扩展功能
文章信息
- 万晴, 宋暖, 黄振东, 薛志静, 庄桂芬, 张瑞玲, 许永玉, 张忠
- WAN Qing, SONG Nuan, HUANG Zhen-dong, XUE Zhi-jing, ZHUANG Gui-fen, ZHANG Rui-ling, XU Yong-yu, ZHANG Zhong
- 盐酸环丙沙星对家蝇幼虫和蛹能量物质含量的影响
- Effect of ciprofloxacin hydrochloride on energy substances in the larvae and pupae of Musca domestica
- 中国媒介生物学及控制杂志, 2019, 30(5): 514-518
- Chin J Vector Biol & Control, 2019, 30(5): 514-518
- 10.11853/j.issn.1003.8280.2019.05.008
-
文章历史
- 收稿日期: 2019-04-05
- 网络出版时间: 2019-08-07 07:00
2 山东省新发传染病溯源与防控协同创新中心, 山东 泰安 271016;
3 山东农业大学植物保护学院, 山东 泰安 271000
2 Shandong Collaborative Innovation Center for the Origin and Control of Emerging Infectious Diseases;
3 Plant Protection College of Shandong Agricultural University
在长期协同进化过程中,昆虫与肠道内共生菌组成了昆虫的微生态系统,大多数昆虫肠道共生菌中的优势菌群为细菌,因此也被称为昆虫肠道共生细菌[1-2]。在昆虫为其肠道细菌提供营养的同时,肠道细菌也可协同调控寄主昆虫的生长发育、生殖能力、免疫抗逆和生态适应等[3-6]。
当昆虫受到不良环境,如重金属、高温、干旱、病原体感染等胁迫时,其体内的主要能量物质(如蛋白质、总糖、脂肪等)会受到影响,从而影响其体内的能量代谢[7-10]。昆虫的肠道共生细菌也容易受到外界环境、食物、病原体感染等因素的影响,反映出肠道微生态对外界环境改变的响应。但昆虫肠道细菌去除后,其体内的能量代谢是否受影响仍未见报道。
家蝇(Musca domestica)是一种重要的病媒生物,可机械性传播细菌、病毒、支原体、真菌、寄生虫卵等多种人兽共患病原体,威胁人类健康[11-12]。研究表明,使用抗生素去除肠道共生细菌后,家蝇生长发育、种群增长和繁殖及抗氧化酶活性均受到严重抑制[13-14],说明去除肠道细菌后,家蝇体内的生理生化、生长繁殖、环境应激等均受到影响,推测其体内的能量物质和能量代谢也会受到影响。因此,本研究在喂食家蝇不同浓度抗生素的基础上,比较家蝇发育过程中体内能量物质的变化,从而推测肠道细菌对家蝇能量代谢的影响。
1 材料与方法 1.1 供试虫源与试剂家蝇为泰山医学院病媒生物与虫媒病实验室饲养的品系;盐酸环丙沙星,纯度>88.5%,由上海源叶生物科技有限公司提供。
1.2 试虫饲养家蝇幼虫的饲料配方及饲养方法见文献[14]。幼虫提供麦麸、奶粉、酵母配成的饲料,饲养于塑料盒内;成虫提供奶粉、红糖水为饲料,饲养于带纱网的养虫笼内。成虫和幼虫均饲养于温度为(25±1)℃,光照周期(L:D)=12 h:12 h的光照培养箱内。
1.3 样品的制备家蝇幼虫1龄幼虫的样品制备采用匀浆法,将1龄幼虫饥饿6 h后,取50只置于预冷的玻璃匀浆器中,加入磷酸缓冲液和少量苯基硫脲,在冰浴中匀浆。匀浆液置于冷冻离心机中,4 ℃ 10 000×g离心10 min,取上清液作为样品,用于实验。
家蝇2~3龄幼虫和蛹均取血淋巴作为检测样品。幼虫血淋巴样品制备采用针刺收集法。首先将幼虫-20 ℃冷冻3~5 min,降低其活动能力,再置于冰浴的干净凹玻片中央,以解剖针刺破其腹部体壁,轻轻挤压虫体(注意不要刺破或压破肠道),待血淋巴流至凹玻片中,以毛细管或微量移液枪快速收集血淋巴,置于含少量苯基硫脲的冰浴离心管内。
