cas系统的本质都为核酸酶,都能根据研究的需要,设计在基因组的特定位置造成DNA双键断裂,但三者进行基因组定点编辑的作用机制却是不同的。其中,发展与运用均较为成熟的锌指蛋白内切核酸酶(ZFN)和类转录激活因子效应物核酸酶(TALEN)技术的原理分别为利用锌指蛋白、TALE蛋白识别特定DNA序列并与之结合[31-33],但CRISPR/Cas系统的作用机制则类似RNA干扰机制(又称为CRISPRi)[17, 23],其作用机制主要包括三个方面,作用机制如图 3所示[14]。总的来说,当噬菌体入侵宿主细胞后,CRISPR/Cas系统中的Cas蛋白复合物可靶向裂解噬菌体基因组内短的原型间隔序列,加工成新的R-S序列后重组到CRISPR簇内(CRISPR位点的5′端)。当噬菌体再次入侵细菌时,CRISPR簇首先转录为长的crRNA(CRISPR RNA)前体,并逐步加工为小的成熟的crRNA-Cas蛋白复合体,以ciRNAs作为模板,通过碱基互补配对精确地与目标DNA(target DNA)相结合,Cas蛋白进而对目标DNA进行断裂、降解[34-36]。
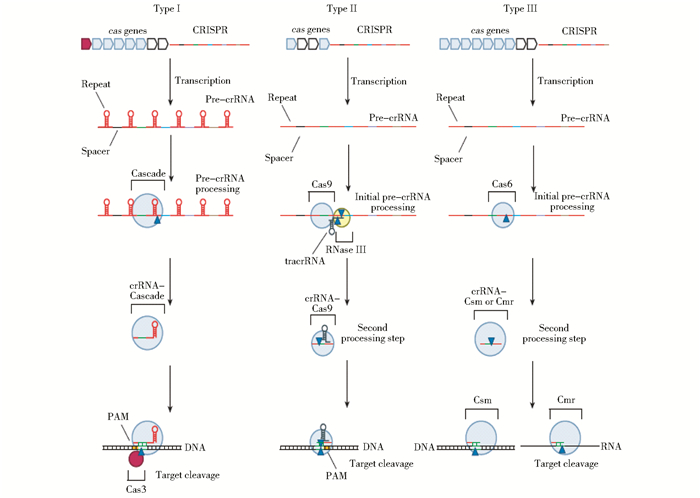
|
| 图 3 Ⅰ、Ⅱ、Ⅲ型CRISPR/Cas系统表达、干扰阶段的作用机制[13] Figure 3 Schematic overview of the Type I, II, and III CRISPR (clustered regularly interspaced short palindromic repeats) expression and interference stages Three main types of CRISPR/Cas (CRISPR-associated) systems are now known, and these display major mechanistic differences. In this schematic overview, the expression and interference stages are depicted for the three different types. Transcription of the CRISPR gives rise to a pre-crRNA molecule, which is subsequently cleaved in the repeat sequence by a Cas6 homolog in Type I (often a subunit of Cascade-like complexes) and Type III systems, and by RNase III and Cas9 in Type II systems. The generated crRNA molecules undergo a further processing event in Type II and Type III systems. Mature crRNA molecules are bound to a Cas protein or protein complex. In the case of Type III-B, the CRISPR RAMP Module (Cmr) complex binds and cleaves complementary RNA, whereas the other types bind and cleave dsDNA (double-stranded DNA). Targets contain a protospacer-adjacent motif (PAM) either downstream (Type I) or upstream (Type II) of the protospacer |
CRISPR/Cas系统干扰的第一阶段不仅是免疫的适应过程也是信息处理的阶段。此阶段的作用机制在不同CRISPR系统中呈高度保守趋势,Cas1和Cas2蛋白为其主要作用蛋白质[37]。此阶段的主要作用机制为:在噬菌体等入侵宿主细胞后,CRISPR系统会从外来的噬菌体或质粒序列中选取一段特定序列(原间隔序列,proto-spacere)插入到前导序列与第一段重复序列之间,而每一次插入活动都会带来重复序列的复制,最终加工成新的同向重复-间隔序列,并非同源重组到CRISPR簇内,从而让宿主获得抵抗相应噬菌体等再次入侵的免疫能力[38-39]。
Bolotin等[40]于2005年研究发现,CRISPR位点中间隔序列的数目与噬菌体敏感性呈负相关,即间隔序列数目越多,宿主细胞对噬菌体的抵抗能力就越强。但新间隔序列的选取并非随机完成。目前,多个研究中不仅发现噬菌体和质粒中存在一系列原间隔序列,而且在其附近鉴定出一系列位于原间隔序列5′端或3′端的邻近基序(protospacer adjacent motif, PAM)[41-42],如表 2所示,相关研究已证明不同CRISPR亚型识别的PAM基序具有亚型特异性[43]。
| Type | Species | PAM(5′-3′) | Typical repeat | CRISPR cluster | References |
| TypeⅠ | Listeriamonocytogenes | Protospacer-NGG | GTTTTAACTACTT ATTATGAAATCT AAAT |
1 | [43] |
| Sulfolobussolfataricus P2 | Protospacer-NGG | GATAATCTCTTA TAGAATTGAAAGb |
7 | [42-44] | |
| Escherichia coli K12 | Protospacer-CTT Protospacer-CAT Protospacer-CCT Protospacer-CTC |
GWGTTCCCCGCG CCAGCGGGGAT AAACCGb |
2 | [43, 45-47] | |
| TypeⅡA | S.thermophilus | TTTYRNNN-protospace | GTTTTTGTACTCT CAAGATTTAAGT AACTGTACAAC |
10 | [40] |
| Streptococcusthermophilus | WTTCTNN-protospace | GTTTTTGTACTCT CAAGATTTAAGT AACTGTACAAC |
10 | [41] | |
| TypeⅡB | S.pyogenes | CCN-protospacer | GTTTTAGAGCTA TGCTGTTTTGAA TGGTCCCAAAACb |
10 | [43] |
| S.thermophilus | CNCCN-protospacer | GTTTTAGAGCTG TGTTGTTTCGAA TGGTTCCAAAAC |
10 | [41] | |
| TypeⅢ | S.solfataricus | No PAM | GATTAATCCCAA AAGGAATTGAAAGb |
7 | [48] |
| Staphylococcusepidermidis | No PAM | GATCGATACCCA CCCCGAAGAAA AGGGGACGAGAACb |
8 | [49] | |
| Note:a, PAM and protospacer correspond to sequence on target strand (i.e. strand that base-pairs with crRNA); b, Direction of CRISPR transcription verified | |||||
2.2 CRISPR的转录、加工
在第一阶段,由于CRISPR系统会从入侵的噬菌体序列中选取一段序列,加工并整合重组到CRISPR簇内,宿主从而获得抵抗噬菌体再次入侵的能力。当噬菌体再次入侵时,CRISPR/Cas系统干扰的第二阶段,即免疫表达过程便会启动。此阶段作用机制在不同CRISPR系统间存在较大差异,且主要作用蛋白质也存在差别。当同类嗜菌体再次入侵宿主细胞时,CRISPR位点转录水平迅速上升[50],CRISPR簇首先由前导序列末端的启动子处转录为包含重复序列和间隔序列的crRNA(CRISPR RNA)前体[51],然后Cas蛋白在Cas内切酶作用下被逐步切割加工成更小的成熟的crRNA。最后,成熟crRNA与Cas蛋白形成具有特殊功能的复合物,即crRNP(crRNA-Cas ribonucleoprotein),参与CRISPR干扰[52]。
此外,研究还表明,CRISPR簇和Cas基因的转录受宿主内多种因素调控[53]。例如,大肠杆菌内,类组蛋白拟核构造蛋白(histone-like nucleoid structuring protein, H-NS)能与CRISPR簇和Cas基因的启动子结合,进而抑制其转录[54-55]。
