文章信息
- 孙欢, 贾海洋, 冯旭东, 刘月芹, 李春
- SUN Huan, JIA HAI-yang, FENG XU-dong, LIU Yue-qin, LI Chun
- 酿酒酵母耐热元器件的筛选
- Screening of Heat-resistant Device in Saccharomyces cerevisiae
- 中国生物工程杂志, 2015, 35(3): 75-83
- China Biotechnology, 2015, 35(3): 75-83
- http://dx.doi.org/10.13523/j.cb.20150311
-
文章历史
- 收稿日期:2014-12-25
- 修回日期:2015-01-22
2. 天津大学系统生物工程教育部重点实验室 天津
2. Key Laboratory of Systems Bioengineering, Ministry of Education, Tianjin University, Tianjin 300072, China
纤维素乙醇作为发展力度最强的生物能源已成为国内外研究热点。利用丰富廉价的木质纤维素生产乙醇可大幅降低原料成本。但是纤维素发酵生产乙醇的方法无论是水解与发酵分段法,还是同步糖化发酵法,最适酶解温度约为50℃。而酿酒酵母的最适发酵温度一般为30℃。因此,纤维素经高温酶解后,需要降到酵母的发酵温度进而增加了生产成本。所以,纤维素乙醇工业急需提高酿酒酵母耐热性[1, 2]。然而目前大部分酿酒酵母不能耐受高温,在发酵过程会受到高温胁迫的影响,使蛋白变性,导致细胞的存活力下降[3]。同时,高温胁迫会诱导细胞产生大量的活性氧自由基(ROS),从而对细胞产生双重危害[4]。这就需要消耗大量的冷却水,因此提高酵母耐热性不仅可以大幅度降低冷却成本[5],而且微生物体内酶的最适催化温度一般要比其最适生长温度高出5~10℃[6]。因此,提高酵母耐热性,可以效地活化反应过程,加速代谢,提高细胞合成效率。此外,提高抗氧化性可以维持酵母细胞及其体内酶的活力,从而更有利于生产。
目前,提高酿酒酵母耐热性已成为国内外的研究热点。传统的驯化法,稳定性差,不能满足大规模生产。通过改变酿酒酵母本身的转录因子或蛋白表达提高酿酒酵母的耐热性,这种方法受限于菌株本身的耐热机制效果并不明显[7, 8]。据报道通过引入外源耐热基因可以有效提高微生物的耐热性[9],但是目前该领域的研究处于初始阶段,并且应用于酿酒酵母不同类型的高温发酵及提高其高温下乙醇合成效率还未见有文献报道。
嗜热栖热菌(Thermus thermophilus)属于古生菌,其最适生长温度为80℃,有一套完整的耐热机制[10],最主要的耐热机制是其含有丰富的热稳定性保护蛋白—热激蛋白(HSP)。HSP对于揭示极端嗜热古菌特殊生命活动的本质及其耐热机制,从系统生物学角度丰富和拓展生命本质活动具有十分重要的意义[11]。在高温环境下,HSP可以帮助热变性的蛋白质和新生肽链进行正确折叠并维持空间构象和功能,从而帮助细胞抵御热胁迫[12]。也有研究发现,HSP可以缓解高温诱导的氧化胁迫的影响[13]从而间接地提高细胞耐热性。HSP一般分为:小分子HSP(15~30kDa)、HSP60、HSP70(68~74kDa)、HSP90(80~90kDa)及HSP100[14]。嗜热栖热菌体内富含上述种类的HSP,为提高酿酒酵母耐热性提供了宝贵的基因资源。
本文依据合成生物学理念,将酿酒酵母组成型启动子FBA1p和终止子SLM5t与嗜热栖热菌热激蛋白groes(hsp10)、hsp20、hsp33、dnak(hsp70)、clpp(hsp100)基因组装成5个热激蛋白元器件,导入酿酒酵母,获得工程菌。并分析热激蛋白对酿酒酵母耐热性及乙醇生产的影响,以期筛选得到在酿酒酵母中有效的耐热元器件。 1 材料与方法 1.1 材 料 1.1.1 菌株与试剂
嗜热栖热菌(CICC编号:10347)购买于中国工业微生物菌种保藏管理中心(CICC),酿酒酵母(Saccharomyces cerevisiae)INVSC1和大肠杆菌TOP 10为实验室保藏菌株。质粒pRS42K购买自德国法兰克福的EUROSCARF。 1.1.2 试剂和培养基
