扩展功能
文章信息
- 赵嘉欣, 王玉姣, 李永香, 母群征, 夏青, 宋秀平, 栗冬梅, 鲁亮, 李贵昌
- ZHAO Jia-xin, WANG Yu-jiao, LI Yong-xiang, MU Qun-zheng, XIA Qing, SONG Xiu-ping, LI Dong-mei, LU Liang, LI Gui-chang
- 小型兽类不同器官恙虫病东方体阳性率调查
- Positive rate of Orientia tsutsugamushi in different organs of small mammals
- 中国媒介生物学及控制杂志, 2021, 32(4): 428-431
- Chin J Vector Biol & Control, 2021, 32(4): 428-431
- 10.11853/j.issn.1003.8280.2021.04.008
-
文章历史
- 收稿日期: 2021-05-14
2 佳木斯大学公共卫生学院, 黑龙江佳木斯 154007;
3 山东第一医科大学公共卫生学院, 山东 泰安 271016
2 School of Public Health, Jiamusi University, Jiamusi, Heilongjiang 154007, China;
3 School of Public Health, Shandong First Medical University, Tai'an, Shandong 271016, China
恙虫病(scrub typhus)是由恙虫病东方体(Orientia tsutsugamushi,Ot)感染引起的自然疫源性疾病,以鼠类为主要传染源,经恙螨幼虫叮咬传播。Ot是一种专性胞内寄生菌,可侵害多个器官。恙虫病疫源地调查或监测中鼠类等小型兽类(小兽)病原学检测最常用标本是脾脏和肝脏[1],然而其他器官也可用于病原学检测。为探究小兽不同器官Ot阳性率有无差别,本文对河南省永城市和北京市平谷区小兽不同器官的Ot阳性率进行分析,为恙虫病的病原学监测提供参考依据。
1 材料与方法 1.1 检测标本2017年10月自永城市采集105只小兽肝、脾、肺、肾、耳样本[2],2018年9-11月在平谷区采集324只小兽肝、脾、耳样本[3]。其中耳部捡取叮咬恙螨最多的部位皮肤,如没有恙螨,则根据有恙螨小兽耳确定恙螨寄生最多的部位。部分样本因采样原因未检测。
1.2 病原学检测使用北京百泰克生物技术有限公司生产的磁珠法DNA提取试剂盒提取小兽标本的DNA。用《恙虫病预防控制技术指南(试行)》(中疾控〔2009〕1号)的巢式PCR引物和方法检测Ot 56 kDa外膜蛋白基因序列。电泳出现特异性条带的PCR产物送北京奥科鼎盛生物技术有限公司测序,序列比对为目标核酸片段者判定为阳性。根据文献[4]从GenBank上下载不同基因型56 kDa蛋白基因序列,建立系统发育树,确定标本Ot基因型。
1.3 数据分析使用Excel 2019、SPSS 22.0软件进行数据整理和统计分析。应用χ2检验比较小兽不同器官的Ot阳性率和基因分型的关系,McNemar检验和Kappa检验对2种小兽器官Ot阳性率进行一致性比较,P < 0.05为差异有统计学意义。
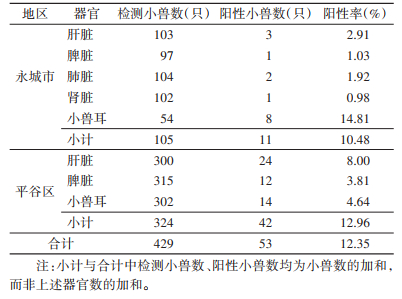
2 结果 2.1 小兽不同器官Ot阳性率永城市和平谷区共检测小兽429只,其中53只阳性,Ot总阳性率为12.35%。其中永城市共检测小兽105只,黑线姬鼠(Apodemus agrarius)检测94只,阳性8只;褐家鼠(Rattus norvegicus)检测5只,阳性1只;小家鼠(Mus musculus)检测3只,阳性2只;臭鼩鼱(Suncus murinus)检测2只、大仓鼠(Tscherskia triton)检测1只均为阴性。对105只小兽的不同器官样本进行巢式PCR检测,11只鼠的15份不同器官样本为阳性。永城市小兽总的Ot阳性率为10.48%,其中小兽耳的阳性率最高,为14.81%,肝、脾、肺、肾的阳性率依次为2.91%、1.03%、1.92%和0.98%。小兽不同器官阳性率之间差异有统计学意义(χ2=17.083,P < 0.001)。两两比较结果显示,小兽耳的阳性率与肝、脾、肺、肾的阳性率差异均有统计学意义(χ2分别为6.371、9.89、8.348和10.501,P分别为0.012、0.002、0.003和0.001),并高于其他器官,肝、脾、肺和肾之间阳性率差异无统计学意义(Fisher检验,P=0.622、0.683、0.621、1.000、1.000、1.000)。