2.3 CRISPR/Cas系统的介导与沉默当CrRNA/Cas蛋白复合体形成后,基于碱基互补配对原则,Cas蛋白可对目标DNA进行断裂、降解,即启动CRISPR/Cas系统干扰的第三阶段-免疫的干扰执行过程。其干扰机制如图 4所示[56]。此阶段的作用机制特点与表达阶段具有共性,不同CRISPR系统的作用机制存在较大差异,主要作用蛋白质也存在差别。在干扰阶段,crRNP以crRNA为向导迅速找到并特异性结合到其互补的外源DNA片段上。当crRNA与原间隔序列互补配对结束时,作用复合体会将外源DNA降解,从而达到保护宿主细胞的目的。然而,成熟crRNA的直接靶标是DNA还是RNA至今是个迷[57-59]。
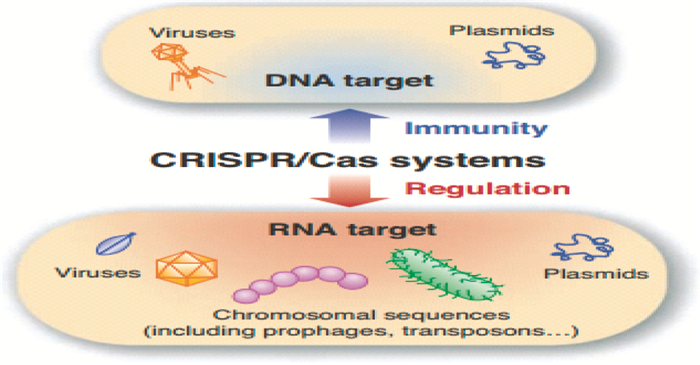
|
| 图 4 CRISPR干扰 Figure 4 CRISPR interference The CRISPR/Cas systems may target either DNA or RNA to interfere with viruses, plasmids, prophages, or other chromosomally encoded sequence |
Cas基因多样性丰富,目前研究已发现的Cas蛋白家族成员超过45个。因此,简单分类很难区分那些同源功能并不相关的Cas蛋白[27]。但按照目前较为公认的RISPR/Cas系统分类方法,即综合干扰执行阶段的差异性、免疫阶段的高度保守性、Cas基因核心元件序列的差异性及Cas蛋白结构等将其划分为3种类型。尽管每种类型的CAISPR/Cas系统均包含精确识别切割位点的特异性非编码RNA和具有核酸酶活性的CRISPR相关Cas基因,但三者在表达和干扰阶段均存在较大区别(图 3)。
3.1 Ⅰ型CRISPR/Cas系统在细菌和古细菌中发现的Ⅰ型CRISPR/Cas系统包含6个Cas蛋白,其中具有解旋酶和核酸酶功能的Cas3蛋白是其特征性蛋白[60]。在表达和干扰阶段,系统内多个Cas基因编码生成具有将长链crRNA前体加工为成熟crRNA功能的Cas蛋白,再与成熟crRNA结合形成能与入侵的外源DNA结合的病毒防御复合物CASCAD(CRISPR associated complex for antivirus defense, CASCD)。当CASCAD与外源DNA结合后,CASCAD内的crRNA会与互补配对的外源DNA链形成R环结构,之后Cas行使核酸酶功能,先识别R环结构再切开先前的互补链。最后,Cas再行使核酸酶、解旋酶功能,切开非互补链,降解靶标DNA[61-62]。
3.2 Ⅱ型CRISPR/Cas系统作为改造最为成功的人工核酸梅,Ⅱ型CRISPR/Cas系统最为简单,运用也最为广泛。其作用机制如图 3所示[11]。其中,由1 409个氨基酸组成,且含有位于蛋白质中间位置的HNH核酸酶结构域和位于氨基端的Ruvc-like结构域的多功能性蛋白-Cas9是其唯一的标志性蛋白质。在干扰执行阶段,HNH核酸酶结构域负责切割与crRNA互补配对的模板链,Ruvc-like结构域则切割有义链[63]。同时,在成熟crRNA与反式编码小RNA(trans-encoded small RNA, tracrRNA)互补配对形成的RNA二聚体指导下,Cas9蛋白对靶位点进行精确切割[64],之后再引入一段目的基因同源序列进行同源交换,修复目的基因。此外,Ⅱ型CRISPR/Cas系统仅存于细菌,其作用的发挥还需RNAseⅢ的参与。
3.3 Ⅲ型CRISPR/Cas系统相比较而言,大多数存在于古细菌的Ⅲ型CRISPR/Cas系统较为复杂。根据靶标的不同,可将其分为TypeⅢA(如激烈嗜热球菌的CRISPR/Cas系统)和TypeⅢB(如表皮葡萄球菌的CRISPR/Cas系统)两种亚型[58]。其中TypeⅢA的靶标为mRNA[58],TypeⅢB的靶标与Ⅰ型、Ⅱ型CRISPR/Cas系统的靶标均为DNA[57]。研究发现,具有RNA酶活性的Cas10蛋白为该系统特征性蛋白[65],它与CASCD的功能相似,主要参与crRNA的成熟和剪切外源DNA。
4 CRISPR/Cas系统的优势传统的基因组编辑技术不仅耗时久且成功率低,在敲除或敲入目的基因时必须经过三个繁琐步骤,即先在胚胎干细胞上完成基因组DNA的遗传修饰,再将修饰后的胚胎干细胞注射至囊胚以获得嵌合体后代,最后通过生殖系统获得突变体动物。而人工核酸酶介导的基因组编辑技术的出现为定点突变技术的发展迎来了新高潮。
ZFN、TALEN及CRISPR/Cas系统的本质都是人工核酸酶。虽然ZFN、TALEN打靶技术已比较成熟,但二者操作技术难控制、载体构建耗时久、成本昂贵。特别是理论上每隔500bp才有一个ZFN位点,每隔35bp才有一个TALEN位点[66],而较理论上每隔8bp就能找到一个靶位点的CRISPR/Cas系统[67],前二者的靶位点数目受到严重限制。CRISPR/Cas系统是新近发展起来的,运用与研究均处于起步阶段,但其具有诸多前两者不可比拟的优势。其不仅基因编辑效率高[68-69]、范围广泛[70]、操作相对简单、成本相对低廉,而且还可实现对真核细胞的基因编辑[12, 71-72];特别是只有一个Cas9作用蛋白的Ⅱ型CRISPR/Cas系统(CRISPR/Cas9系统),其载体构建相对较为简单。同时,CRISPR/Cas系统是一个天然存在于原核生物里的RNA干扰系统,由crRNA指导其介导的基因编辑,对靶序列的识别依赖于RNA与DNA的碱基互补配对,相对蛋白质对DNA序列的识别,其基因组定点编辑要精确得多。
目前研究表明,CRISPR/Cas系统的基因导入效率达到51%~79%,远大于TALEN的0%~34%[73];CRISPR/Cas系统可同时对两个位点切割,并在两个位点同时引入LoxP序列以构建条件性敲除的位点,缩短突变动物获得时间[12]。此外,还有研究表明,CRISPR/Cas系统具有可逆性[74]。
5 CRISPR/Cas系统的应用及其在畜禽遗传改良中的运用前景基因组定点编辑技术的运用不仅可以打破物种界限,还可以扩大基因的利用范围。作为最新发展起来的基因组定点编辑技术,CRISPR/Cas系统正被广泛运用于基因治疗[75-76]、基因功能研究[77-79]以及建立与人类疾病相关的动物模型[80-81]等方面的研究。目前,已有的成功典型研究实例如表 3所示。畜禽主要作为人类日常食用肉品而发挥作用,但截至目前,尚未在世界范围内建立一个成熟的转基因动物评价体系,消费者、学界对转基因食品的安全性也未达成共识。此外,外源随机整合的转基因在受体内可控性差,很难获取理想且表型稳定的动物。因此,转基因技术在畜禽遗传改良方面的运用是较为缓慢的。
| Specise | Cell/tissue | Gene | Description | Reference |
| Human | 293T cell | HBV cccDNA | Cas9/sgRNA组合特异性地使HBV病毒DNA总量水平降低1 000倍、乙肝病毒cccDNA水平降低10倍, 且可突变灭活大部分残余病毒DNA | [84] |
| Human | HeLa, 293T cell | E6, E7 | 在E6或E7基因中删除和插入突变,可影响细胞周期,造成细胞凋亡。Cas9/sgRNA组合可有效地影响HPV引起的癌症的治疗效果 | [85] |
| Mouse | Embryonic stem | More than five genes | 提供了一套详细的可在胚胎水平将核酸注入细胞质中,在4周内便可获得基因修饰小鼠的操作方法 | [86] |
| Swine | Embryo | Rosa-26 | 鉴定、标记了猪Rosa26基因座并开发了一种Cre-inducible EGFP reporter pig line | [87] |
| Danio rerio | Embryo | fh1, tia11, gsk3b, etc | 一种定制的CRISPR/Cas系统可被用于特异性地修饰斑马鱼,这种简单但功能强大的技术也可运用于其他物种研究 | [88] |
| Human | SACC-83 cell | FN | CRISPR/Cas9系统可以通过排除源于FN基因的EDA外显子来调控pro-oncogenic剪接,从而抑制癌细胞的扩散转移 | [89] |
| Cynomolgus monkey | Embryo | PPAR-γ, Rag1 | 运用Cas9 mRNA和sgRNA进行单细胞层面的胚胎共注射,实现食蟹猴基因修饰 | [90] |
杂交是目前畜禽遗传改良的主要手段。与ZFN相比,CRISPR/Cas系统在畜禽遗传改良方面的运用进展缓慢[82-83],但CRISPR/Cas系统在其他研究运用中展现出的诸多优势决定了其在畜禽遗传改良方面,特别是肉质改良、生长性状改良、抗病性改良等方面的运用依然值得期待。