家蝇蛹的取样方法采用活体离心法。将蛹置于0.5 ml离心管内,200×g离心5 min,轻轻取出家蝇蛹,保持蛹离心时的状态,不倒置,以解剖针挑开围蛹壳,刺破内表皮后,以微量移液枪吸取蛹内的血淋巴,收集到含少量苯基硫脲的冰浴离心管内。家蝇2~3龄幼虫和蛹的血淋巴,均以4 ℃,1 000×g离心5 min,收集上清液,用于实验。
1.4 血淋巴可溶性蛋白浓度的测定按Bradford[15]法进行测定。取1.3中制备的样品,以0.7%的生理盐水按适当比例稀释。以考马斯亮蓝G-250染色液染色(考马斯亮蓝G-250染色液配置:称取100 mg考马斯亮蓝G-250,溶于50 ml 90%乙醇溶液中,加入85%的磷酸溶液100 ml,最后用蒸馏水定容至1 000 ml,过滤掉残渣即得,常温放置不超过1个月),用分光光度计测定样品在波长为595 nm下的吸光度(A)值,并以牛血清蛋白为标准蛋白制作标准曲线。不同组的每种虫态均设3次重复。
1.5 总糖含量的测定参照孙虹霞等[8]的方法。取1.3中制备的样品,加入磺基水杨酸溶液至最终浓度为10%,1 000×g离心5 min,除去蛋白质沉淀,取上清液1 ml,加入0.2%蒽酮浓硫酸试剂5 ml,在沸水浴中加热10 min,冷却后测定A620。以葡萄糖制作标准曲线。不同组的每种虫态均设3次重复。
1.6 脂质的测定采用磷酸香草醛法[16]。取1.3中制备的样品20 μl,以去离子水补足至100 μl,加入0.5 ml氯仿,室温静止10 min;然后缓缓加入0.5 ml浓硫酸,沸水中加热10 min,冷却至室温。最后加入1 ml磷酸香草醛溶液(13 mmol/L香草醛溶于14 mol/L磷酸中),显色30 min后,测定A547,以胆固醇制作标准曲线。不同组的每种虫态均设3次重复。
1.7 热量值的计算参照Graney和Giesy[17]的方法,按照脂肪的热量值为9.5 cal/mg,糖的热量值为4.3 cal/mg,蛋白质的热量值为4.1 cal/mg来计算热量值。
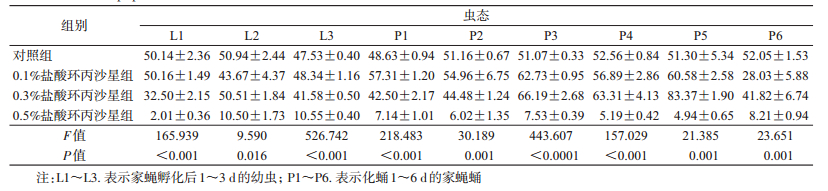
2 结果 2.1 不同浓度盐酸环丙沙星对家蝇幼虫和蛹体内蛋白质含量的影响喂食不同浓度的盐酸环丙沙星后,同日龄家蝇幼虫和蛹体内蛋白质含量差异均有统计学意义(表 1),且随着喂食盐酸环丙沙星浓度的提高,幼虫和蛹体内蛋白质含量总体呈下降趋势。其中0.5%盐酸环丙沙星组1日龄家蝇幼虫体内蛋白质的含量最低为(2.01±0.36)mg/ml,降为对照组1日龄幼虫体内蛋白质含量的4.00%;5日龄蛹体内蛋白质含量最低为(4.94±0.65)mg/ml,降为对照组5日龄蛹体内蛋白质含量的10.00%。
|
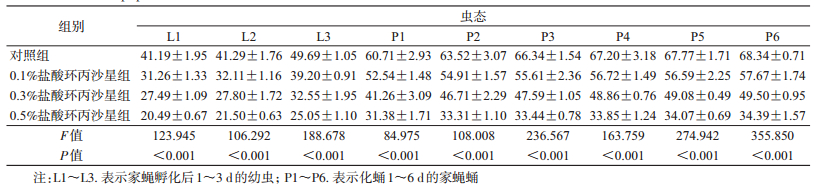
喂食不同浓度盐酸环丙沙星后,同日龄家蝇幼虫和蛹体内脂肪含量差异有统计学意义(表 2),且随着盐酸环丙沙星浓度的提高,其体内脂肪含量均呈下降趋势。0.5%盐酸环丙沙星组1日龄家蝇幼虫体内的脂肪含量最低,为(20.49±0.67) mg/ml,而对照组3日龄幼虫体内脂肪含量最高,为(49.69±1.05) mg/ml。0.5%盐酸环丙沙星组1日龄家蝇蛹体内脂肪含量最低,为(31.38±1.71) mg/ml,而对照组6日龄蛹体内脂肪含量最高,为(68.34±0.71) mg/ml(表 2)。0.1%、0.3%和0.5%组幼虫体内脂肪含量约下降为对照组的75.00%、65.00%和50.00%,蛹体内脂肪含量约下降为对照组的85.00%、75.00%和50.00%。
|
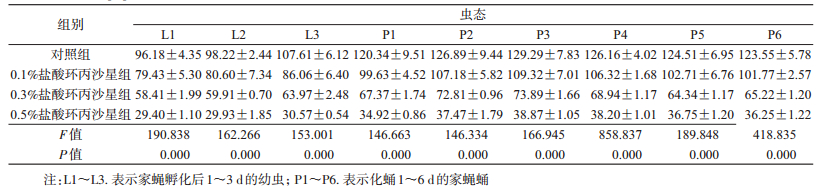
喂食不同浓度盐酸环丙沙星后,同日龄家蝇幼虫和蛹体内总糖含量差异均有统计学意义(表 3),且随着盐酸环丙沙星处理浓度的提高,呈逐渐下降趋势。喂食0.5%盐酸环丙沙星组1~3日龄家蝇幼虫体内总糖含量相近,分别为(29.40±1.10)、(29.93±1.85)和(30.57±0.54) mg/ml。喂食含0.5%盐酸环丙沙星的1日龄家蝇蛹体内总糖含量最低,为(34.92±0.86) mg/ml,而2~6日龄蛹体内总糖含量差异不大,分别为(37.47±1.79)、(38.87±1.05)、(38.20±1.01)、(36.75±1.20)和(36.25±1.22) mg/ml。0.1%、0.3%和0.5%组幼虫和蛹体内总糖含量约下降为对照组的80.00%、60.00%和30.00%。
