文章信息
- 魏金梅, 范小琴, 熊海庭, 高学娟, 刘小会, 刘朗夏
- WEI Jin-mei, FAN Xiao-qin, XIONG Hai-ting, GAO Xue-juan, LIU Xiao-hui, LIU Lang-xia
- hnRNPK与Nef相互作用并有利于细胞表面CD4的表达
- hnRNPK Interacts with Nef and Facilitates the Cell Surface Expression of CD4
- 中国生物工程杂志, 2015, 35(4): 17-22
- China Biotechnology, 2015, 35(4): 17-22
- http://dx.doi.org/10.13523/j.cb.20150403
-
文章历史
- 收稿日期:2015-02-15
- 修回日期:2015-03-06
Nef蛋白是艾滋病病毒HIV的辅助蛋白,包含206个氨基酸,相对分子质量为25kDa~27kDa,对于艾滋病的发病机理至关重要[1, 2, 3]。早期研究发现,Nef蛋白能够提高HIV病毒在宿主细胞内的复制及感染力[1, 4]。在病毒感染的早期,Nef能通过内吞作用下调宿主细胞表面受体CD4,CD4受体的下调抑制了CD4与gp120的结合从而促进病毒释放[5, 6, 7]。另外,Nef能与多种信号蛋白结合,如与Pak2的结合能影响F-actin的重排[8, 9];该蛋白高度保守的脯氨酸富集区PxxP(57-80aa)与Src家族激酶的SH3结合,从而抑制或激活它们的催化活性[10, 11]。
hnRNPK是hnRNP家族的一种RNA结合蛋白,分子量为60kDa,它参与多种生物功能的调控,如信号转导、基因表达等[12, 13]。该蛋白包含3个KH (K homology)区和1个蛋白质相互区KI。KH区能与DNA、RNA结合,参与染色体重塑、基因转录、RNA剪接及蛋白质翻译等过程[13, 14, 15]。与Nef相似,KI区存在脯氨酸富集区,它能与含SH3结构的Scr家族激酶相互结合,包括Lck、Lyn等[14, 15, 16, 17]。Yoo等[17]发现hnRNPK能与N-WASP相互作用,调节细胞的粘附及传播。有研究表明,hnRNPK能调节多种细胞骨架相关基因的表达[18, 19],敲低hnRNPK可抑制TGF-β1引起的F-actin的极化现象[20]。
上述hnRNPK与Nef的结构与功能特性特别是他们与细胞骨架的关系,以及Nef下调细胞表面CD4的表达的机制提示我们,hnRNPK也可能参与调节细胞表面CD4的表达。另有研究报道,hnRNPK与Nef存在相互作用,Nef通过hnRNPK增强Tat介导的HIV的转录活性[21],但其相互作用对宿主细胞表面CD4的表达是否有影响并不清楚。本研究通过GST-pulldown证实了两者的相互作用,并证明hnRNPK与Nef对细胞表面CD4的表达有相反的调节作用,两者之间有可能存在涉及复杂调控的非直接关系,这为进一步阐明细胞表面CD4表达水平的调节机制提供线索。
1 材料与方法 1.1 材 料 1.1.1 质粒、菌株及细胞质粒pGEX-4T-1、pGEX-4T-GST-Nef、pCMV-N-Flag-Nef、pmCherry-N1、大肠杆菌E.coli DH5α、大肠杆菌E.coli BL21及HeLa-CD4细胞均由本实验室保存。
1.1.2 分子生物学相关材料试剂限制性内切酶EcoR Ι、BamH Ι、PrimeSTAR HS DNA聚合酶、T4 DNA连接酶等工具酶及DNA Marker购自TaKaRa公司;质粒提取和凝胶回收试剂盒购自天根公司;IPTG、溶菌酶、谷胱甘肽琼脂糖珠购自美国GE公司;DMEM高糖培养基购自美国Gibco公司;胎牛血清(FBS)购自德国PAA公司;转染试剂Lipofectamine 2000购自Invitrogen公司;PVDF膜、ECL显色液购自美国Bio-Rad公司;单克隆小鼠抗mCherry购自Abbkine公司,单克隆小鼠抗GAPDH购自北京中杉金桥公司,单克隆小鼠抗hnRNPK购自Santa Cruz公司,CD4 -FITC antibodies购自Miltenyi Biotec公司;其他试剂均为国产或进口分析纯。 1.2 方 法 1.2.1 GST蛋白及GST-Nef融合蛋白的表达与纯化分别将pGEX-4T-1和pGEX-4T-GST-Nef转化至E.coli BL21感受态细胞,挑取阳性单克隆菌落置于含Amp的LB培养液中,37℃,200 r/min,过夜培养。次日按1∶100的比例扩大培养,至OD600为0.6~0.8时加入终浓度为0.2 mmol/L 的IPTG,室温诱导6~8 h。离心收集菌体,用含溶菌酶及PMSF 的EBC裂解液(150 mmol/L NaCl,50 mmol/L Tris,1%Triton,pH7.6)重悬菌体,超声破碎后,高速离心收集上清液。上清用Glutathione-Sepharose 4B 亲和层析法纯化目的蛋白。将结合有目的蛋白的GST、GST-Nef球珠重悬于EBC中,各取10 μl珠子悬液进行SDS-PAGE及考马斯亮蓝染色,分析蛋白纯化情况。