5.1 肉质改良上的运用前景由于生活水平的限制,过去很长一段时间里人们对畜禽的遗传改良主要集中在生长速率和生产效率上,但随着生活水平的逐步提高,人们越来越注重畜禽的肉质风味。肉质的优劣受多个因素调控,特别是基因水平的调控。例如,研究已发现线粒体2, 4-双烯酰辅酶A还原酶(2, 4-dienoyl-COA reductase 1, DECR1)可能与猪脂肪沉积和肉质性状有直接关系[91-92],MRF(myogenic regulatory factors)家族基因参与肌纤维的调控[93-95],而肌肉抑制素Myostatin则会通过抑制骨骼肌细胞的增殖、分化来减少肌纤维数目[96],猪酸肉基因(rendement napole gene, RN)和氟烷基因(halothane gene, Hal)会引起PSE肉的产生[97-98]。因此,通过CRISPR/Cas系统在畜禽敲除有害肉质的基因或导入利于肉质的基因,如跨物种在畜禽敲入小秀丽线虫中可编码ω-3多不饱和脂肪酸的Fat-1基因,对肉质的改良显得尤为重要。最近,中国科学家利用CRISPR/Cas9,对抑制狗骨骼肌生长的基因(myostatin, MSTN)进行敲出,培育出两只肌肉发达的"大力神"狗,成功构建了世界首个基因敲出狗模型[99]。
5.2 生长性状改良方面的运用前景从遗传角度而言,高生长速率和高瘦肉率很难兼得,但二者的确是畜禽遗传改良一直追求的目标。研究发现,畜禽生长性状同样受多个遗传因子调控,如类胰岛素生长因子1(insulin-like growth factor-1, IGF-1)[100-101]、黑素皮质素受体4(melanocortin-4 receptor gene, MC4R)[102-103]和FRZB基因(Frizzled motif associated with bone development gene)[104-105]。之前的研究发现。猪的IFG2内含子2的SNP可调节IGF2在猪肌肉组织中的表达,显著提高其瘦肉率[106]。随着基因编辑技术的不断更新发展,基因对畜禽生长性状的正向调控或负向调控的效果将得到大大增强或减弱。
5.3 抗病性改良上的运用前景CRISPR/Cas9在细胞水平上可简便高效地实现多个位点的切割[80],因此可利用CRISPR/Cas9实现一次性将多个SNP位点引入种畜基因组,缩短育种时间。抗病营养和抗病育种是近几年畜禽研究的热点领域。利用基因组定点编辑技术,特别是CRISPR/Cas系统导入可提高畜禽抗病力的基因或敲除畜禽致病性基因是从遗传本质上改良畜禽抗病性的有效手段。研究发现,正常阮蛋白PrPc被其异构体PrPsc转化后可导致疯牛病,未来可利用CRISPR/Cas系统敲除PRNP基因[107-108]以降低牛群疯牛病发病率,或敲入MYH基因提高畜禽的免疫应答能力。
6 结语发现广泛存在于细菌、古细菌的CRISPR/Cas系统是目前炙手可热的新型基因组定点修饰技术。虽然目前其很多作用机制还不完全明晰,运用也大多局限在细胞水平,但相比较于传统的基因组编辑技术,ZFN、TALEN和CRISPR/Cas系统具有诸多的优势,如基因编辑效率高且构建方法简单、可实现多基因打靶且成本偏低等。
综上所述,CRISPR/Cas系统的出现极大地推进了生命科学的发展,其在基因功能研究、基因治疗、人类疾病模式动物的构建及畜禽遗传改良方面的运用值得深入研究,未来研究成果依然值得我们期待。
| [1] | Mussolino C, Cathomen T. RNA guides genome engineering. Nat Biotechnol , 2013, 31 (3) : 208–209. DOI:10.1038/nbt.2527 |
| [2] | Pan Y, Xiao L, Li A S, et al. Biological and biomedical applications of engineered nucleases. Molecular Biotechnology , 2013, 55 (1) : 54–62. DOI:10.1007/s12033-012-9613-9 |
| [3] | Sander J D, Joung J K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol , 2014, 32 (4) : 347–355. DOI:10.1038/nbt.2842 |
| [4] | Ramalingam S, London V, Kandavelou K, et al. Generation and genetic engineering of human induced pluripotent stem cells using designed zinc finger nucleases. Stem Cells Dev , 2013, 22 (4) : 595–610. DOI:10.1089/scd.2012.0245 |
| [5] | Dong P, Yu F, Fan X, et al. Inhibition of ATIR by shRNA prevents collagen synthesis in hepatic stellate cells. Molecular and Cellular Biochemistry , 2010, 344 (1-2) : 195–202. DOI:10.1007/s11010-010-0542-2 |
| [6] | Carroll D, Beumer K J. Genome engineering with TALENs and ZFNs:repair pathways and donor design. Methods , 2014, 69 (2) : 137–141. DOI:10.1016/j.ymeth.2014.03.026 |
| [7] | Bedell V M, Wang Y, Campbell J M, et al. In vivo genome editing using a high-efficiency TALEN system. Nature , 2012, 491 (7422) : 114–118. DOI:10.1038/nature11537 |
| [8] | Sung Y H, Baek I J, Kim D H, et al. Knockout mice created by TALEN-mediated gene targeting. Nat Biotechnol , 2013, 31 (1) : 23–24. DOI:10.1038/nbt.2477 |
| [9] | Matzke M A, Mosher R A. RNA-directed DNA methylation:an epigenetic pathway of increasing complexity. Nature Reviews Genetics , 2014, 15 (6) : 394–408. DOI:10.1038/nrg3683 |
| [10] | Wang H, Yang H, Shivalila C S, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell , 2013, 153 (4) : 910–918. DOI:10.1016/j.cell.2013.04.025 |
| [11] | Chylinski K, Makarova K S, Charpentier E, et al. Classification and evolution of typeⅡCRISPR-Cas systems. Nucleic Acids Res , 2014, 42 (10) : 6091–6105. DOI:10.1093/nar/gku241 |
| [12] | Cong L, Ran F A, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems. Science , 2013, 339 (6121) : 819–823. DOI:10.1126/science.1231143 |
| [13] | Alaiyan B, Ilyayev N, Stojadinovic A, et al. Differential expression of colon cancer associated transcript1(CCAT1) along the colonic adenoma-carcinoma sequence. BMC cancer , 2013, 13 (1) : 196. DOI:10.1186/1471-2407-13-196 |
| [14] | Westra E R, Swarts D C, Staals R H, et al. The CRISPRs, they are a-changin:how prokaryotes generate adaptive immunity. Annu Rev Genet , 2012, 46 : 311–339. DOI:10.1146/annurev-genet-110711-155447 |
