扩展功能
文章信息
- 陈翰明, 陈辉莹, 高景鹏, 李凯利, 杨振洲, 彭恒, 马雅军
- CHEN Han-ming, CHEN Hui-ying, GAO Jing-peng, LI Kai-li, YANG Zhen-zhou, PENG Heng, MA Ya-jun
- 我国黄土高原延伸地带利什曼病流行区的传播媒介小生境调查
- Ecological niches of sandfly (Diptera: Psychodidae) in the extension region of Loess Plateau, China: an endemic focus of visceral leishmaniasis
- 中国媒介生物学及控制杂志, 2019, 30(6): 597-602
- Chin J Vector Biol & Control, 2019, 30(6): 597-602
- 10.11853/j.issn.1003.8280.2019.06.001
-
文章历史
- 收稿日期: 2019-07-02
- 网络出版时间: 2019-10-15 09:48
2 解放军疾病预防控制中心, 北京 100071;
3 海军军医大学基础医学院, 上海 200433
2 Institute of Disease Control and Prevention, People's Liberation Army of China;
3 Department of Medical Microbiology and Parasitology, College of Basic Medical Sciences, Naval Medical University
白蛉是隶属于毛蠓科(Psychodidae)的一类小型吸血昆虫,全世界已记录8属900余种,我国的白蛉有5属40余种,个别蛉种的分类地位仍有争议[1-2]。部分白蛉可传播利什曼病、巴尔通体病和白蛉热等疾病。内脏利什曼病曾流行于我国长江以北的16个省(直辖市、自治区),经过大力防治,至20世纪70年代,东部和中部平原地带已达到清除标准[3]。但近年的疫情显示,我国西部的陕西、山西、内蒙古、新疆、甘肃和四川6省(自治区)的病例报告从未间断,且病例数和分布呈现上升与扩散趋势,偶有流行暴发点[4]。其原因除了城镇化速度加快、人员流动频繁及生态环境改变外,与流行区传播媒介白蛉的生态习性不甚了解也有密切关系。我国河南与山西省的黄土高原延伸地带是内脏利什曼病的丘陵犬源型流行区,当地的传播媒介是中华白蛉(Phlebotomus chinensis),以往的初步调查显示,中华白蛉白天在村外的窑洞或土洞栖息,日落后进入村庄寻找血源[1]。河南省自1983年之后便无内脏利什曼病病例报告[3],但2013年又开始出现本地病例[5];而山西省的内脏利什曼病病例报告近5年呈现逐年上升趋势,如长治和阳泉市的报告病例每年从几例到数十例。