1.2.2 GST-pulldown验证Nef与hnRNPK的相互作用37℃,5% CO2培养HeLa-CD4,细胞汇合率达80%~90%后收集细胞,加入EBC裂解液(含1 mmol/L蛋白酶抑制剂PI、1mmol/L PMSF、10mmol/L NaF、1mmol/L Na3VO4)裂解,4℃高速离心20 min,收集上清,用BCA法测定蛋白浓度。各取50 μg纯化的GST、GST-Nef蛋白与1.5 mg HeLa-CD4全细胞裂解液4℃旋转孵育过夜,2500 r/min,4℃离心5 min,弃上清,珠子用EBC清洗3~5次,加入30 μl 1×SDS loading buffer 沸水煮10 min,SDS-PAGE电泳后用单克隆鼠抗hnRNPK进行Western blot检测。
1.2.3 pmCherry-N1-Nef真核表达载体的构建根据Nef基因序列设计引物,引物序列如下:F:5′-CGGAATTCTGATGGGTGGCAAG TGGTCA-3′(下划线为EcoR Ι酶切位点);R:5′- CGGGATCCTGGCAGTTCTTG
AAGTACT-3′(下划线为BamH Ι酶切位点)。以pCMV-N-Flag-Nef为模板,PCR扩增Nef片段,凝胶回收PCR产物。用EcoR Ι和BamH Ι双酶切pmCherry-N1质粒及回收的Nef片段,凝胶回收双酶切产物后用T4 DNA连接酶连接,连接产物转化至E.coli DH5α,抽提质粒进行双酶切及电泳鉴定,选取阳性克隆进行测序。 1.2.4 HeLa-CD4细胞的转染、干扰及细胞流式技术处于对数生长期的HeLa-CD4细胞按60%汇合率铺板于6孔板,16 h后按Invitrogen公司Lipofectamine 2000转染试剂说明书进行转染及hnRNPK的siRNA干扰实验,hnRNPK干扰片段及干扰对照片段序列如下。
siRNA-hnRNPK 1: 5′-UAUUAAGGCUCUCCGUACATT-3′ 5′-UGUACGGAGAGCCUUAAUATT-3′
siRNA-hnRNPK 2: 5′-CCUUAUGAUCCCAACUUUUTT-3′ 5′-AAAAGUUGGGAUCAUAAGGTT-3′
siRNA-control: 5′-UUCUCCGAACGUGUCACGUTT-3′ 5′-ACGUGACACGUUCGGAGAATT-3′
实验分4组:(1)转染pmCherry-N1并加siRNA-control;(2)转染pmCherry-N1并加siRNA-hnRNPK;(3)转染pmCherry-N1-Nef并加siRNA-control;(4)转染pmCherry-N1-Nef并加siRNA-hnRNPK。转染及干扰36 h后收集细胞,取部分细胞用单克隆鼠抗mCherry、hnRNPK进行Western blot检测转染及干扰效果,剩余细胞与CD4-FITC 抗体孵育后,用BD AccuriTM C6流式细胞仪分析细胞表面CD4表达水平。该实验进行3次独立重复。
1.2.5 免疫印迹(Western blot)样品经SDS-PAGE电泳后电转于PVDF膜上,TBST洗膜3次,5%脱脂奶粉室温封闭1 h,加入用5%脱脂奶粉稀释的一抗(1∶1000),4℃过夜孵育,TBST洗膜3次,加入相应二抗室温轻摇1h,TBST洗膜3次,用ECL发光液进行显影。
2 结 果 2.1 GST蛋白及GST-Nef融合蛋白的表达与纯化分别将pGEX-4T-1和pGEX-4T-GST-Nef转化至E.coli BL21,IPTG诱导表达,收集菌体超声破碎,上清用Glutathione-Sepharose 4B亲和层析法纯化GST和GST-Nef融合蛋白,SDS-PAGE电泳及考马斯亮蓝染色法检测蛋白纯化情况。结果见图 1,泳道2、8分别表明成功诱导表达了GST和GST-Nef蛋白,GST分子量为26kDa,GST-Nef分子量约为52kDa,泳道6和泳道12表明成功纯化出GST、GST-Nef蛋白。
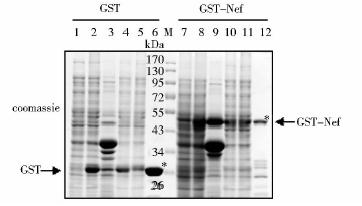
|
| 图 1 GST和GST-Nef蛋白的表达与纯化 Fig. 1 The Expression and purification of GST and GST-Nef 1: pGEX-GST without IPTG induction; 2: pGEX-GST with IPTG induction; 3: The pellet of cell lysates; 4:The supernatant of cell lysates; 5: The flow-through; 6: GST protein-beads after purification; M: Protein marker; 7: pGEX-GST-Nef without IPTG induction; 8: pGEX-GST-Nef with IPTG induction; 9: The pellet of cell lysates; 10:The supernatant of cell lysates; 11: The flow-through; 12: GST protein-beads after purification |
