扩展功能
文章信息
- 宋暖, 黄振东, 薛志静, 万晴, 庄桂芬, 张瑞玲, 许永玉, 张忠
- SONG Nuan, HUANG Zhen-dong, XUE Zhi-jing, WAN Qing, ZHUANG Gui-fen, ZHANG Rui-ling, XU Yong-yu, ZHANG Zhong
- 喂食盐酸环丙沙星对家蝇生长发育和种群繁殖的影响
- Effects of ciprofloxacin hydrochloride on the individual growth and development and population reproduction of Musca domestica
- 中国媒介生物学及控制杂志, 2019, 30(3): 300-305
- Chin J Vector Biol & Control, 2019, 30(3): 300-305
- 10.11853/j.issn.1003.8280.2019.03.016
-
文章历史
- 收稿日期: 2018-12-22
- 网络出版时间: 2019-4-23 16:05
2 山东农业大学植物保护学院, 山东 泰安 271000;
3 山东省新发传染病溯源与防控协同创新中心, 山东 泰安 271016
2 Plant Protection College, Shandong Agricultural University;
3 Shandong Collaborative Innovation Center for the Origin and Control of Emerging Infectious Diseases
昆虫及其肠道菌在长期的协同进化过程中,共同构建了昆虫的微生态系统,而昆虫肠道中共生菌在调控寄主的生殖、免疫、生长发育、寄主适应等方面发挥着重要功能[1-4]。昆虫体内的共生菌可分为初级和次级共生菌,初级共生菌主要与寄主的生殖、发育和疾病传播有关,严格进行垂直传播[5-7];次级共生菌多存在于昆虫肠道内,主要与寄主的适应性有关,可进行水平传播和垂直传播,称为肠道共生菌,而大多数昆虫肠道共生菌中的优势菌群为细菌,有时也统称为昆虫肠道共生细菌[4, 8-9]。因昆虫的肠道共生细菌易受到外界环境、食物、病原体感染等因素的影响,可反映出昆虫肠道微生态对外界环境改变的响应,因此昆虫肠道共生细菌组成与功能已成为新的研究热点。
家蝇(Musca domestica)是一种重要的病媒生物,可机械性传播细菌、病毒、支原体、真菌、寄生虫卵等病原体[10-12],干扰人的生活,威胁人类健康,影响养殖业的安全发展。同时,家蝇肠道共生细菌的组成已有相关研究[13-14],但对于肠道细菌去除后对家蝇生长发育和种群繁殖的影响仍未见相关报道。
1 材料与方法 1.1 供试家蝇与药剂供试家蝇为泰山医学院病媒生物与虫媒病实验室饲养的品系。盐酸环丙沙星,纯度>88.5%,由上海源叶生物科技有限公司提供。
1.2 试虫饲养家蝇幼虫饲养条件:恒温培养箱温度(25±1) ℃,相对湿度(70±5)%,光照周期(L : D)为12 h : 12 h,饲料配方见表 1,其中麦麸和水均经灭菌处理。
家蝇成虫饲养于35 cm×35 cm×35 cm的养虫笼中,置于恒温培养箱饲养,培养条件为温度(25±1) ℃,相对湿度(70±5)%,光照周期(L : D)为12 h : 12 h。对照组成虫提供奶粉和红糖作为补充营养,同时提供饮水。实验组成虫提供奶粉和红糖作为补充营养,同时提供含有0.001、0.003、0.005 g/ml盐酸环丙沙星的饮水。
1.3 家蝇生命表实验将家蝇产的新鲜卵块分别移入洁净的培养皿(ϕ9 cm)中,对照组培养皿底铺有用无菌水湿润的滤纸,实验组培养皿底铺有用不同浓度盐酸环丙沙星溶液湿润的滤纸。培养皿口覆封口膜,并用解剖针扎若干小孔,保持通风,24 h后观察记录孵化卵的数量,统计家蝇卵的发育历期和孵化数。待孵化后,挑取不同滤纸上发育一致的初孵蛆虫100头,用毛笔轻轻挑至盛有同等含量盐酸环丙沙星饲料配方的100 ml烧杯中(表 1)。每天08:00观察各组幼虫发育情况并记录死亡幼蛆的数量。待幼虫化蛹后,单头放入有通气孔的5 ml离心管中,记录蛹的历期及成虫羽化数。各组成虫羽化后,组内雌、雄配对饲养于口径7.0 cm的350 ml塑料杯中,杯内放有红糖和产卵基质,处理组分别提供同样浓度盐酸环丙沙星的饮用水,对照组提供无菌水。以纱布封口,每杯放置1对成虫。每天定时记录各组雌蝇产卵量直至成虫死亡。