沃尔巴克氏体(Wolbachia spp.)是细胞内共生细菌,隶属于α变形杆菌门(alpha-Proteobacteria),立克次体目(Rickettsiales),已在多种节肢动物体内发现[6],已报道有76.00%的昆虫有沃尔巴克氏体共生[7]。沃尔巴克氏体可以通过垂直传递传播,与宿主间存在复杂的相互作用,如诱导宿主的孤雌生殖、雄性不育、胞质不相容和增强宿主免疫力等[8-10]。最近的研究表明,沃尔巴克氏体可以作为媒介生物防控的重要策略[11]。有研究发现,在罗蛉属(Genus Lutzomyia)[12-14]和白蛉属(Genus Phlebotomus)[13, 15-16]种类中均有沃尔巴克氏体共生,课题组之前也从河南省的中华白蛉体内分离到沃尔巴克氏体,但在分布于四川省九寨沟的中华白蛉群体中未分离到[16],提示沃尔巴克氏体感染率可能与地理分布有关。沃尔巴克氏体与宿主白蛉之间的相互作用目前也知之甚少,需要进一步研究以了解白蛉与其相关微生物的生态学意义。本研究在我国黄土高原延伸地带的河南和山西省部分地区采集白蛉,阐明其孳生地与栖息环境,并鉴定白蛉的种类,同时分子检测白蛉体内沃尔巴克氏体感染情况,研究结果可为制定有效的白蛉传播疾病防控策略提供科学依据。
1 材料与方法 1.1 样本采集2015年7月在河南省陕县的窑洞和鸡舍,2017年6、7月,在山西省阳泉市的杂物间和养鸡场及武乡县的牲畜圈,布置诱虫灯(MYFS-HJY-1,东莞厚积电子科技有限公司)进行通宵捕获(17:00-08:00),每个地点布灯4~5盏,每次2~3晚,并结合电动吸蚊器人工采集,采集地的详细信息见表 1,同时观察和了解当地的地理环境。现场依据外部形态特征分拣白蛉,并按照雌、雄、吸血的个体分别计数,干燥保存带回实验室。
现场随机挑选新鲜白蛉样本置于解剖镜下,持针解剖头部的口腔、咽甲及雌蛉尾部的受精囊,移至显微镜下观察并拍照,根据形态特征鉴定至种[1]。
1.2.2 分子鉴定将干燥的单只白蛉置60 μl DNAzol中,按照说明书抽提基因组DNA,扩增线粒体DNA细胞色素b(mitochondrial DNA cytochrom b,mtDNA Cyt b)的部分序列,参照文献[17]合成引物,正向(CB1)序列:5′-TAT GTA CTA CCA TGA GGA CAA ATA TC-3′,反向(CB3-R3A)序列:5′-GCT AAT TAC TCC TCC TAA CTT ATT-3′。PCR反应体系:1×KOD FX Neo缓冲液、0.4 mmol/L dNTPs、0.3 μmol/L引物、0.5 U KOD FX Neo酶和2.0 μl模板,总体积25 μl。反应条件:94 ℃ 2 min;94 ℃ 30 s,45 ℃ 30 s,72 ℃ 1 min,4个循环;94 ℃ 30 s,51 ℃ 30 s,72 ℃ 1 min,30个循环;72 ℃ 8 min。扩增产物经1%琼脂糖凝胶(5 μl /50 ml Gelred)电泳检测后,送至铂尚生物技术(上海)有限公司,应用四色荧光标记的双脱氧末端终止法,以正向引物进行测序。测定的序列在美国国立生物技术信息中心(NCBI)网站的GenBank中进行BLAST比对,确定白蛉的种类。
1.3 沃尔巴克氏体的16S rRNA部分片段扩增以1个白蛉个体,或者4个个体、10个个体为一组进行检测,依据课题组分离自中华白蛉的沃尔巴克氏体16S rRNA部分片段(KX363667.1)设计合成[16],引物序列:正向(WF-1)5′-CCA AGG CAA TGA TCT ATA GCT GA-3′;反向(WR-1)5′-GGA ATT CCT CTA TCC TCT TTC AAT-3′。反应体系和反应条件同mtDNA Cyt b扩增,扩增产物在1%琼脂糖(5 μl /50 ml Gelred)凝胶电泳检测,随机选择阳性扩增的条带测定序列,分析序列以验证扩增条带是否为预期的目的片段。