| [15] | Labrie S J, Samson J E, Moineau S. Bacteriophage resistance mechanisms. Nat Rev Microbiol , 2010, 8 (5) : 317–327. DOI:10.1038/nrmicro2315 |
| [16] | Barrangou R, Fremaux C, Deveau H, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science , 2007, 315 (5819) : 1709–1712. DOI:10.1126/science.1138140 |
| [17] | Marraffini L A, Sontheimer E J. CRISPR interference:RNA-directed adaptive immunity in bacteria and archaea. Nature Reviews Genetics , 2010, 11 (3) : 181–190. DOI:10.1038/nrg2749 |
| [18] | Lillestøl R, Redder P, Garrett R A, et al. A putative viral defence mechanism in archaeal cells. Archaea , 2006, 2 (1) : 59–72. DOI:10.1155/2006/542818 |
| [19] | Mojica F J, García-Martínez J, Soria E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. Journal of Molecular Evolution , 2005, 60 (2) : 174–182. DOI:10.1007/s00239-004-0046-3 |
| [20] | Magadán A H, Dupuis M-È, Villion M, et al. Cleavage of phage DNA by the Streptococcus thermophilus CRISPR3-Cas system. PLoS One , 2012, 7 (7) : e40913. DOI:10.1371/journal.pone.0040913 |
| [21] | Bland C, Ramsey T L, Sabree F, et al. CRISPR recognition tool (CRT):a tool for automatic detection of clustered regularly interspaced palindromic repeats. BMC Bioinformatics , 2007, 8 (1) : 209. DOI:10.1186/1471-2105-8-209 |
| [22] | Jansen R, Embden J, Gaastra W, et al. Identification of genes that are associated with DNA repeats in prokaryotes. Molecular Microbiology , 2002, 43 (6) : 1565–1575. DOI:10.1046/j.1365-2958.2002.02839.x |
| [23] | Deveau H, Garneau J E, Moineau S. CRISPR/Cas system and its role in phage-bacteria interactions. Annual review of Microbiology , 2010, 64 : 475–493. DOI:10.1146/annurev.micro.112408.134123 |
| [24] | Makarova K S, Grishin N V, Shabalina S A, et al. A putative RNA-interference-based immune system in prokaryotes:computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action. Biology Direct , 2006, 1 (1) : 7. DOI:10.1186/1745-6150-1-7 |
| [25] | Jansen R, van Embden J D, Gaastra W, et al. Identification of a novel family of sequence repeats among prokaryotes. Omics:A Journal of Integrative Biology , 2002, 6 (1) : 23–33. DOI:10.1089/15362310252780816 |
| [26] | Pourcel C, Salvignol G, Vergnaud G. CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology , 2005, 151 (3) : 653–663. DOI:10.1099/mic.0.27437-0 |
| [27] | Haft D H, Selengut J, Mongodin E F, et al. A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR/Cas subtypes exist in prokaryotic genomes. PLoS Computational Biology , 2005, 1 (6) : e60. DOI:10.1371/journal.pcbi.0010060 |
| [28] | Makarova K S, Haft D H, Barrangou R, et al. Evolution and classification of the CRISPR-Cas systems. Nature Reviews Microbiology , 2011, 9 (6) : 467–477. DOI:10.1038/nrmicro2577 |
| [29] | Han D, Krauss G. Characterization of the endonuclease SSO2001 from Sulfolobus solfataricus P2. FEBS Letters , 2009, 583 (4) : 771–776. DOI:10.1016/j.febslet.2009.01.024 |
| [30] | Wiedenheft B, Zhou K, Jinek M, et al. Structural basis for DNase activity of a conserved protein implicated in CRISPR-mediated genome defense. Structure , 2009, 17 (6) : 904–912. DOI:10.1016/j.str.2009.03.019 |
| [31] | Beane J D, Lee G K, Zheng Z, et al. Clinical scale zinc finger nuclease (ZFN)-driven gene-editing of PD-1 in tumor infiltrating lymphocytes (TIL) for the potential treatment of metastatic melanoma. Journal for Immunotherapy of Cancer , 2014, 2 (Suppl 3) : P2. DOI:10.1186/2051-1426-2-S3-P2 |
| [32] | Wood A J, Lo T-W, Zeitler B, et al. Targeted genome editing across species using ZFNs and TALENs. Science , 2011, 333 (6040) : 307–307. DOI:10.1126/science.1207773 |
| [33] | Umasankar P K, Ma L, Thieman J R, et al. A clathrin coat assembly role for the muniscin protein central linker revealed by TALEN-mediated gene editing. eLife , 2014, 10 (3) : 1–33. |