1.4 家蝇两性生命表的调查与制作分析用Excel 2010软件按照Chi和Liu[15]发表的两性年龄-龄期生命表和Chi[16]的多行矩阵法,将种群结构用两性年龄-龄期发育速率矩阵、生长矩阵、存活率矩阵、死亡分布矩阵等表示,并计算种群内禀增长率(r)、种群净生殖率(R0)、年龄特征存活率(lx)、周限增长率(λ)等种群参数。
种群参数用生命表分析软件TWOSEX-MSChart系统进行分析。r=Σe-r(x+1)lxmx,其中x为实验天数,lx为每天家蝇的存活百分率,mx为特定年龄生殖率;λ=exp(r)。
1.5 统计学处理采用SPSS 18.0软件进行数据的方差分析,不同处理组及对照组间分析采用多重比较,两两比较采用t检验,以软件SigmaPlot 12.0进行绘图。P<0.05为差异有统计学意义。
2 结果 2.1 不同浓度盐酸环丙沙星对家蝇各虫态发育历期的影响盐酸环丙沙星对家蝇卵期无影响,处理组和对照组的蝇卵均在24 h完成孵化,不同浓度盐酸环丙沙星对卵的发育差异无统计学意义(F=2.065,df=3,P>0.05)。喂食含不同浓度盐酸环丙沙星的饲料,家蝇幼虫的发育历期显著延长(F=308.978,df=3,P=0.000)(表 2),而0.1%和0.3%处理组间差异无统计学意义(t=1.770,P=0.079)。抗生素处理组中有个别家蝇幼虫期可延长至10 d,而对照组平均为6 d,说明喂食盐酸环丙沙星对家蝇幼虫的生长发育有阻滞作用。喂食0.5%盐酸环丙沙星处理组的雌虫平均寿命为17.19 d,比对照组缩短约11 d,雄虫为17.31 d,比对照组缩短约9 d。雌虫和雄虫的0.1%和0.3%处理组间差异有统计学意义(P=0.000)(表 2)。
|
取食盐酸环丙沙星能显著延长家蝇雌虫产卵前期(F=9.690,df=3,P<0.001),但喂食抗生素的处理组间差异无统计学意义,均为6 d左右。喂食0.5%盐酸环丙沙星家蝇单雌产卵量约为(137.37±24.22)粒,显著低于对照组(638.76±39.32)粒(t=10.196,P=0.000),0.1%和0.3%处理组间差异无统计学意义(t=0.230,P=0.982)(表 3)。喂食抗生素盐酸环丙沙星后能显著缩短家蝇成虫寿命,浓度越高,效果越显著。处理组的家蝇成虫寿命比对照组分别短约4、7和11 d(表 2)。

|
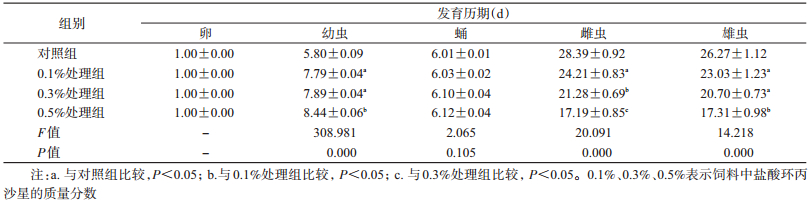
特定年龄阶段存活率(Sij)是指新生卵存活至x天j虫态的概率。特定年龄存活率研究表明,家蝇不同处理间个体发育速率明显不同,且各虫态存在重叠现象。0.1%、0.3%和0.5%处理组各虫态的存活率均明显低于对照组,浓度越高,存活率越低。用0.1%、0.3%和0.5%盐酸环丙沙星处理后,卵孵化率分别为81.73%、77.45%和68.75%,而对照组卵孵化率为96.43%。喂食0.5%抗生素盐酸环丙沙星导致幼虫化蛹和羽化的一致性降低(图 1)。
|
| 图 1 喂食不同浓度盐酸环丙沙星对家蝇特定年龄阶段存活率的影响 Figure 1 Effects of different concentrations of ciprofloxacin hydrochloride on the age-stage survival rate of Musca domestica |
| |
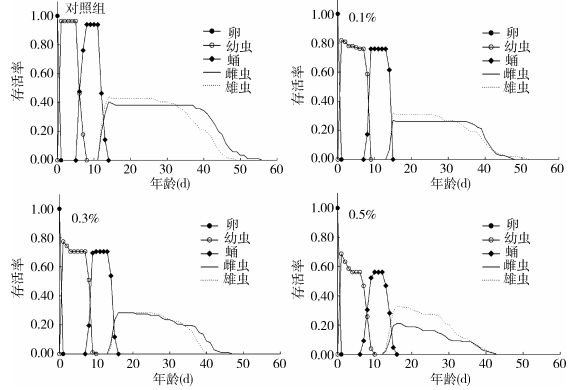
以含有盐酸环丙沙星的饲料饲养家蝇,其雌蝇的生殖力和总产卵量均明显降低,0.5%处理组的影响最为明显,mx均低于10,而0.1%和0.3%处理组间差异较小。对照组和0.1%、0.3%、0.5%处理组家蝇整个发育阶段的死亡率分别为15.48%、41.35%、43.14%和46.09%(图 2)。其中,lx指所有家蝇新生卵存活至第x天的概率,mx指所有家蝇个体第x天的平均生殖力,lxmx指所有个体第x天的繁殖总数。
|
| 注:lx.表示所有家蝇新生卵存活至第x天的概率;mx.表示所有家蝇个体第x天的平均生殖力;lxmx.表示所有个体第x天的繁殖总数 图 2 喂食不同浓度盐酸环丙沙星家蝇特定年龄存活率、日均繁殖率及繁殖总数 Figure 2 The age-stage survival rate (lx), mean daily reproductive rate (mx), and total reproductive number (lxmx) of Musca domestica fed with different concentrations of ciprofloxacin hydrochloride |