2 结果 2.1 河南和山西省黄土高原延伸地带的白蛉小生境采集地河南省陕县和山西省阳泉市、武乡县具有类似的丘陵地理环境,海拔为895~1 050 m,是黄土高原的延伸地带(图 1A、B、C),采集地村庄的建筑为土窑洞或水泥砖瓦结构(图 1D、E、F),人主要居住在砖瓦/水泥房屋,少数仍以窑洞为居室,多数窑洞用于养殖畜禽或已废弃。农户院落、村庄的道路和村庄周围多为裸露的土地。村庄中可见有集中于窑洞或砖瓦房内半封闭养殖,或散养的鸡、羊、猪、犬、鸭、鹅、驴和牛等(图 1G、H、I、J、K、L)。

|
| 注:A~C.地貌和村貌;D~F.废弃的砖瓦房和窑洞;G~L.集中或散养的羊、鸡、猪、驴、犬、鹅、鸭等 图 1 河南和山西省黄土高原延伸地带的白蛉小生境 Figure 1 The ecological niches of sandflies in the extension regions of the Loess Plateau in Henan and Shanxi provinces |
| |
在河南省陕县通宵采集3晚,共获得白蛉2 273只,其中雌蛉在鸡舍中占79.60%(1 635/2 054),在窑洞和院落占93.15%(204/219),均高于雄蛉(表 1)。在山西省阳泉市(养鸡场、杂物间和院落)和武乡县(牲畜圈)分别通宵采集3晚和2晚,分别捕获白蛉3 599和2 850只,雌蛉所占比例均远大于雄蛉,其中在养鸡场诱集的雌蛉占99.60%,吸血的个体占雌蛉的74.01%(表 1)。
本研究的采集场所除院落外,均为人居的窑洞、半封闭的窑洞或砖瓦房环境内养殖的畜禽圈舍,动物及人为白蛉提供了主要的血源,故采集的雌蛉个体(吸血的比例也较高)明显多于雄性。观察白蛉在当地的可能血源动物,包括村庄中圈养或散养的鸡、羊、猪、犬、鸭、鹅、驴和牛等,以及周围活动的人。白蛉的孳生地应是采集场所的地面、院子角落、废弃窑洞、杂物间或村庄周围的疏松土壤,成虫产卵于孳生地,幼虫和蛹在其中生长发育,蛹羽化至成虫后,寻找血源动物。
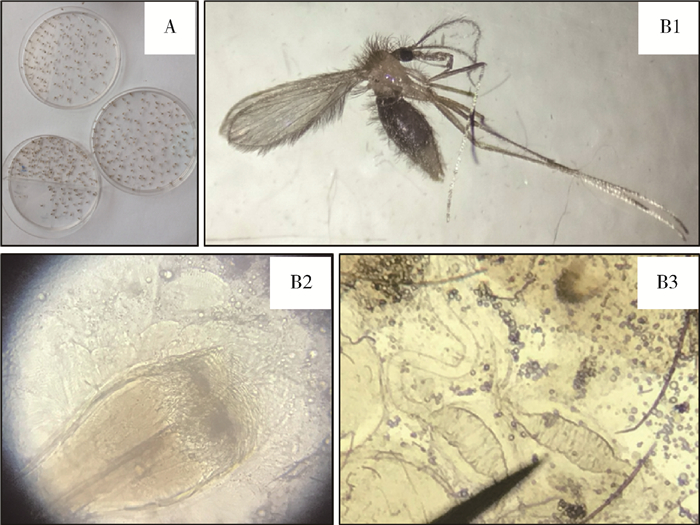
2.2 白蛉种类鉴定和优势种群在现场随机检视50只白蛉个体的形态特征,带回实验室以分子特征鉴定108只白蛉。依据成虫体表银灰色(图 2A、B1),口腔无色板,口甲退化;咽甲前排有许多“V”形尖齿,靠近前中部大而分散,后部小而密集,基部密集横脊(图 2B2);雌性白蛉受精囊囊体纺锤状,囊体不完全分节,分13~14节,各节交接处呈三角形等特征(图 2B3),形态鉴定的50只均为中华白蛉。分析108只白蛉的mtDNA Cytb序列特征,除采自河南省陕县的1只为鳞喙司蛉(Sergentomyia squamirostris)(MF966807.1)外,显示107只均为中华白蛉(MF966800.1)。从种类鉴定的结果看,中华白蛉为当地的优势种。
|
| 注:A.现场挑拣的白蛉个体;B1.中华白蛉成虫(雌);B2.咽甲;B3.受精囊 图 2 2015年7月和2017年6、7月采集的白蛉样本(A)和中华白蛉的形态特征(B1~3) Figure 2 Sandfly samples collected in July 2015 and in June and July, 2017 (A) and morphological characteristics of Phlebotomus chinensis (B) |