RNA提取试剂盒和qPCR试剂盒分别为OMGA和罗氏公司产品。质粒提取试剂盒及DNA凝胶回收试剂盒分别为北京博迈德生物技术有限公司和北京百泰克生物技术有限公司产品。Pfu DNA 聚合酶、限制性内切酶、T4 DNA 连接酶为TaKaRa公司产品。G418购于北京赛百盛基因有限公司。2′,7′-二氯二乙酸酯荧光素(2′,7′-H2DCFDA)购自北京百灵威公司,引物由生工生物工程(上海)有限公司(北京合成部)合成。含有50 μg/ml Amp抗生素的LB (10 g/L胰化蛋白胨,5 g/L酵母提取物,10 g/L NaCl)培养基用于大肠杆菌的转化子筛选、培养和提取重组质粒。YPD(20 g/L胰蛋白胨,10 g/L酵母提取物,20 g/L葡萄糖)培养基用于培养酿酒酵母,含300 μg/ml G418的YPD培养基,用于酿酒酵母转化子筛选、培养。 1.2 方 法 1.2.1 酿酒酵母工程菌的构建用嗜热栖热菌基因组扩增所有热激蛋白基因片段,并用酿酒酵母基因组扩增出启动子FBA1p和终止子SLM5t片段。扩增所需引物(表 1),PCR参数:95℃8 min,30 个循环(95℃ 1 min,57℃1min,72℃ 2min)。重叠延伸PCR连接耐热元件与调控元件,用BamH I和EcoRI对含有G418抗性基因的pRS42K穿梭质粒和基因片段酶切,在4℃,T4 DNA连接酶进行连接,转化大肠杆菌TOP 10,筛选出阳性克隆。提取重组质粒并电击转化酿酒酵母,用含G418固体YPD平板筛选阳性克隆。
| 引物名称 | 序列(5′~3′) |
| groes-F | ATGGCCGCGGAGGTGAAGAC |
| groes-R | AGGTTCACCTCCTTTACTGCA |
| hsp20-F | ATGATGCGGTTTGATCCCTT |
| hsp20-R | ATTACTCCACCTGCACCTGGA |
| hsp33-F | ATGGGACGGATCCTTAGGGG |
| hsp33-R | ATCAAGCCTGGGGGCGGGTCT |
| groel-F | ATGGCGAAGATCCTGGTGTT |
| groel-R | TAACGCCGTCCCCGGCACTTAG |
| dnak-F | ATGGCCAAGGCAGTGGGCAT |
| dnak-R | ATGGGCCCTCCTTGGGGGGAG |
| clpp-F | ATGGTCATACCCTACGTCAT |
| clpp-R | ACTAGGCCTCTTCACGGGTCA |
在30℃下培养36 h的酿酒酵母种子液以初始OD660为0.1转接到含50 ml YPD液体培养基的250 ml三角瓶中(含300 μg/ml G418),将细胞置于30℃恒温摇床中培养12h,之后在42℃培养24h。收集不超过2×107个酵母细胞,用RNA提取试剂盒提取RNA。用NanoDrop 2000c确定RNA浓度,每个样品的RNA终浓度调至500ng,用于反转录合成cDNA。根据罗氏的LightCycler SYBR Green I Master试剂盒添加用于荧光定量PCR的反应体系。用罗氏96孔荧光定量PCR仪进行反应,其中用管家基因ACT1作为内参。 1.2.3 梯度升温培养种子液培养及转接同1.2.2 ,将酵母于30℃培养12h,接着把温度提高到35℃,并每12h提高2 ℃,整个发酵持续72 h。培养过程中,每12h取样,用分光度计测定OD660,利用OD660的变化值以表征酿酒酵母在梯度高温培养下的生长状况。 1.2.4 恒定高温培养 种子液培养及转接同1.2.2 。于30℃培养12h,将温度提高到37℃ 和42℃,发酵持续72h。每12h取样并测定OD660。对36 h和72 h所取样品按10-1比例进行梯度稀释,取100μl菌液涂布在YPD固体培平板上,在30℃培养箱中培养2~3天后菌落计数,以CFU/ml为单位计算存活率(CFU是菌落形成单位),存活率=菌落数×稀释倍数×10(CFU/ml)。点滴平板法操作如下:分别对37 ℃ 和42℃培养60h后的菌液取样以10-1进行梯度稀释,然后每个梯度取2.5μl菌液滴到YPD固体平板上,30℃培养2~3天,观察菌落生长情况。 1.2.5 高温下酿酒酵母体内ROS水平的测定 种子液培养及转接方法同1.2.2 ,于30℃摇床中培养12h。用pH 7.4磷酸钾盐缓冲液(PBS)冲洗细胞并稀释。