前期研究通过免疫共沉淀实验证明了Nef与hnRNPK在Jurkat细胞里存在相互作用[21]。为了验证这一发现,我们进行GST-pulldown实验。分别取50 μg纯化于Glutathione-Sepharose 4B上的GST和GST-Nef与1.5 mg HeLa-CD4的全细胞裂解液孵育,反复洗涤后进行SDS-PAGE电泳及Western blot分析,用单克隆鼠抗hnRNPK检测GST-Nef与hnRNPK的结合情况。结果显示(图 2),GST-Nef蛋白能够与细胞内表达的hnRNPK特异性结合,而阴性对照GST不能特异性结合hnRNPK,从而证实了Nef与hnRNPK存在相互作用。
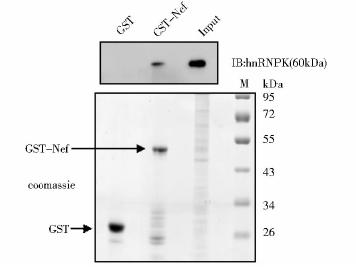
|
| 图 2 GST-pulldown验证Nef与hnRNPK的相互作用Fig. 2 The interaction between Nef and hnRNPK determined by GST-pulldown Used 50 μg the purified GST and GST-Nef incubated with 1.5 mg whole cell lysis from HeLa-CD4 cell respectively. After sufficient cleaning the sample was subjected to SDS-PAGE electrophoresis. coomassie blue staining detected the presence of GST and GST-Nef. Western blot showed the GST-Nef could bond to hnRNPK,but not GST |
以pCMV-N-Flag-Nef为模板,PCR扩增获得的目的片段Nef经EcoR Ι和BamH Ι双酶切后,用T4 DNA连接酶将其插入到载体pmCherry-N1中,连接产物转化至E.coli DH5α,挑取3个单克隆扩大培养,抽提质粒并进行双酶切鉴定。结果(图 3a)显示,③号质粒(泳道7、8)成功将目的片段Nef插入pmCherry-N1载体中,将③号质粒送Invitrogen公司测序,测序结果表明,重组质粒序列完全正确,图 3b为部分测序结果。
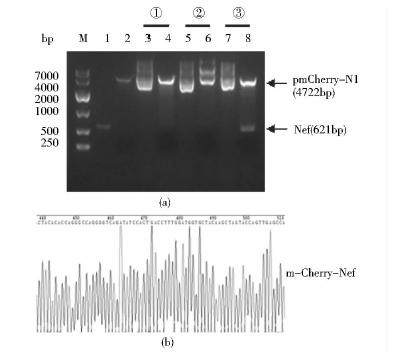
|
| 图 3 重组质粒pmCherry-N1-Nef的构建Fig. 3 The construction of the recombinant plasmid pmCherry-N1-Nef (a) Identification of pmCherry-N1-Nef by dual-enzyme digestion M: DNA marker; 1: Enzyme-digested product Nef; 2: The pmCherry-N1 plasmid digested by EcoR Ι and BamH Ι; 3,5,7: pmCherry-N1-Nef plasmid from different monoclonal; 4,6,8: Dual-enzyme digestion of different pmCherry-N1-Nef recombinant plasmid (b)Partial sequencing results of pmCherry-N1-Nef plasmid |
为探究hnRNPK是否参与调节细胞表面CD4的表达,以及Nef是否通过hnRNPK调节细胞表面CD4的表达,对HeLa-CD4细胞进行转染及siRNA干扰实验,实验组别设置见方法。Western blot检测转染及干扰效果,结果(图 4a)显示,质粒pmCherry-N1 和pmCherry-N1-Nef成功转染并表达目的蛋白,另外,hnRNPK的干扰效果非常明显。流式细胞术检测细胞表面CD4表达水平,结果见图 4(b)(c),①③组相比,Nef的表达使细胞表面CD4表达水平大约降低了75%;②④组相比表明,敲低hnRNPK的情况下,Nef的表达以同样的幅度下调细胞表面CD4表达水平;比较 ①②组说明,没有Nef时敲低hnRNPK,细胞表面CD4表达水平降低了50%;③④组相比显示,Nef存在时,hnRNPK的敲低依然使细胞表面CD4表达水平明显下降。结果表明hnRNPK参与调节细胞表面CD4的表达,其调节作用与Nef相反,二者的作用可能独立不相关,但也有可能是涉及复杂调控的非直接关系。

|