| |
本研究在河南省陕县、山西省阳泉市和武乡县采集白蛉虽仅为2或3晚,但收集的个体数量较多,初步统计白蛉密度,河南省陕县、山西省阳泉市和武乡县分别为151.53、299.92和356.25只/(灯·晚),每年的6-7月是当地白蛉活动的高峰季节。
2.3 白蛉体内沃尔巴克氏体的检测检测结果显示,河南省陕县13组样本中9组沃尔巴克氏体感染阳性;山西省阳泉市9组样本中的6组白蛉为阳性,武乡县的10组白蛉样本均为阳性。在单只检测的白蛉样本中,感染率分别为51.85%(56/108,阳泉市)和73.15%(79/108,武乡县)(表 2)。分析测定沃尔巴克氏体序列,种类为沃尔巴克属未定种Wolbachia spp.(KX363667.1)。

|
研究证实,白蛉可以适应不同的小生境,并有广泛的吸血对象[18-21],制定有效的白蛉控制措施必须了解当地白蛉的孳生地和栖息场所。本研究在河南和山西省采集的白蛉,雌蛉比例显著大于雄蛉,是因为布灯位置几乎均为白蛉的吸血场所,检测发现的血源不仅只是采集点的动物种类,甚至单只白蛉的胃血来源动物不止1种,提示当地白蛉或在不同的血源动物间飞行(课题组未发表数据);本研究虽然各诱虫灯也捕获到一定比例的雄蛉,但比例较低,提示吸血场所同时也是孳生地,由于白蛉的飞行距离有限,院落周边和角落,邻近的废弃窑洞、土洞等应是当地白蛉的孳生地,非常分散。作者收集了部分疑似孳生地的疏松土壤带回实验室培养,未获成功。我国四川省九寨沟的自然环境为山区,白蛉的孳生地为半山的石洞,在其中采集到的雄蛉数量远大于雌蛉,证实其为主要的孳生地,相对集中[22];在石洞中喷洒杀虫剂,极大地降低了白蛉密度[23]。然而不同地区白蛉的小生境有所差异,应采取不同的治理方案,本研究河南与山西省的白蛉孳生地分散且难以查找,提示当地应以在白蛉血源动物的养殖场所喷洒杀虫剂为主,并需加强个人防护避免叮咬。
白蛉对血源动物无严格的选择,吸血对象广泛,甚至检测到可吸取冷血动物(如蜥蜴)的血液[24-25]。不同地区的白蛉对血源动物的选择也明显不同,在四川省九寨沟首选的是猪,其次是鸡和犬[22]。本研究河南和山西省的白蛉血源动物从观察来看,主要是鸡、牛、人、犬和羊等,从白蛉的血源动物分析(课题组未发表资料),山西省两地的蛉媒病流行风险明显大于河南和四川省。无症状的犬通常是最为重要的利什曼原虫保虫宿主,有报道各地犬体内利什曼原虫(Leishmania spp.)的阳性率高达77.21%(甘肃省文县)、50.00%(四川省九寨沟县)和41.90%(四川省黑水县)[26-28],山西省1959年曾报告犬的阳性率仅为0.01%[1],近期的初步检测发现已升高至5.00%以上(课题组未发表数据),可见当地存在较高的内脏利什曼病流行风险。
本研究在河南和山西省的中华白蛉群体中均检测到沃尔巴克氏体共生菌,沃尔巴克氏体在现场白蛉的感染率较高,而在四川省九寨沟县的中华白蛉体内却未发现[16],白蛉的共生菌种类和丰度与小生境密切相关,上述共生的沃尔巴克氏体对白蛉生长繁殖的影响、对利什曼原虫感染的作用[29],及其对当地内脏利什曼病流行的影响等,需进一步探讨。
志谢 河南省疾病预防控制中心(CDC)张红卫,河南省陕县CDC苏春利,山西省CDC程璟侠、赵俊英、代培芳、田晓东等在现场采集白蛉时给予帮助,特此志谢| [1] |
熊光华, 金长发, 管立人. 中国的白蛉[M]. 北京: 科学出版社, 2016: 23-60.