| |
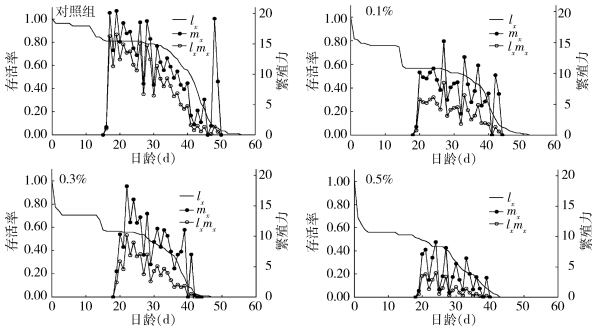
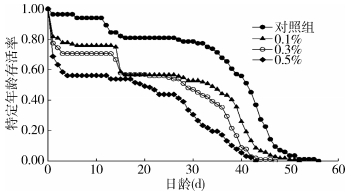
家蝇的期望寿命曲线是指某一特定年龄阶段的家蝇个体发育至成虫死亡存活时间的期望值。由图 3可知,用盐酸环丙沙星处理后,家蝇存活率随处理浓度升高而下降。而由家蝇每个虫态的期望寿命可知,对照组新生卵和0.1%、0.3%、0.5%处理组新生卵的期望寿命依次降低,分别为36.07、25.85、23.04和18.03 d,雌虫的期望寿命也由32.03 d下降至19.88 d(图 4)。
|
| 图 3 喂食不同浓度盐酸环丙沙星家蝇特定年龄存活率 Figure 3 The age-stage survival rate of Musca domestica fed with different concentrations of ciprofloxacin hydrochloride |
| |

|
| 图 4 喂食不同浓度盐酸环丙沙星的家蝇期望寿命 Figure 4 The life expectancy of Musca domestica fed with different concentrations of ciprofloxacin hydrochloride |
| |
喂食盐酸环丙沙星不同浓度家蝇种群的r和λ与对照组比较显著减小;家蝇种群的R0对照组最高,0.1%和0.3%组次之,0.5%处理组最小,仅为对照组的11.21%(表 4)。其中,R0=F·(Nf/N),N为实验过程中卵的总数,F为羽化后的雌虫数量。
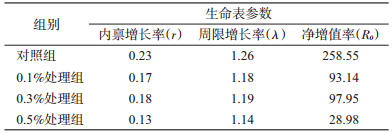
|
盐酸环丙沙星是第3代氟喹诺酮类合成抗生素,具有广谱抗菌活性,对革兰阴性菌效果明显,而家蝇肠道内大部分细菌为革兰阴性菌,如肺炎克雷伯菌属、气单胞菌属、志贺菌、摩根菌和雷氏普罗维登斯菌等。
本研究对喂食不同浓度盐酸环丙沙星家蝇的生长发育和繁殖生物学参数进行了研究。实验结果表明,盐酸环丙沙星对家蝇的卵期和蛹期无显著影响,但随着喂食抗生素浓度的增加,家蝇幼虫的发育历期显著延长,蛹重下降,成虫寿命缩短,特定年龄阶段存活率和单雌产卵量随之降低,说明部分去除家蝇肠道内共生菌对其生长发育和繁殖是不利的。通过TWOSEX-MSChart两性生命表研究表明,喂食盐酸环丙沙星家蝇的r、λ和R0均明显低于对照组,说明盐酸环丙沙星可通过部分去除肠道共生细菌显著抑制家蝇种群的增长。
家蝇幼虫肠道中细菌种类繁多[17],但主要是通过氧化食物中的脂肪酸和氨基酸来满足自身的能量需要[18],已有研究表明,家蝇幼虫在无菌环境中不能完成正常生长[19],添加细菌则可助其完成生活史发育[20],且添加多种不同细菌对家蝇的发育更有益[21]。因此,肠道细菌可能协助分解家蝇幼虫生活环境中的有机物质,为其发育提供必需的营养[22-23]或环境中的细菌直接作为家蝇幼虫食物[21]。本研究表明,以盐酸环丙沙星去除部分肠道细菌后,家蝇的生长发育和种群繁衍均受到显著抑制,且随着处理浓度的提高,抑制效果更加明显,可能与家蝇肠道细菌可协助分解食物为其提供营养有关。
| [1] |
Ludvigsen J, Porcellato D, Amdam GV, et al. Addressing the diversity of the honeybee gut symbiont Gilliamella:description of Gilliamella apis sp. nov., isolated from the gut of honeybees (Apis mellifera)[J]. Int J Syst Evol Microbiol, 2018, 68(5): 1762-1770. DOI:10.1099/ijsem.0.002749 |
| [2] |
Engel P, Moran NA. The gut microbiota of insects:diversity in structure and function[J]. FEMS Microbiol Rev, 2013, 37(5): 699-735. DOI:10.1111/1574-6976.12025 |