| 图 4 敲低hnRNPK或表达Nef对细胞表面CD4表达的影响Fig. 4 Analyses of the effect of hnRNPK knockdown or Nef expression on the cell surface epression of CD4 (a)Western blot analyses showing the transient expression of mCherry-Nef and the siRNA interference of hnRNPK in HeLa-CD4 cells (b) Assays of CD4 cell surface expression by flow cytometry The data was analysed by Flow-Jo software (c) Mean fluorescence intensity of cell surface CD4 of three independent experiments The unpaired T test by GraphPad Prism5 software(* P<0.05,*** P<0.001). were performed for the statistical analysis |
HIV Nef蛋白作为病毒的辅助蛋白之一,在病毒感染的早期大量合成,对病毒的复制及感染力至关重要[1, 2, 3]。它能够下调宿主细胞表面受体CD4、CD28及组织相容性复合物[6, 22]。其N端能结合多种信号蛋白复合物,包括Lck、Src、PKCδ、Eed等,简称Nef相关激酶复合物(NAKC)[23, 24]。有研究表明,hnRNPK也是NAKC 成员之一,它通过Eed与Nef间接相互作用,导致Erk1/2的激活,进而增强Tat介导的病毒的转录活性[21]。另有文献报道,Nef与hnRNPK都存在能与Src家族激酶相互作用的脯氨酸富集区,且两者都与F-actin细胞骨架相关[8, 11, 14, 20]。这促使我们探究hnRNPK是否也参与细胞表面CD4表达的调节,且其调节机制是否与Nef的作用相关。
本研究采用GST-pulldown技术鉴定Nef与hnRNPK的相互作用。首先纯化GST和GST-Nef融合蛋白,看其能否沉淀细胞内源表达的hnRNPK,结果显示,GST-Nef能够与内源的hnRNPK结合,而阴性对照GST不能,证明Nef与hnRNPK确实存在相互作用。为了进一步研究hnRNPK是否参与调节细胞表面CD4的表达,以及Nef是否通过hnRNPK调节细胞表面CD4的表达,我们先构建了带红色荧光标签的真核表达载体pmCherry-N1-Nef,将其转染到稳定表达CD4的HeLa细胞中,并联合hnRNPK的敲低实验,采用流式细胞术分析细胞表面CD4的表达水平。结果显示,不管是否有Nef,hnRNPK的敲低都使细胞表面CD4表达水平明显下降;另一方面,不管是否敲低hnRNPK,Nef的表达都使细胞表面CD4表达水平下调约75%。这些结果表明hnRNPK参与调节细胞表面CD4的表达,其调节作用与Nef相反,本研究提示Nef不是单纯通过hnRNPK下调细胞表面CD4的表达。
在病毒感染的早期,Nef与细胞连接蛋白复合物和NBP1蛋白形成复合物,该复合物再与CD4的胞浆尾区双亮氨酸结合,然后通过内吞作用将复合物转运到溶酶体,促进CD4的降解[5, 6, 25]。有研究表明,细胞受体的内吞作用依赖于细胞骨架[26],Nef与Pak2结合影响细胞骨架actin的重排,且Nef能调节actin调节器N-WASP的活性[8, 9]。另有研究表明,hnRNPK调节多种细胞骨架相关基因的表达[18, 19],并通过作用于RNA及RNA结合蛋白调节细胞的传播及迁移[27],在A549细胞中敲低hnRNPK能抑制TGF-β1引起的F-actin的极化,显示hnRNPK对细胞骨架的调节作用[20],特别是hnRNPK能结合并抑制N-WASP 的活性[17]。这提示我们,hnRNPK与Nef对细胞表面CD4表达的调节可能存在非直接关系,两者涉及复杂调控机制,可能通过N-WASP 及细胞骨架建立联系;另一方面,hnRNPK可能通过调节CD4的转录及翻译起调节作用,后期研究可从这两方面进行试验,以确定两者相互作用对细胞表面CD4表达的影响。
本文验证了hnRNPK与Nef的相互作用,并首次证实了hnRNPK有利于细胞表面CD4的表达,这为进一步阐明细胞表面CD4表达水平的调节机制提供线索,为抗HIV的发展提供新思路和新靶点。
| [1] | Kestler H W, Ringler D J, Mori K, et al. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell, 1991, 65(4): 651-662. |
| [2] | Amorim N A, da Silva E M, de Castro R O, et al. Interaction of HIV-1 Nef protein with the host protein Alix promotes lysosomal targeting of CD4 receptor. The Journal of Biological Chemistry, 2014, 289(40): 27744-27756. |
| [3] | Baur A S. HIV-Nef and AIDS pathogenesis: are we barking up the wrong tree? Trends Microbiol, 2011, 19(9): 435-440. |