每个样品再添加5μl/ml溶解在 DMSO溶液中的2mmol/L 2,7′-dichlorofluoroscein diacetate (2′,7′-H2DCFDA)。之后于30 ℃避光孵育15 min。然后用PBS冲洗细胞三遍。将细胞分别于30℃和42 ℃摇床培养30 min。最后通过荧光分光光度计(Fluoromax 4 Spectrofluorometer,Horiba,USA)测定并记录各个样品在激发波长为504 nm和发射波长为524 nm下的荧光值[15]。相对ROS水平=(30 ℃酵母的荧光强度/42 ℃酵母的荧光强度)×100%。 1.2.6 酿酒酵母H2O2抗性的测定 种子液培养及转接方法同1.2.2 (转接起始OD660为0.2),向培养液中加入终浓度为2mmol/L的H2O2于30℃培养1 h。每隔15min取100μl菌液梯度稀释涂布在YPD固体平板上,在30℃培养箱中培养2~3天后进行菌落计数并计算存活率。存活率=(H2O2处理后酵母菌落数/0min对照菌落数)×100%[16]。 1.2.7 耐热酿酒酵母高温乙醇发酵 种子液培养及转接方法同1.2.2 ,但转接后的YPD培养基含有40 g/L葡萄糖,于30℃摇床中培养12h,之后将培养温度提高到40℃,发酵持续60h。在此发酵过程中保证厌氧条件。每隔12h取样,用分光度计测定测定OD660值。发酵培养液离心后,上清液稀释到合适的倍数,进样器取25 μl用SBA生物传感分析仪测定乙醇产量。乙醇得率(%)=[乙醇浓度/(40-葡萄糖残余浓度)×0.51] ×100%。 2 结果与讨论 2.1 热激蛋白元器件的构建与初步筛选
嗜热栖热菌基因组(NC_006461.1)已经测序成功,利用生物信息学分析嗜热微生物耐热基因发现,其富含热激蛋白基因(HSP70、HSP60、sHSP等)[17],为提高酿酒酵母耐热性提供了丰富的耐热基因资源。基于此,通过克隆嗜热栖热菌基因组得到5个热激蛋白基因,分别为groes(hsp10)、hsp20、hsp33、dnak(hsp70)、clpp(hsp100)。并与酿酒酵母组成型启动子(FBA1p)和终止子(SLM5t)连接,构建热激蛋白元器件,并将它们转入酿酒酵母,得到的工程菌列于表 2。
| 热激蛋白类型 | 热激蛋白基因 | 热激蛋白元器件a | 酿酒酵母工程菌 |
| Control | |||
| Heat shock protein 10 | groes | FBA1p-groes-SLM5t | S. c-GroES |
| Heat shock protein 20 | hsp20 | FBA1p-hsp20-SLM5t | S. c-HSP20 |
| Heat shock protein 33 | hsp33 | FBA1p-hsp33-SLM5t | S. c-HSP33 |
| Heat shock protein 70 | dnak | FBA1p-dnak-SLM5t | S. c-Dnak |
| Heat shock protein 100 | clpp | FBA1p-clpp-SLM5t | S. c-ClpP |
| a:热激蛋白元器件:启动子(FBA1p)+热激蛋白基因+终止子(SLM5t) | |||
将酿酒酵母的ACT1基因作为内参,通过荧光定量PCR(qPCR)计算出基因的相对转录水平,结果表明异源热激蛋白基因42 ℃下在酿酒酵母中均可以转录,但是转录水平不同,在相同启动子-终止子控制下,dnak转录水平最高,而hsp20转录水平最低(图 1)。
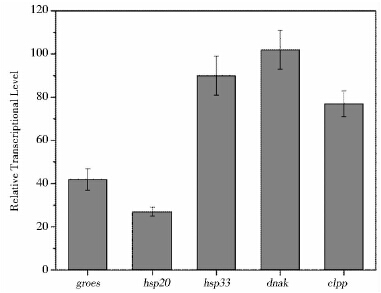
|
| 图 1 异源热激蛋白基因在酿酒酵母体内的相对转录水平(ACT1基因作为内参) Fig. 1 The relative transcriptional levels of heterogeous hsp genes in S. cerevisiae under 42 ℃ (ACT1 as the reference gene |