|
| [2] |
杨曼尼, 马雅军. 我国白蛉的分类现状[J]. 国际医学寄生虫病杂志, 2008, 35(1): 46-49. DOI:10.3760/cma.j.issn.1673-4122.2008.01.011 |
| [3] |
Guan LR, Wu ZX. Historical experience in the elimination of visceral leishmaniasis in the plain region of Eastern and Central China[J]. Infect Dis Poverty, 2014, 3: 10. DOI:10.1186/2049-9957-3-10 |
| [4] |
Wang JY, Cui G, Chen HT, et al. Current epidemiological profile and features of visceral leishmaniasis in people's republic of China[J]. Parasit Vectors, 2012, 5: 31. DOI:10.1186/1756-3305-5-31 |
| [5] |
杨书丽, 宋录军, 马改青, 等. 河南省安阳市4例黑热病病例调查[J]. 中华地方病学杂志, 2018, 37(12): 1027. DOI:10.3760/cma.j.issn.2095-4255.2018.12.020 |
| [6] |
Werren JH, Zhang W, Guo LR. Evolution and phylogeny of Wolbachia:reproductive parasites of arthropods[J]. Proc Biol Sci, 1995, 261(1360): 55-63. DOI:10.1098/rspb.1995.0117 |
| [7] |
Jeyaprakash A, Hoy MA. Long PCR improves Wolbachia DNA amplification:wsp sequences found in 76% of sixty-three arthropod species[J]. Insect Mol Biol, 2000, 9(4): 393-405. DOI:10.1046/j.1365-2583.2000.00203.x |
| [8] |
Zug R, Hammerstein P. Wolbachia and the insect immune system:what reactive oxygen species can tell us about the mechanisms of Wolbachia-host interactions[J]. Front Microbiol, 2015, 6: 1201. DOI:10.3389/fmicb.2015.01201 |
| [9] |
Jaenike J, Brekke TD. Defensive endosymbionts:a cryptic trophic level in community ecology[J]. Ecol Lett, 2011, 14(2): 150-155. DOI:10.1111/j.1461-0248.2010.01564.x |
| [10] |
Werren JH, Baldo L, Clark ME. Wolbachia:master manipulators of invertebrate biology[J]. Nat Rev Microbiol, 2008, 6(10): 741-751. DOI:10.1038/nrmicro1969 |
| [11] |
Mingchay P, Sai-Ngam A, Phumee A, et al. Wolbachia supergroups A and B in natural populations of medically important filth flies (Diptera:Muscidae, Calliphoridae, and Sarcophagidae) in Thailand[J]. Southeast Asian J Trop Med Public Health, 2014, 45(2): 309-318. DOI:10.1016/j.socscimed.2013.11.018 |
| [12] |
Azpurua J, De La Cruz D, Valderama A, et al. Lutzomyia sand fly diversity and rates of infection by Wolbachia and an exotic Leishmania species on Barro Colorado Island, Panama[J]. PLoS Negl Trop Dis, 2010, 4(3): e627. DOI:10.1371/journal.pntd.0000627 |
| [13] |
Da Rocha NO, Lambert SM, Dias-Lima AG, et al. Molecular detection of Wolbachia pipientis in natural populations of sandfly vectors of Leishmania infantum in endemic areas:first detection in Lutzomyia longipalpis[J]. Med Vet Entomol, 2018, 32(1): 111-114. DOI:10.1111/mve.12255 |
| [14] |
Ono M, Braig HR, Munstermann LE, et al. Wolbachia infections of phlebotomine sand flies (Diptera:Psychodidae)[J]. J Med Entomol, 2001, 38(2): 237-241. DOI:10.1603/0022-2585-38.2.237 |
| [15] |
Karimian F, Vatandoost H, Rassi Y, et al. Wsp-based analysis of Wolbachia strains associated with Phlebotomus papatasi and P. sergenti (Diptera:Psychodidae) main cutaneous leishmaniasis vectors, introduction of a new subgroup wSerg[J]. Pathog Glob Health, 2018, 112(3): 152-160. DOI:10.1080/20477724.2018.1471438 |
| [16] |