平谷区共检测小兽324只,其中黑线姬鼠检测185只,阳性9只,小家鼠检测115只,阳性21只,中华姬鼠(A. draco)检测7只,阳性1只;山东小麝鼩(Crocidura shantungensi)检测5只,阳性1只;大仓鼠和北社鼠(Niviventer confucianus)各检测6只,均为阴性。42只小兽的49份不同器官样本为阳性。总的Ot阳性率为12.96%,其中肝脏的阳性率最高为8.00%,脾脏为3.81%,小兽耳为4.64%。小兽不同器官阳性率之间差异有统计学意义(χ2=6.619,P=0.033)。两两比较结果显示,肝脏与脾脏的阳性率差异有统计学意义(χ2=5.818,P=0.022),且肝脏的阳性率高于脾脏。肝脏、脾脏与小兽耳的阳性率差异均无统计学意义(χ2=2.88、0.519,P=0.090、0.471)。
两地小兽总的Ot阳性率差异无统计学意义(χ2=0.494,P=0.504),分别比较小兽不同器官Ot阳性率,两地小兽的肝脏、脾脏阳性率差异无统计学意义(χ2=3.175、1.874,P=0.107、0.207),小兽耳的阳性率差异有统计学意义(χ2=6.525,P=0.011),两地小兽不同器官Ot阳性率见表 1。
|
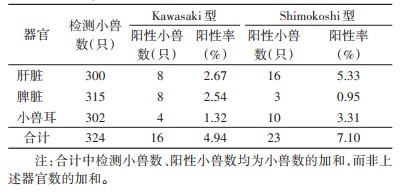
永城市鼠体Ot为Kawasaki型,平谷区则包括Kawasaki和Shimokoshi型2种,故对平谷区小兽不同器官感染的Ot基因分型进行分析。Kawasaki型和Shimokoshi型的总阳性率分别为4.94%和7.10%(表 2),差异无统计学意义(χ2=1.337,P=0.248)。相同器官不同Ot基因型别的阳性率差异均无统计学意义(χ2=2.778、2.313、2.632,P=0.096、0.128、0.105)。Kawasaki型在小兽不同器官的阳性率差异无统计学意义(χ2=1.560,P=0.455),Shimokoshi型在小兽不同器官的阳性率差异有统计学意义(χ2=9.662,P=0.007),即肝脏的阳性率高于小兽耳及脾脏。Shimokoshi型在小兽不同器官的阳性率两两比较结果显示,肝脏与脾脏、脾脏与小兽耳的阳性率差异均有统计学意义(χ2=9.850、4.159,P=0.002、0.041),且肝脏的阳性率高于脾脏,小兽耳高于脾脏。肝脏与小兽耳的阳性率差异无统计学意义(χ2=1.489,P=0.222)。
|
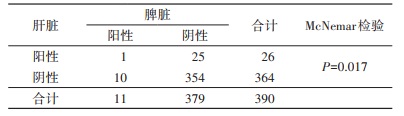
永城市阳性小兽标本中有27.27%(3/11)是2个以上器官同时阳性,平谷区为14.29%(6/42)。因永城市和平谷区小兽的肝脏、脾脏Ot阳性率差异无统计学意义,将两地肝、脾检测结果合并后进行McNemar检验(表 3),结果显示肝脏、脾脏Ot阳性率差异有统计学意义(P=0.017),2个器官检测的阳性率有差异,肝脏的阳性率高于脾脏。Kappa值为0.015,Kappa检验结果无统计学意义(P=0.744)。
|
Ot可以侵犯多种哺乳动物的不同细胞,当动物被恙螨幼虫叮咬后,Ot通过叮咬处侵入宿主体内,首先在被叮咬部位皮肤中被真皮树突状细胞和激活的单核-巨噬细胞吞噬,引起局部炎症反应,然后以活化的树突状细胞和单核细胞作为传播载体[5],随着血液或淋巴转移到局部淋巴结和全身组织器官,并侵入小血管内皮细胞及其周边的单核-吞噬细胞中,引起细胞凋亡,造成多种器官的功能损害[6-7]。Ot在人体内可侵害多种器官的多种细胞,例如3例死于战争期间的病例尸检显示Ot分布于心、肺、脑、肾、胰腺和皮肤等器官的内皮细胞及肝和脾的巨噬细胞中[8]。而在动物模型中也可观察到Ot的广泛分布,如Keller等[9]在小鼠足垫皮内注射Ot,14 d后在肝、脾、肺、肾、心、脑和淋巴结等多种器官中均出现Ot 47 kDa蛋白核酸拷贝数的高峰,此后开始下降,但28 d仍可检出。Soong等[10]采用耳侧皮内注射感染小鼠动物模型,发现Ot可在小鼠血液、淋巴结、肝、脾、肺、耳中持续至少84 d,但拷贝数很低。上述研究说明Ot不仅可在哺乳动物宿主的多种器官中增殖,且持续时间很长,并有拷贝数的差异。后两项研究均为实验室动物模型感染实验,但在野外动物自然感染Ot以后不同器官Ot感染率情况的横断面调查尚无报道。