发酵过程中,如果不控温,发酵温度会不断升高,为模拟真实发酵过程,采用先梯度升温(35~43℃)培养对热激蛋白元器件进行初步筛选。除S. c-HSP20和S. c-ClpP的生长状况不如对照,其余的工程菌耐热性均有一定提高(图 2)。其中S. c-GroES在72h时OD660提高了29.2%。研究表明[18, 19],GroES在所有的热激蛋白中最先进行结构解析,可以与GroEL形成GroESL系统,该系统可以帮助非天然构象的蛋白质进行正确折叠对提高细胞耐热性发挥着重要作用。此外,梯度高温下S. c-GroES的生长曲线基本与生长在30℃的对照重合。初步筛选结果说明热激蛋白元器件FBA1p-groes-SLM5t可以作为酿酒酵母耐热元器件以有效提高其耐热性。
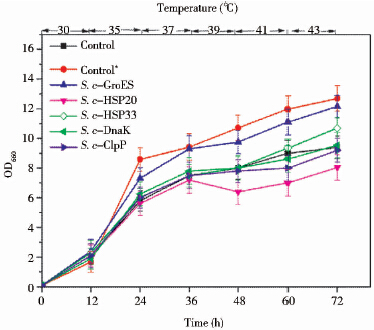
|
| 图 2 先30 ℃培养12 h,接着梯度高温(35~45 ℃)培养,酿酒酵母在此种培养策略下的生长状况(Control*:30 ℃培养的对照 Fig. 2 The cell growth of S. cerevisiae cultured at 30 ℃ at initial 12 h and then at the gradually enhanced high temperature from 35℃to 45℃ (Control*: the control yeast incubated at constant 30 ℃) |
根据以上结果,将筛选得到耐热性较好的工程菌S. c-GroES在恒定高温37℃(酵母的热激温度)[20]和42℃(酵母的致死温度)[21]下,进行进一步的耐热验证。在37℃时培养36h后,S. c-GroES的生长状况明显优于对照,并且在72h时OD660相比于对照提高了15%(图 3a)。42 ℃,72 h时S. c-GroES的OD660比对照高出57%(图 3b)。此外,42 ℃酵母生长状况均比37 ℃酵母较差,如42 ℃对照在72 h的OD660比37 ℃下降了74.3%,然而工程菌S. c-GroES只下降了65%。恒定高温培养(37 ℃和42 ℃)与梯度升温培养共同证明了耐热元器件FBA1p-groes-SLM5t可以使酿酒酵母应用于不同的高温培养类型,赋予酵母良好耐热性。
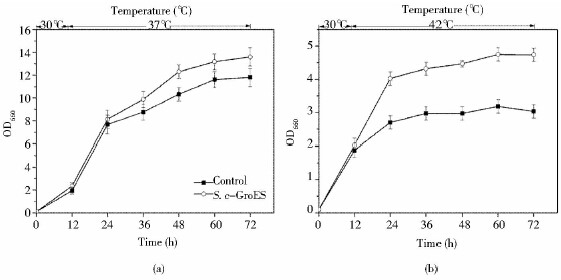
|
| 图 3 酿酒酵母工程菌S. c-GroES和对照酵母在37℃(a)和42℃(b)下的生长状况 Fig. 3 The cell growth ofthe engineered S. cerevisae,S. c-GroES and the control yeast cultured at 37℃(a) and 42℃ (b) |
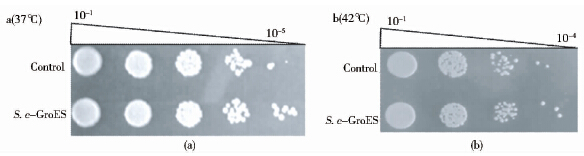
菌株的耐热性一般从高温下的生长状态和细胞存活率两个方面考察。所以分别测定了37℃和42℃下酵母存活率。37℃,S. c-GroES存活率均比对照高(图 4a)。随着时间的延长,工程菌的存活率优势越来越明显。42℃,36h时S. c-GroES细胞存活率是对照的3倍(图 4b)。这一结果与上述37 ℃和42℃培养下酵母生长状态很好的符合,S. c-GroES表现出良好的耐热性。同时,通过点滴平板实验发现(图 5),37℃培养的菌液经过10-5梯度稀释后对照只长出一个菌落,而S. c-GroES却形成较多的菌落形态。42℃点滴平板法也可以得到相同的结论。同时,S. c-GroES经过37 ℃和42℃培养后仍可以形成正常的菌落形态,说明高温培养没有破坏细胞生长和代谢。
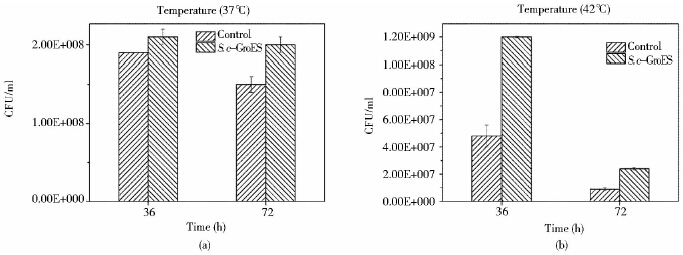
|
| 图 4 酿酒酵母工程菌S. c-GroES和对照酵母在37℃(a)和42℃(b)下的细胞存活率 Fig. 4 The cell growth ofthe engineered S. cerevisae,S. c-GroES and the control yeast cultured at 37℃(a) and 42℃ (b) |
|
| 图 5 点滴平板法考察酿酒酵母工程菌S. c-GroES 和对照酵母在37℃(a)和42℃(b)下的菌落形成情况 Fig. 5 Investigation of colony formation of the engineered S. cerevisae,S. c-GroES and the control yeast by spottedplate method at 37℃ (a)and 42℃(b) |