Li KL, Chen HY, Jiang JJ, et al. Diversity of bacteriome associated with Phlebotomus chinensis (Diptera:Psychodidae) sand flies in two wild populations from China[J]. Sci Rep, 2016, 6: 36406. DOI:10.1038/srep36406 |
| [17] |
Zhang L, Ma YJ. Identification of Phlebotomus chinensis (Diptera:Psychodidae) inferred by morphological characters and molecular markers[J]. Entomotaxonomia, 2012, 34(1): 71-80. |
| [18] |
Ready PD. Biology of phlebotomine sand flies as vectors of disease agents[J]. Annu Rev Entomol, 2013, 58: 227-250. DOI:10.1146/annurev-ento-120811-153557 |
| [19] |
Garlapati RB, Abbasi I, Warburg A, et al. Identification of bloodmeals in wild caught blood fed Phlebotomus argentipes (Diptera:Psychodidae) using cytochrome b PCR and reverse line blotting in Bihar, India[J]. J Med Entomol, 2012, 49(3): 515-521. DOI:10.1603/ME11115 |
| [20] |
Warburg A, Faiman R. Research priorities for the control of phlebotomine sand flies[J]. J Vector Ecol, 2011, 36(Suppl 1): S10-16. DOI:10.1111/j.1948-7134.2011.00107.x |
| [21] |
Abbasi I, Cunio R, Warburg A. Identification of blood meals imbibed by phlebotomine sand flies using cytochrome b PCR and reverse line blotting[J]. Vector Borne Zoonotic Dis, 2009, 9(1): 79-86. DOI:10.1089/vbz.2008.0064 |
| [22] |
Chen HY, Li KL, Shi H, et al. Ecological niches and blood sources of sand fly in an endemic focus of visceral leishmaniasis in Jiuzhaigou, Sichuan, China[J]. Infect Dis Poverty, 2016, 5: 33. DOI:10.1186/s40249-016-0126-9 |
| [23] |
金长发, 何生全, 洪玉梅, 等. 防制中华白蛉对控制内脏利什曼病的效果追踪[J]. 中国寄生虫学与寄生虫病杂志, 2004, 22(6): 338-339. DOI:10.3969/j.issn.1000-7423.2004.06.006 |
| [24] |
Siripattanapipong S, Leelayoova S, Ninsaeng U, et al. Detection of DNA of Leishmania siamensis in Sergentomyia (Neophlebotomus) iyengari (Diptera:Psychodidae) and molecular identification of blood meals of sand flies in an affected area, southern Thailand[J]. J Med Entomol, 2018, 55(5): 1277-1283. DOI:10.1093/jme/tjy069 |
| [25] |
Gebresilassie A, Abbasi I, Aklilu E, et al. Host-feeding preference of Phlebotomus orientalis (Diptera:Psychodidae) in an endemic focus of visceral leishmaniasis in northern Ethiopia[J]. Parasit Vectors, 2015, 8: 270. DOI:10.1186/s13071-015-0883-5 |
| [26] |
Wang JY, Ha Y, Gao CH, et al. The prevalence of canine Leishmania infantum infection in western China detected by PCR and serological tests[J]. Parasit Vectors, 2011, 4: 69. DOI:10.1186/1756-3305-4-69 |
| [27] |
Zhao GH, Yin K, Zhong WX, et al. Epidemiological investigation of asymptomatic dogs with Leishmania infection in Southwestern China where visceral leishmaniasis is intractable[J]. Korean J Parasitol, 2016, 54(6): 797-801. DOI:10.3347/kjp.2016.54.6.797 |
| [28] |
汪俊云, 陈生邦, 高春花, 等. 甘肃文县婴儿利什曼原虫无症状感染犬的检测[J]. 中国人兽共患病学报, 2006, 22(8): 734-737. DOI:10.3969/j.issn.1002-2694.2006.08.011 |
| [29] |
Van Den Hurk AF, Hall-Mendelin S, Pyke AT, et al. Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti[J]. PLoS Negl Trop Dis, 2012, 6(11): e1892. DOI:10.1371/journal.pntd.0001892 |
 2019, Vol. 30
2019, Vol. 30