| [34] | Barrangou R, Fremaux C, Deveau H, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science , 2007, 315 (5819) : 1709–1712. DOI:10.1126/science.1138140 |
| [35] | Li T, Du B. CRISPR-Cas system and coevolution of bacteria and phages. Hereditas , 2011, 33 (3) : 213–218. |
| [36] | Garneau J E, Dupuis M-È, Villion M, et al. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature , 2010, 468 (7320) : 67–71. DOI:10.1038/nature09523 |
| [37] | Beloglazova N, Brown G, Zimmerman M D, et al. A novel family of sequence-specific endoribonucleases associated with the clustered regularly interspaced short palindromic repeats. Journal of Biological Chemistry , 2008, 283 (29) : 20361–20371. DOI:10.1074/jbc.M803225200 |
| [38] | Koonin E V, Wolf Y I. Genomics of bacteria and archaea:the emerging dynamic view of the prokaryotic world. Nucleic Acids Research , 2008, 36 (21) : 6688–6719. DOI:10.1093/nar/gkn668 |
| [39] | Sorek R, Kunin V, Hugenholtz P. CRISPR-a widespread system that provides acquired resistance against phages in bacteria and archaea. Nature Reviews Microbiology , 2008, 6 (3) : 181–186. DOI:10.1038/nrmicro1793 |
| [40] | Bolotin A, Quinquis B, Sorokin A, et al. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology , 2005, 151 (8) : 2551–2561. DOI:10.1099/mic.0.28048-0 |
| [41] | Horvath P, Romero D A, Coûté-Monvoisin A C, et al. Diversity, activity, and evolution of CRISPR loci in Streptococcus thermophilus. Journal of Bacteriology , 2008, 190 (4) : 1401–1412. DOI:10.1128/JB.01415-07 |
| [42] | Lillestøl R K, Shah S A, Brügger K, et al. CRISPR families of the crenarchaeal genus Sulfolobus:bidirectional transcription and dynamic properties. Molecular Microbiology , 2009, 72 (1) : 259–272. DOI:10.1111/mmi.2009.72.issue-1 |
| [43] | Mojica F, Diez-Villasenor C, Garcia-Martinez J, et al. Short motif sequences determine the targets of the prokaryotic CRISPR defence system. Microbiology , 2009, 155 (3) : 733–740. DOI:10.1099/mic.0.023960-0 |
| [44] | Gudbergsdottir S, Deng L, Chen Z, et al. Dynamic properties of the Sulfolobus CRISPR/Cas and CRISPR/Cmr systems when challenged with vector-borne viral and plasmid genes and protospacers. Molecular Microbiology , 2011, 79 (1) : 35–49. DOI:10.1111/j.1365-2958.2010.07452.x |
| [45] | Swarts D C, Mosterd C, Van Passel M W, et al. CRISPR interference directs strand specific spacer acquisition. PloS One , 2012, 7 (4) : e35888. DOI:10.1371/journal.pone.0035888 |
| [46] | Westra E R, van Erp P B, Künne T, et al. CRISPR immunity relies on the consecutive binding and degradation of negatively supercoiled invader DNA by Cascade and Cas3. Molecular Cell , 2012, 46 (5) : 595–605. DOI:10.1016/j.molcel.2012.03.018 |
| [47] | Semenova E, Jore M M, Datsenko K A, et al. Interference by clustered regularly interspaced short palindromic repeat (CRISPR) RNA is governed by a seed sequence. Proceedings of the National Academy of Sciences , 2011, 108 (25) : 10098–10103. DOI:10.1073/pnas.1104144108 |
| [48] | Zhang J, Rouillon C, Kerou M, et al. Structure and mechanism of the CMR complex for CRISPR-mediated antiviral immunity. Molecular Cell , 2012, 45 (3) : 303–313. DOI:10.1016/j.molcel.2011.12.013 |
| [49] | Marraffini L A, Sontheimer E J. Self versus non-self discrimination during CRISPR RNA-directed immunity. Nature , 2010, 463 (7280) : 568–571. DOI:10.1038/nature08703 |
| [50] | Agari Y, Sakamoto K, Tamakoshi M, et al. Transcription profile of Thermus thermophilus CRISPR systems after phage infection. Journal of Molecular Biology , 2010, 395 (2) : 270–281. DOI:10.1016/j.jmb.2009.10.057 |
| [51] | Kunin V, Sorek R, Hugenholtz P. Evolutionary conservation of sequence and secondary structures in CRISPR repeats. Genome Biol , 2007, 8 (4) : R61. DOI:10.1186/gb-2007-8-4-r61 |
| [52] | Marraffini L A, Sontheimer E J. Self versus non-self discrimination during CRISPR RNA-directed immunity. Nature , 2010, 463 (7280) : 568–571. DOI:10.1038/nature08703 |