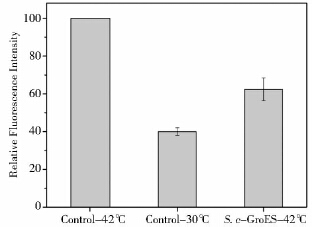
高温会诱导酵母细胞积累大量的ROS,对细胞造成损伤并对发酵生产带来极不利的影响[22]。细胞受到氧化胁迫后,会有大量的HSP基因上调,HSP可抑制产生ROS的关键酶NADPH氧化酶或直接增加内源性过氧化酶水平来清除氧自由基[23]。如果耐热元器件FBA1p-groes-SLM5t能清除高温诱导的ROS,将更有利于发酵行业的发展。42 ℃热激后S. c-GroES的ROS水平比42 ℃对照降低37.6%(图 6),虽比30 ℃高,但是从一定程度上清除了高温诱导的ROS。
|
| 图 6 42 ℃和30 ℃对照酿酒酵母与42℃酿酒酵母工程菌S. c-GroES相对ROS水平 Fig. 6 The relative ROS levles of the controlyeastcultured at 42 ℃ and 30 ℃ and the engineered S. cerevisae,S. c-GroES cultured at 42℃ |
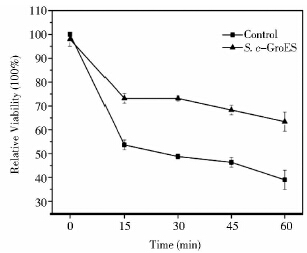
上述结果表明,FBA1p-groes-SLM5t可以降低高温诱导的ROS。为了进一步验证S. c-GroES具备抗氧化性,又考察了30 ℃下,S. c-GroES对H2O2的抗性。在H2O2处理下,酵母存活率都有一定下降,然而S. c-GroES存活率下降缓慢,且在1h时,存活率是对照的1.62倍。表明,S. c-GroES具备了一定抗氧化性能。结合上述高温培养结果表明耐热元器件在缓解热胁迫的同时对细胞的抗氧化性也有帮助。
|
| 图 7 H2O2作用下对照酿酒酵母与酿酒酵母工程菌S. c-GroES细胞存活率 Fig. 7 The cell viability ofthe congtrolyeast and the engineered S. cerevisae,S. c-GroES treated with H2O2 |
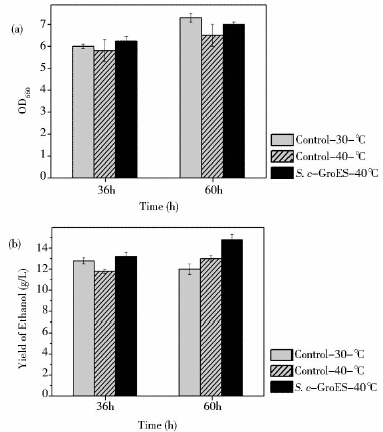
上述结果表明,S. c-GroES的耐热性得到了提高。鉴于此,考察了酿酒酵母40 ℃发酵乙醇的能力,进而对耐热元器件进行功能验证。如图 8a所示,36 h和72 h时,30 ℃对照和40 ℃的S. c-GroES的OD660均比40 ℃对照高,虽然工程菌比30 ℃对照稍差,但差别不大。由于此次实验的培养条件为高温与厌氧所以导致整体的生长状况不如上述梯度高温和恒定高温发酵。在初始葡萄糖浓度一定,批式发酵过程中,36 h时30 ℃对照乙醇产量高于40 ℃对照,然而在60 h其乙醇产量下降并低于40 ℃对照这一现象与已有研究一致(图 8b)[24]。但是60h时,其OD660最高(图 8a),这说明细胞浓度高但对应的乙醇产量却降低了。由于酵母营养缺乏时也可以将乙醇做为碳源[25],30℃是酵母的最适生长温度可以最有效利用碳源进行生长。所以可能是因为酵母将大部分营养用于生长,并且将乙醇做为碳源导致30 ℃酵母生长状况良好而后期乙醇产量不高。
|
| 图 8 对照酵母(30℃和40℃)与酿酒酵母工程菌S. c-GroES(40 ℃)乙醇合成效率验证,菌株生长状况(a)和乙醇产量(b) Fig. 8 The verification of ethanol synthetic efficiency in the control yeast(30℃ and40℃) and the engineered S. cerevisae,S. c-GroES(40℃) by growth condition (a) and ethanol yield (b) |
36 h时S. c-GroES和30 ℃对照乙醇产量均比40 ℃对照高。并且60 h时,S. c-GroES乙醇产量最高,比30 ℃对照和40 ℃对照分别高出25%和13.8%(图 8b)。此外,36h时,S.c-GroES的乙醇得率达到64.7%,比30 ℃对照和40 ℃对照均有一定地提高,说明酿酒酵母工程菌S. c-GroES可以更加有效地利用底物合成乙醇(表 3)。提高温度可以有效提高酶的催化活性和细胞代谢水平[6],同时耐热元器件FBA1p-groes-SLM5t可以有效地保护细胞免受热胁迫的影响,所以S. c-GroES的乙醇产量及单位细胞乙醇产量最高。因此,FBA1p-groes-SLM5t可以有效地提高酿酒酵母耐热性和乙醇合成效率。