|
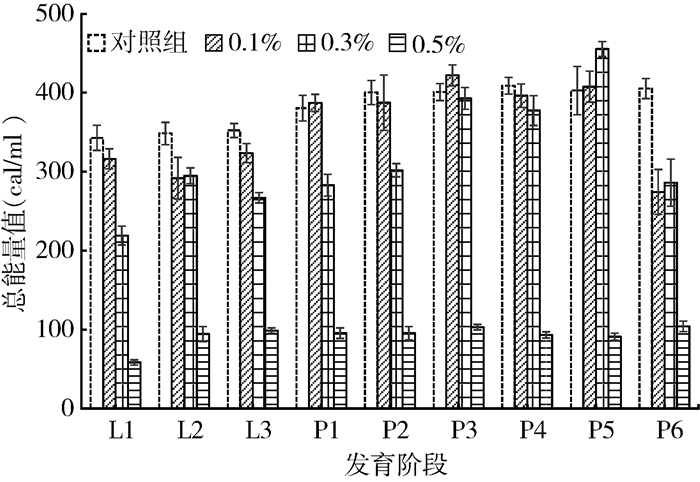
喂食不同浓度盐酸环丙沙星后,家蝇幼虫和蛹体内热量值均随盐酸环丙沙星喂食浓度的升高而明显下降(F=12.551,P<0.001)。其中,对照组体内的热量值均高于0.1%、0.3%和0.5%盐酸环丙沙星组(t=13.093、11.294、8.601,均P<0.001);0.1%组体内的热量值高于0.3%、0.5%盐酸环丙沙星组(t=10.290、7.842,均P<0.001);0.3%组体内的热量值高于0.5%组(t=6.919,P<0.001)(图 1)。
|
| 注:L1~L3.表示家蝇孵化后1~3 d的幼虫;P1~P6.表示化蛹1~6 d的家蝇蛹 图 1 不同浓度盐酸环丙沙星对家蝇幼虫和蛹体内总热量值的影响 Figure 1 Influence of different concentrations of ciprofloxacin hydrochloride on total caloric values of the larvae and pupae of Musca domestica |
| |
家蝇幼虫期体内热量值随盐酸环丙沙星喂食浓度的升高明显下降(F=3.737,P<0.001)。对照组体内的热量值高于0.1%、0.3%和0.5%盐酸环丙沙星组(t=6.652、6.218、4.477,均P<0.001);0.1%盐酸环丙沙星组体内的热量值高于0.3%(t=5.865,P<0.001)和0.5%组(t=5.865、4.301,均P<0.001);0.3%盐酸环丙沙星组体内的热量值高于0.5%盐酸环丙沙星组(t=4.260,P<0.001)(图 1)。
家蝇蛹期体内热量值也随盐酸环丙沙星处理浓度的升高而下降(F=8.732,P=0.021)。其中,对照组体内的热量值高于0.1%、0.3%和0.5%盐酸环丙沙星组(t=11.348、9.468、7.300,均P<0.001);0.1%盐酸环丙沙星组家蝇蛹体内的热量值高于0.3%和0.5%组(t=8.553、6.520,均P<0.001);0.3%组体内的热量值显著高于0.5%组(t=5.593,P<0.001)(图 1)。
3 讨论生物体内的蛋白质、脂肪和糖类起着重要的储能和供能作用,也是最为基本的三大营养物质,为生物体的生长发育和种群繁衍提供必要的能量,而能量是所有生物生长和繁殖的首要限制性因素[18]。有研究表明,当昆虫肠道细菌去除或菌群失调后,其生长发育和种群繁衍均受到显著抑制[13-14],且更易受到昆虫病原微生物或媒介病毒的感染[19-20]。
本实验室前期研究也表明,去除肠道细菌后,家蝇的生长发育、种群繁衍、抗氧化酶活性及对病原体的抵抗力均显著下降[13-14]。如喂食盐酸环丙沙星后,家蝇的卵孵化率下降,幼虫期发育迟缓,个体变小,成虫期产卵前期延长,产卵量下降,期望寿命缩短,种群的内禀增长率、净增长率、周限增长率均下降[13],说明家蝇的个体发育和种群繁衍均受到明显抑制,但其内在驱动力或关键因素仍未明确。本研究表明,去除肠道细菌后,家蝇幼虫和蛹体内的蛋白质、脂肪和糖类三大营养物质含量和体内的热量值(能量)均明显下降。故此推测,去除肠道细菌后,家蝇生长发育和种群繁衍受限的重要因素可能是其体内三大营养物质的合成受到抑制,造成能量来源不足,故此其个体生长发育延缓,种群繁衍能力下降。因此,家蝇的肠道细菌可作为对其种群控制的重要靶标,通过在诱集物质中加入少量抗生素,即可达到有效控制种群的目的,定点释放的抗生素用量低,可完全回收,且使用方便,成本低廉,不污染环境。去除肠道细菌在影响家蝇能量物质合成的同时,还可降低其对病原真菌(如球孢白僵菌等)的抵抗力,如能将抗生素与杀蝇真菌合理配合使用,可达到更为有效控制蝇类的目的。
| [1] |