| [53] | Mojica F J, Díez-Villaseñor C. The on-off switch of CRISPR immunity against phages in Escherichia coli. Molecular Microbiology , 2010, 77 (6) : 1341–1345. DOI:10.1111/j.1365-2958.2010.07326.x |
| [54] | Pul Ü, Wurm R, Arslan Z, et al. Identification and characterization of E.coliCRISPR-cas promoters and their silencing by H-NS. Molecular Microbiology , 2010, 75 (6) : 1495–1512. DOI:10.1111/mmi.2010.75.issue-6 |
| [55] | Westra E R, Pul V, Heidrich N, et al. H-NS-mediated repression of CRISPR-based immunity in Escherichia coli K12 can be relieved by the transcription activator LeuO. Molecular Microbiology , 2010, 77 (6) : 1380–1393. DOI:10.1111/j.1365-2958.2010.07315.x |
| [56] | Horvath P, Barrangou R. CRISPR/Cas, the immune system of bacteria and archaea. Science , 2010, 327 (5962) : 167–170. DOI:10.1126/science.1179555 |
| [57] | Marraffini L A, Sontheimer E J. CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science , 2008, 322 (5909) : 1843–1845. DOI:10.1126/science.1165771 |
| [58] | Hale C R, Zhao P, Olson S, et al. RNA-guided RNA cleavage by a CRISPR RNA-Cas protein complex. Cell , 2009, 139 (5) : 945–956. DOI:10.1016/j.cell.2009.07.040 |
| [59] | Van der Oost J, Brouns S J. RNAi:prokaryotes get in on the act. Cell , 2009, 139 (5) : 863–865. DOI:10.1016/j.cell.2009.11.018 |
| [60] | Sinkunas T, Gasiunas G, Fremaux C, et al. Cas3 is a single-stranded DNA nuclease and ATP-dependent helicase in the CRISPR/Cas immune system. The EMBO journal , 2011, 30 (7) : 1335–1342. DOI:10.1038/emboj.2011.41 |
| [61] | Wiedenheft B, van Duijn E, Bultema J B, et al. RNA-guided complex from a bacterial immune system enhances target recognition through seed sequence interactions. Proceedings of the National Academy of Sciences , 2011, 108 (25) : 10092–10097. DOI:10.1073/pnas.1102716108 |
| [62] | Haurwitz R E, Jinek M, Wiedenheft B, et al. Sequence-and structure-specific RNA processing by a CRISPR endonuclease. Science , 2010, 329 (5997) : 1355–1358. DOI:10.1126/science.1192272 |
| [63] | Friedland A E, Tzur Y B, Esvelt KM, et al. Heritable genome editing in C.elegans via a CRISPR-Cas9 system.Nature Methods. Nature Methods , 2013, 10 (8) : 741–743. DOI:10.1038/nmeth.2532 |
| [64] | Deltcheva E, Chylinski K, Sharma C M, et al. CRISPR RNA maturation by trans-encoded small RNA and host factor RNaseⅢ. Nature , 2011, 471 (7340) : 602–607. DOI:10.1038/nature09886 |
| [65] | Anantharaman V, Iyer L M, Aravind L.Discovery notes presence of a classical RRM-fold palm domain in Thg1-type 3'-5'nucleic acid polymerases and the origin of the GGDEF and CRISPR polymerase domains, 2010, 5(43):1-9. |
| [66] | Cermak T, Doyle E L, Christian M, et al. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Research , 2011, 39 (12) : H1. |
| [67] | Jiang W, Bikard D, Cox D, et al. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nature Biotechnology , 2013, 31 (3) : 233–239. DOI:10.1038/nbt.2508 |
| [68] | Mali P, Yang L, Esvelt K M, et al. RNA-guided human genome engineering via Cas9. Science , 2013, 339 (6121) : 823–826. DOI:10.1126/science.1232033 |
| [69] | Hwang W Y, Fu Y, Reyon D, et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nature Biotechnology , 2013, 31 (3) : 227–229. DOI:10.1038/nbt.2501 |
| [70] | Brouns S J, Jore M M, Lundgren M, et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science , 2008, 321 (5891) : 960–964. DOI:10.1126/science.1159689 |
| [71] | Jinek M, East A, Cheng A, et al. RNA-programmed genome editing in human cells. Elife , 2013, 2 (e00471) : 1–9. |
| [72] | Cho S W, Kim S, Kim J M, et al. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nature Biotechnology , 2013, 31 (3) : 230–232. DOI:10.1038/nbt.2507 |
| [73] | Ding Q, Regan S N, Xia Y, et al. Enhanced efficiency of human pluripotent stem cell genome editing through replacing TALENs with CRISPRs. Cell Stem Cell , 2013, 12 (4) : 393. DOI:10.1016/j.stem.2013.03.006 |