| 酿酒酵母 | 葡萄糖浓度(g/L) | 乙醇得率(%) | ||
| 12 h | 24h | 36 h | 36 h | |
| Control-30 ℃ | 27.5±0.42 | 0.16±0.01 | 0 | 62.7% |
| Control-40 ℃ | 29.3±0.39 | 0.25±0.02 | 0 | 57% |
| S. c-GroES | 28±0.67 | 0.13±0.01 | 0 | 64.7% |
本研究基于合成生物学的方法提高酿酒酵母耐热性与乙醇合成效率。通过分析嗜热栖热菌热激蛋白基因,设计并构建了5个热激蛋白元器件,并导入酿酒酵母获得工程菌。
梯度升温培养初步筛选出性能较好的耐热元器件FBA1p-groes-SLM5t,并利用恒定高温培养进一步验证了FBA1p-groes-SLM5t能有效地提高酵母耐热性。在热激温度37℃和热致死温度42℃下,S. c-GroES细胞存活率均比对照高,例如42℃,48h的存活率是对照的3倍。此外,42℃ S. c-GroES的ROS水平比对照低37.6%,并且H2O2处理1 h后存活率是对照的1.62倍,说明耐热元器件培养在缓解酵母热胁迫的同时对抗氧化也有一定的帮助
对导入耐热元器件的酿酒酵母(S. c-GroES)进行40 ℃乙醇发酵,结果表明其乙醇产量比30 ℃对照和40℃对照分别提高了25%和13.8%。该研究为提高酿酒酵母耐热性和生物合成效率,降低生产成本提供了新思路。
| [1] | Abdel-Banat B M A, Hoshida H, Ano A, et al. High-temperature fermentation: how can processes for ethanol production at high temperatures become superior to the traditional process using mesophilic yeast? Applied Microbiology and Biotechnology, 2010, 85(4): 861-867. |
| [2] | Yanase S, Hasunuma T, Yamada R, et al. Direct ethanol production from cellulosic materials at high temperature using the thermotolerant yeast Kluyveromyces marxianus displaying cellulolytic enzymes. Applied Microbiology and Biotechnology, 2010, 88(1): 381-388. |
| [3] | Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death, Molecular Cell, 2010, 40(2), 253-266. |
| [4] | Davidson J F, Whyte B, Bissinger P H, et al. Oxidative stress is involved in heat-induced cell death in Saccharomyces cerevisiae, PNAS, 1996, 93(10), 5116-5121. |
| [5] | Kenny A, Hitzig W. Bone marrow transplantation for severe combined immunodeficiency disease, European Journal of Pediatrics, 1979, 131(3), 155-177. |