Spiteller D, Dettner K, Bolan W. Gut bacteria may be involved in interactions between plants, herbivores and their predators:microbial biosynthesis of N-acylglutamine surfactants as elicitors of plant volatiles[J]. Biol Chem, 2000, 381(8): 755-762. DOI:10.1515/BC.2000.096 |
| [2] |
Dharne MS, Gupta AK, Rangrez AY, et al. Antibacterial activities of multi drug resistant Myroides odoratimimus bacteria isolated from adult flesh flies (Diptera:sarcophagidae) are independent of metallo beta-lactamase gene[J]. Braz J Microbiol, 2008, 39(2): 397-404. DOI:10.1590/S1517-838220080002000035 |
| [3] |
Crotti E, Balloi A, Hamdi C, et al. Microbial symbionts:a resource for the management of insect-related problems[J]. Microb Biotechnol, 2012, 5(3): 307-317. DOI:10.1111/j.1751-7915.2011.00312.x |
| [4] |
Engel P, Moran NA. The gut microbiota of insects-diversity in structure and function[J]. FEMS Microbiol Rev, 2013, 37(5): 699-735. DOI:10.1111/1574-6976.12025 |
| [5] |
Shi WB, Syrenne R, Sun JZ, et al. Molecular approaches to study the insect gut symbiotic microbiota at the 'omics' age[J]. Insect Sci, 2010, 17(3): 199-219. DOI:10.1111/j.1744-7917.2010.01340.x |
| [6] |
相辉, 黄勇平. 肠道微生物与昆虫的共生关系[J]. 昆虫学报, 2008, 45(5): 687-693. DOI:10.3969/j.issn.0452-8255.2008.05.003 |
| [7] |
Wu GX, Ye GY, Hu C, et al. Accumulation of cadmium and its effects on growth, development and hemolymph biochemical compositions in Boettcherisca peregrina larvae (Diptera:Sarcophagidae)[J]. Insect Sci, 2006, 13(1): 31-39. DOI:10.1111/j.1744-7917.2006.00065.x |
| [8] |
孙虹霞, 夏嫱, 唐文成, 等. Ni2+胁迫对斜纹夜蛾幼虫血淋巴中能量物质水平的适应性调节[J]. 昆虫学报, 2010, 53(4): 361-368. DOI:10.16380/j.kcxb.2010.04.006 |
| [9] |
谢春, 吴建伟, 国果, 等. 镉对家蝇幼虫血淋巴能量物质的影响[J]. 环境与健康杂志, 2013, 30(4): 304-307. DOI:10.16241/j.cnki.1001-5914.2013.04.001 |
| [10] |
张慧, 吴圣勇, 王晓青, 等. 球孢白僵菌对葱蝇成虫血淋巴蛋白质及游离氨基酸的影响[J]. 中国农业科学, 2017, 50(3): 591-598. |
| [11] |