| [74] | Qi L S, Larson M H, Gilbert L A, et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell , 2013, 152 (5) : 1173–1183. DOI:10.1016/j.cell.2013.02.022 |
| [75] | Schwank G, Koo B-K, Sasselli V, et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell , 2013, 13 (6) : 653–658. DOI:10.1016/j.stem.2013.11.002 |
| [76] | Wu Y, Liang D, Wang Y, et al. Correction of a genetic disease in mouse via use of CRISPR-Cas9. Cell Stem Cell , 2013, 13 (6) : 659–662. DOI:10.1016/j.stem.2013.10.016 |
| [77] | Raghavan A, Peters D, Kuperwasser N, et al. Functional characterization of a Cis-eQTL locus for plasma cholesterol using CRISPR/Cas genome editing in human pluripotent stem cells. Arteriosclerosis, Thrombosis, and Vascular Biology , 2014, 34 (Suppl 1) : A242–A242. |
| [78] | Chen B, Gilbert L A, Cimini B A, et al. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell , 2014, 156 (1) : 373. |
| [79] | Deans J R, Titova N V, Wickramaratne A, et al.SAT-272:Verifying affinity altering SNPs with Crispr/Cas system in HepG2 Cells.2015. |
| [80] | Wang H, Yang H, Shivalila C S, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell , 2013, 153 (4) : 910–918. DOI:10.1016/j.cell.2013.04.025 |
| [81] | Li W, Teng F, Li T, et al. Simultaneous generation and germline transmission of multiple gene mutations in rat using CRISPR-Cas systems. Nature Biotechnology , 2013, 31 (8) : 684–686. DOI:10.1038/nbt.2652 |
| [82] | Carlson D F, Tan W, Lillico S G, et al. Efficient TALEN-mediated gene knockout in livestock. Proceedings of the National Academy of Sciences , 2012, 109 (43) : 17382–17387. DOI:10.1073/pnas.1211446109 |
| [83] | Yu S, Luo J, Song Z, et al. Highly efficient modification of beta-lactoglobulin (BLG) gene via zinc-finger nucleases in cattle. Cell Research , 2011, 21 (11) : 1638–1640. DOI:10.1038/cr.2011.153 |
| [84] | Kennedy E M, Bassit LC, Mueller H, et al. Suppression of hepatitis B virus DNA accumulation in chronically infected cells using a bacterial CRISPR/Cas RNA-guided DNA endonuclease. Virology , 2015, 2 (476) : 196–205. |
| [85] | Kennedy E M, Kornepati A V, Goldstein M, et al. Inactivation of the human papillomavirus E6 or E7 gene in cervical carcinoma cells by using a bacterial CRISPR/Cas RNA-guided endonuclease. J Virol , 2014, 88 (20) : 11965–11972. DOI:10.1128/JVI.01879-14 |
| [86] | Yang H, Wang H, Jaenisch R. Generating genetically modified mice using CRISPR/Cas-mediated genome engineering. Nat Protoc , 2014, 9 (8) : 1956–1968. DOI:10.1038/nprot.2014.134 |
| [87] | Li X, Yang Y, Bu L, et al. Rosa26-targeted swine models for stable gene over-expression and Cre-mediated lineage tracing. Cell Res , 2014, 24 (4) : 501–504. DOI:10.1038/cr.2014.15 |
| [88] | Hwang W Y, Fu Y, Reyon D, et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol , 2013, 31 (3) : 227–229. DOI:10.1038/nbt.2501 |
| [89] | Wang H C, Yang Y, Xu S Y, et al. The CRISPR/Cas system inhibited the pro-oncogenic effects of alternatively spliced fibronectin extra domain A via editing the genome in salivary adenoid cystic carcinoma cells. Oral Dis , 2015, 21 (5) : 608–618. DOI:10.1111/odi.2015.21.issue-5 |
| [90] | Niu Y, Shen B, Cui Y, et al. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell , 2014, 156 (4) : 836–843. DOI:10.1016/j.cell.2014.01.027 |
| [91] | Ramírez O, Quintanilla R, Varona L, et al. DECR1 and ME1 genotypes are associated with lipid composition traits in Duroc pigs. Journal of Animal Breeding and Genetics , 2014, 131 (1) : 46–52. DOI:10.1111/jbg.2013.131.issue-1 |
| [92] | Hua T, Wu D, Ding W, et al. Studies of Human 2, 4-Dienoyl CoA Reductase Shed New Light on Peroxisomalβ-Oxidation of Unsaturated Fatty Acids. Journal of Biological Chemistry , 2012, 287 (34) : 28956–28965. DOI:10.1074/jbc.M112.385351 |