| [4] | Jere A, Fujita M, Adachi A, et al. Role of HIV-1 Nef protein for virus replication in vitro. Microbes and Infection/Institut Pasteur, 2010, 12(1): 65-70. |
| [5] | Grzesiek S, Stahl S J, Wingfield P T, et al. The CD4 determinant for downregulation by HIV-1 Nef directly binds to Nef. Mapping of the Nef binding surface by NMR. Biochemistry, 1996, 35(32): 10256-10261. |
| [6] | Arganaraz E R, Schindler M, Kirchhoff F, et al. Enhanced CD4 down-modulation by late stage HIV-1 nef alleles is associated with increased env incorporation and viral replication. Journal Of Biological Chemistry, 2003, 278(36): 33912-33919. |
| [7] | Hanna Z, Priceputu E, Hu C Y, et al. HIV-1 Nef mutations abrogating downregulation of CD4 affect other Nef functions and show reduced pathogenicity in transgenic mice. Virology, 2006, 346(1): 40-52. |
| [8] | Haller C, Rauch S, Michel N, et al. The HIV-1 pathogenicity factor Nef interferes with maturation of stimulatory T-lymphocyte contacts by modulation of N-Wasp activity. The Journal of Biological Chemistry, 2006, 281(28): 19618-19630. |
| [9] | Renkema G H, Manninen A, Mann D A, et al. Identification of the Nef-associated kinase as p21-activated kinase 2. Current Biology: CB, 1999, 9(23): 1407-1410. |
| [10] | Asamitsu K, Morishima T, Tsuchie H, et al. Conservation of the central proline-rich (PxxP) motifs of human immunodeficiency virus type 1 Nef protein during the disease progression in two hemophiliac patients. Febs Lett, 2000, 467(2-3): 366. |
| [11] | Saksela K, Cheng G, Baltimore D. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. The EMBO Journal, 1995, 14(3): 484-491. |
| [12] | Tsai P L, Chiou N T, Kuss S, et al. Cellular RNA binding proteins NS1-BP and hnRNP K regulate influenza A virus RNA splicing. Plos Pathog, 2013, 9(6):e1003460. |
| [13] | Szczyrba J, Nolte E, Hart M, et al. Identification of ZNF217, hnRNP-K, VEGF-A and IPO7 as targets for microRNAs that are downregulated in prostate carcinoma. International Journal of Cancer, 2013, 132(4): 775-784. |
| [14] | Bomsztyk K, Denisenko O, Ostrowski J. hnRNP K: one protein multiple processes. BioEssays, 2004, 26(6): 629-638. |
| [15] | Liepelt A, Mossanen J C, Denecke B, et al. Translation control of TAK1 mRNA by hnRNP K modulates LPS-induced macrophage activation. Rna, 2014, 20(6): 899-911. |