本研究中野外捕获的小兽肝、脾、肺、肾和耳均可检出Ot 56 kDa蛋白基因,证实在野外Ot感染黑线姬鼠、小家鼠等多种鼠类以后可侵入多种器官,与实验室小鼠模型的研究结果一致[9-11]。
永城市小兽肝、脾、肺、肾Ot阳性率无差异,而平谷区肝、脾Ot阳性率则存在差异。通过比较平谷区Kawasaki和Shimokoshi基因型小兽不同器官的阳性率,发现平谷区Kawasaki基因型小兽肝、脾、耳阳性率并无差别,与永城市单一的Kawasaki型一致,而平谷区小兽肝、脾阳性率的差异可能是由Shimokoshi基因型的阳性率不同造成的,其原因还有待后续研究。
永城市仅检测了携带恙螨的小兽耳,但携带和未携带恙螨的小兽耳可能存在Ot感染率差异,从而造成永城市小兽耳Ot阳性率高于其他4种器官,也高于平谷区全部检查小兽耳的阳性率。而且平谷区的小兽耳阳性率依然高于脾,小兽耳比较高的阳性率提示我们在鼠体病原学监测中,可以只检测鼠耳中的Ot,这样即可将恙螨监测与Ot病原学监测结合起来,捕获鼠以后只需剪下鼠耳,不需解剖和保存脏器,可以减少鼠体解剖的操作和生物安全风险。但小兽耳皮肤的取样应靠近恙螨叮咬的部位。
永城市和平谷区均发现同一只小兽的不同器官同时阳性的概率较小,且两地肝、脾合并后配对检测结果不一致。推测原因是小兽各器官在感染早期或后期Ot拷贝数较低[10],此时巢式PCR较少的加样量可能导致56 kDa蛋白基因拷贝数较低,从而出现假阴性结果。通过检测多种器官可以提高检测方法的灵敏性,提高小兽Ot的检出率。
本研究仅针对永城市和平谷区2个秋季型恙虫病疫源地,其媒介均为小盾纤恙螨(Leptotrombidium scutellare),宿主为黑线姬鼠和小家鼠[2-3],但在其他宿主、媒介和Ot基因型不同的疫源地内宿主各器官感染率情况,今后需在不同类型疫源地开展相关研究。
利益冲突 无
| [1] |
Wangroongsarb P, Saengsongkong W, Petkanjanapong W, et al. An application of duplex PCR for detection of Leptospira spp. and Orientia tsutsugamushi from wild rodents[J]. Jpn J Infect Dis, 2008, 61(5): 407-409. |
| [2] |
李贵昌, 李永香, 陈传伟, 等. 河南省永城市恙虫病自然疫源地调查研究[J]. 中国媒介生物学及控制杂志, 2019, 30(3): 255-258. G C, Li YX, Chen CW, et al. An investigation of natural focus of scrub typhus in Yongcheng, Henan province, China[J]. Chin J Vector Biol Control, 2019, 30(3): 255-258. DOI:10.11853/j.issn.1003.8280.2019.03.006 |
| [3] |
李贵昌, 王玉姣, 母群征, 等. 北京市平谷区恙虫病自然疫源地宿主、媒介和病原体调查[J]. 中国媒介生物学及控制杂志, 2021, 32(3): 291-297. Li GC, Wang YJ, Mu QZ, et al. An investigation of the hosts, vectors, and pathogens of scrub typhus in Pinggu natural focus of Beijing, China[J]. Chin J Vector Biol Control, 2021, 32(3): 291-297. |