Scott JG, Liu N, Kristensen M, et al. A case for sequencing the genome of Musca domestica (Diptera:Muscidae)[J]. J Med Entomol, 2009, 46(2): 175-182. DOI:10.1603/033.046.0202 |
| [12] |
刘小改, 杨亚军, 廖秋菊, 等. 稻纵卷叶螟肠道细菌群落结构与多样性分析[J]. 昆虫学报, 2016, 59(9): 965-976. DOI:10.16380/j.kcxb.2016.09.006 |
| [13] |
宋暖.去除肠道共生细菌对家蝇发育和丽蝇蛹集金小蜂寄生作用的影响[D].泰安: 山东农业大学, 2014. http://cdmd.cnki.com.cn/Article/CDMD-10434-1014341670.htm
|
| [14] |
宋暖, 黄振东, 薛志静, 等. 盐酸环丙沙星对家蝇幼虫和蛹体内抗氧化酶活力的影响[J]. 中国媒介生物学及控制杂志, 2018, 29(5): 448-452. DOI:10.11853/j.issn.1003.8280.2018.05.007 |
| [15] |
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding[J]. Anal Biochem, 1976, 72(1/2): 248-254. DOI:10.1016/0003-2697(76)90527-3 |
| [16] |
Nakamatsu Y, Tanaka T. Venom of ectoparasitoid, Euplectrus sp. near plathypenae (Hymenoptera:Eulophidae) regulates the physiological state of Pseudaletia separata (Lepidoptera:Noctuidae) host as a food resource[J]. J Insect Physiol, 2003, 49(2): 149-159. DOI:10.1016/S0022-1910(02)00261-5 |
| [17] |
Graney RL, Giesy Jr JP. Effects of long-term exposure to pentachlorophenol on the free amino acid pool and energy reserves of the freshwater amphipod Gammarus pseudolimnaeus bousfield (crustacea, amphipoda)[J]. Ecotoxicol Environ Saf, 1986, 12(3): 233-251. DOI:10.1016/0147-6513(86)90015-1 |
| [18] |
Wallace DC, Fan WW. Energetics, epigenetics, mitochondrial genetics[J]. Mitochondrion, 2010, 10(1): 12-31. DOI:10.1016/j.mito.2009.09.006 |
| [19] |
Wang SB, Dos-Santos ALA, Huang W, et al. Driving mosquito refractoriness to Plasmodium falciparum with engineered symbiotic bacteria[J]. Science, 2017, 357(6358): 1399-1402. DOI:10.1126/science.aan5478 |
| [20] |
Xi ZY, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection[J]. PLoS Pathog, 2008, 4(7): e1000098. DOI:10.1371/journal.ppat.1000098 |
 2019, Vol. 30
2019, Vol. 30