| [93] | Zanou N, Gailly P. Skeletal muscle hypertrophy and regeneration:interplay between the myogenic regulatory factors (MRFs) and insulin-like growth factors (IGFs) pathways. Cellular and Molecular Life Sciences , 2013, 70 (21) : 4117–4130. DOI:10.1007/s00018-013-1330-4 |
| [94] | Zhu C, Gi G, Tao Z, et al. Development of skeletal muscle and expression of myogenic regulatory factors during embryonic development in Jinding ducks (Anas platyrhynchos domestica). Poultry Science , 2014, 93 (5) : 1211–1216. DOI:10.3382/ps.2013-03695 |
| [95] | Choi Y, Suh Y, Ahn J, et al. Muscle hypertrophy in heavy weight Japanese quail line:Delayed muscle maturation and continued muscle growth with prolonged upregulation of myogenic regulatory factors. Poultry Science , 2014, 93 (9) : 2271–2277. DOI:10.3382/ps.2013-03844 |
| [96] | McPherron A C, Lee S J. Double muscling in cattle due to mutations in the myostatin gene. Proceedings of the National Academy of Sciences , 1997, 94 (23) : 12457–12461. DOI:10.1073/pnas.94.23.12457 |
| [97] | Hamilton D N, Ellis M, Miller K D, et al. The effect of the Halothane and Rendement Napole genes on carcass and meat quality characteristics of pigs. Journal of Animal Science-Menasha Then Albany Then Champaign Illinois , 2000, 78 (11) : 2862–2867. |
| [98] | Gispert M, Faucitano L, Oliver M, et al. A survey of pre-slaughter conditions, halothane gene frequency, and carcass and meat quality in five Spanish pig commercial abattoirs. Meat Science , 2000, 55 (1) : 97–106. DOI:10.1016/S0309-1740(99)00130-8 |
| [99] | Zou Q, Wang X, Liu Y, et al. Generation of gene-target dogs using CRISPR/Cas9 system. Journal of Molecular Cell Biology , 2015, 7 (6) : 580–583. DOI:10.1093/jmcb/mjv061 |
| [100] | Davies K T, Tsagkogeorga G, Bennett N C, et al. Molecular evolution of growth hormone and insulin-like growth factor 1 receptors in long-lived, small-bodied mammals. Gene , 2014, 549 (2) : 228–236. DOI:10.1016/j.gene.2014.07.061 |
| [101] | Standen P, Sferruzzi-Perri A N, Taylor R, et al. Maternal insulin-like growth factor 1 and 2 differentially affect the renin-angiotensin system during pregnancy in the guinea pig. Growth Hormone & IGF Research , 2015, 25 (3) : 141–147. |
| [102] | Klimenko A, Usatov A, Getmantseva L, et al. Effects of melanocortin-4 receptor gene on growth and meat traits in pigs raised in russia. American Journal of Agricultural and Biological Sciences , 2014, 9 (2) : 232. DOI:10.3844/ajabssp.2014.232.237 |
| [103] | Zuo B, Liu G, Peng Y, et al. Melanocortin-4 receptor (MC4R) polymorphisms are associated with growth and meat quality traits in sheep. Molecular Biology Reports , 2014, 41 (10) : 6967–6974. DOI:10.1007/s11033-014-3583-x |
| [104] | Chu Q, Cai L, Fu Y, et al. Dkk2/Frzb in the dermal papillae regulates feather regeneration. Developmental Biology , 2014, 387 (2) : 167–178. DOI:10.1016/j.ydbio.2014.01.010 |
| [105] | Wang Z, Li Q, Zhang B, et al. Single nucleotide polymorphism scanning and expression of the FRZB gene in pig populations. Gene , 2014, 543 (2) : 198–203. DOI:10.1016/j.gene.2014.04.023 |
| [106] | Van Laere A S, Nguyen M, Braunschweig M, et al. A regulatory mutation in IGF2 causes a major QTL effect on muscle growth in the pig. Nature , 2003, 425 (6960) : 832–836. DOI:10.1038/nature02064 |
| [107] | Houston F, Goldmann W, Foster J, et al. Comparative susceptibility of New Zealand sheep with a range of PRNP genotypes to challenge with bovine spongiform encephalopathy and scrapie. In:PRION; 2014:Landes Bioscience 1806 Rio Grandest, Austin, TX 78702 USA , 2014 : 102–102. |
| [108] | Czarnik U, Strychalski J, Barcewicz M, et al. The effect of insertion/deletion polymorphisms within the promoter and intron 1 sequences of the PRNP gene on the breeding value of Holstein-Friesian bulls. Animal Science Papers and Reports , 2015, 33 (1) : 13–22. |
 2016, Vol. 36
2016, Vol. 36