| [6] | Shahsavarai H, Hasegawa D, Yokota D, et al. Enhanced bio-ethanol production from cellulosic materials by semi-simultaneous saccharification and fermentation using high temperature resistant Saccharomyces cerevisiaeTJ14. J Biosci Bioeng, 2013, 115 (1):20-23. |
| [7] | Chen H Y, Chu Z M, ZhangY,et al. Over expression and characterization of the recombinant small heat shock protein from Pyrococcus furiosus. Biotechnology Letters, 2006, 14 (28):1089-1094. |
| [8] | Li D C, Yang F, Lu B,et al. Thermotolerance and molecular chaperone function of the small heat shock protein HSP20 from hyperthermophilic archaeon, Sulfolobus solfataricusP2. Cell Stress and Chaperones, 2011,17(1): 103-108. |
| [9] | Jia H Y, Fan Y, Feng X D, et al. Enhancing stress-resistance for efficient microbial biotransformations by synthetic biology. Frontiers in Bioengineering and Biotechnology, 2014, 2. |
| [10] | Henne A, Brüggemann H, Raasch C, et al. The genome sequence of the extreme thermophile Thermus thermophilus. Nature Biotechnology, 2004, 22(5): 547-553. |
| [11] | Laksanalamai P, Whitehead T A, Robb F T. Minimal protein-folding systems in hyperthermophilic archaea. Nat Rev Microbiol, 2004, 2(4): 315-324. |
| [12] | Hilary A S, Ashleigh R B, Tonya L S,et al.Three heat shock proteins are essential for rotifer thermotolerance.Journal of Experimental Marine Biology and Ecology, 2012, 413: 1-6. |
| [13] | Runde S, Molière N, Heinz A, et al. The role of thiol oxidative stress response in heat‐induced protein aggregate formation during thermotolerance in Bacillus subtilis. Molecular Microbiology, 2014, 91(5):1036-1052. |
| [14] | Horwich A L. Molecular chaperones in cellular protein folding: the birth of a field. Cell, 2014, 157(2): 285-288. |
| [15] | Davidson J F, Whyte B, Bissinger P H, et al. Oxidative stress is involved in heat-induced cell death in Saccharomycescerevisiae. PNAS, 1996, 93(10): 5116-5121. |