| [4] |
Kelly DJ, Fuerst PA, Ching WM, et al. Scrub typhus: the geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi[J]. Clin Infect Dis, 2009, 48(Suppl 3): S203-230. DOI:10.1086/596576 |
| [5] |
Paris DH, Phetsouvanh R, Tanganuchitcharnchai A, et al. Orientia tsutsugamushi in human scrub typhus eschars shows tropism for dendritic cells and monocytes rather than endothelium[J]. PLoS Negl Trop Dis, 2012, 6(1): e1466. DOI:10.1371/journal.pntd.0001466 |
| [6] |
Díaz FE, Abarca K, Kalergis AM. An update on host-pathogen interplay and modulation of immune responses during Orientia tsutsugamushi infection[J]. Clin Microbiol Rev, 2018, 31(2): e00076-17. DOI:10.1128/CMR.00076-17 |
| [7] |
陈香蕊. 恙虫病和恙虫病东方体[M]. 北京: 军事医学科学出版社, 2001: 65-70. Chen XR. Scrub typhus and Orientia tsutsugamushi[M]. Beijing: Military Medical Science Press, 2001: 65-70. |
| [8] |
Moron CG, Popov VL, Feng HM, et al. Identification of the target cells of Orientia tsutsugamushi in human cases of scrub typhus[J]. Mod Pathol, 2001, 14(8): 752-759. DOI:10.1038/modpathol.3880385 |
| [9] |
Keller CA, Hauptmann M, Kolbaum J, et al. Dissemination of Orientia tsutsugamushi and inflammatory responses in a murine model of scrub typhus[J]. PLoS Negl Trop Dis, 2014, 8(8): e3064. DOI:10.1371/journal.pntd.0003064 |
| [10] |
Soong L, Mendell NL, Olano JP, et al. An intradermal inoculation mouse model for immunological investigations of acute scrub typhus and persistent infection[J]. PLoS Negl Trop Dis, 2016, 10(8): e0004884. DOI:10.1371/JOURNAL.PNTD.0004884 |
| [11] |
Shelite TR, Saito TB, Mendell NL, et al. Hematogenously disseminated Orientia tsutsugamushi-infected murine model of scrub typhus[J]. PLoS Negl Trop Dis, 2014, 8(7): e2966. DOI:10.1371/journal.pntd.0002966 |
 2021, Vol. 32
2021, Vol. 32