| [16] | Ciarlo M, Benelli R, Barbieri O, et al. Regulation of neuroendocrine differentiation by AKT/hnRNPK/AR/beta-catenin signaling in prostate cancer cells. International Journal of Cancer, 2012, 131(3): 582-590. |
| [17] | Yoo Y, Wu X, Egile C, et al. Interaction of N-WASP with hnRNPK and its role in filopodia formation and cell spreading. The Journal of Biological Chemistry, 2006, 281(22): 15352-15360. |
| [18] | Liu Y Y, Szaro B G. hnRNP K post-transcriptionally co-regulates multiple cytoskeletal genes needed for axonogenesis. Development, 2011, 138(14): 3079-3090. |
| [19] | Nagano K, Bornhauser B C, Warnasuriya G, et al. PDGF regulates the actin cytoskeleton through hnRNP-K-mediated activation of the ubiquitin E(3)-ligase MIR. EMBO Journal, 2006, 25(9): 1871-1882. |
| [20] | Li L P, Lu C H, Chen Z P, et al. Subcellular proteomics revealed the epithelial-mesenchymal transition phenotype in lung cancer. Proteomics, 2011, 11(3): 429-439. |
| [21] | Wolf D, Witte V, Clark P, et al. HIV Nef enhances Tat-mediated viral transcription through a hnRNP-K-nucleated signaling complex. Cell Host Microbe, 2008, 4(4): 398-408. |
| [22] | Greenway A L, Holloway G, McPhee D A, et al. HIV-1 Nef control of cell signalling molecules: multiple strategies to promote virus replication. J Biosciences, 2003, 28(3): 323-335. |
| [23] | Witte V, Laffert B, Rosorius O, et al. HIV-1 Nef mimics an integrin receptor signal that recruits the polycomb group protein Eed to the plasma membrane. Mol Cell, 2004, 13(2): 179-190. |
| [24] | Wolf D, Giese S I, Witte V, et al. Novel (n)PKC kinases phosphorylate Nef for increased HIV transcription, replication and perinuclear targeting. Virology, 2008, 370(1): 45-54. |
| [25] | Aiken C, Konner J, Landau N R, et al. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell, 1994, 76(5): 853-864. |
| [26] | Kornilova E S. Receptor-mediated endocytosis and cytoskeleton. Biochemistry-Moscow, 2014, 79(9): 865-878. |
| [27] | de Hoog C L, Foster L J, Mann M. RNA and RNA binding proteins participate in early stages of cell spreading through spreading initiation centers. Cell, 2004, 117(5): 649-662. |
 2015, Vol. 35
2015, Vol. 35