| [16] | Ueom J, Kwon S, Kim S, et al. Acquisition of heat shock tolerance by regulation of intracellular redox states. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 2003, 1642(1): 9-16. |
| [17] | Ohtani N, Tomita M, Itaya M. An extreme thermophile, Thermus thermophilus, is a polyploid bacterium. Journal of Bacteriology, 2010, 192(20): 5499-5505. |
| [18] | Vabulas R M, Raychaudhuri S, Hayer-Hartl M, et al. Protein folding in the cytoplasm and the heat shock response, Cold Spring Harbor Perspectives in Biology, 2010,2(12), 1-18. |
| [19] | Guisbert E, Herman C, Lu C Z, et al. A chaperone network controls the heat shock response in E. coli. Genes & Development, 2004, 18(22): 2812-2821. |
| [20] | Holubáǐová A, Müller P, Svoboda A. A response of yeast cells to heat stress: cell viability and the stabilitz of cytoskeletal structures. Scripta Medica (BRNO), 2000,73 (6): 381-392. |
| [21] | Vabulas R M, Raychaudhuri S, Hayer-Hartl M, et al. Protein folding in the cytoplasm and the heat shock response, Cold Spring Harbor Perspectives in Biology,2010, 2(12):a004390. |
| [22] | Gómez Pastor R, Pérez Torrado R, Cabiscol E, et al. Reduction of oxidative cellular damage by overexpression of the thioredoxin TRX2 gene improves yield and quality of wine yeast dry active biomass. Microb Cell Fact, 2010, 9(9):doi:10.1186/1475-2859-9-9. |
| [23] | Jamieson D J. Oxidative stress responses of the yeast Saccharomyces cerevisiae. Yeast, 1998, 14(16): 1511-1527. |
| [24] | Lin Y, Zhang W, Li C, et al. Factors affecting ethanol fermentation using Saccharomyces cerevisiae BY4742. Biomass and bioenergy, 2012, 47: 395-401. |
| [25] | Turcotte B, Liang X B, Robert F, et al. Transcriptional regulation of nonfermentable carbon utilization in budding yeast. FEMS Yeast Research, 2010, 10(1): 2-13. |
 2015, Vol. 35
2015, Vol. 35