| [3] |
Inagaki T, Matsuura K. Extended mutualism between termites and gut microbes:nutritional symbionts contribute to nest hygiene[J]. Sci Nat, 2018, 105(9/10): 52. DOI:10.1007/s00114-018-1580-y |
| [4] |
Lee JB, Park KE, Lee SA, et al. Gut symbiotic bacteria stimulate insect growth and egg production by modulating hexamerin and vitellogenin gene expression[J]. Dev Comp Immunol, 2017, 69: 12-22. DOI:10.1016/j.dci.2016.11.019 |
| [5] |
Rodríguez-Ruano SM, Škochová V, Rego ROM, et al. Microbiomes of North American triatominae:the grounds for Chagas disease epidemiology[J]. Front Microbiol, 2018, 9: 1167. DOI:10.3389/fmicb.2018.01167 |
| [6] |
Dharne MS, Gupta AK, Rangrez AY, et al. Antibacterial activities of multi drug resistant Myroides odoratimimus bacteria isolated from adult flesh flies (Diptera:sarcophagidae) are independent of metallo beta-lactamase gene[J]. Braz J Microbiol, 2008, 39(2): 397-404. DOI:10.1590/S1517-838220080002000035 |
| [7] |
Scott JG, Liu N, Kristensen M, et al. A case for sequencing the genome of Musca domestica (Diptera:Muscidae)[J]. J Med Entomol, 2009, 46(2): 175-182. DOI:10.1603/033.046.0202 |
| [8] |
刘小改, 杨亚军, 廖秋菊, 等. 稻纵卷叶螟肠道细菌群落结构与多样性分析[J]. 昆虫学报, 2016, 59(9): 965-976. DOI:10.16380/j.kcxb.2016.09.006 |
| [9] |
陈勃生, 鲁兴萌, 邵勇奇. 鳞翅目昆虫肠道微生物的多样性及其与宿主的相互作用[J]. 昆虫学报, 2017, 60(6): 710-722. DOI:10.16380/j.kcxb.2017.06.011 |
| [10] |
陈丹, 张瑞玲, 刘婧, 等. 蝇类携带病原体研究进展[J]. 中国病原生物学杂志, 2016, 11(8): 765-768. DOI:10.13350/j.cjpb.160821 |
| [11] |
陈丹.不同生境家蝇体表携带细菌的多样性研究及家蝇携菌能力评估[D].泰安: 泰山医学院, 2016.
|
| [12] |
薛志静, 张瑞玲, 庄桂芬, 等. 家蝇携带真菌的研究进展[J]. 中国媒介生物学及控制杂志, 2017, 28(4): 396-399. DOI:10.11853/j.issn.1003.8280.2017.04.025 |
| [13] |
刘婧, 陈丹, 庄桂芬, 等. 进食对家蝇成虫肠道共生细菌组成和数量的影响[J]. 中国病原生物学杂志, 2017, 12(3): 238-241. DOI:10.13350/j.cjpb.170310 |
| [14] |
刘婧, 陈丹, 庄桂芬, 等. 家蝇发育过程中肠道可培养共生细菌的分离与鉴定[J]. 中国寄生虫学与寄生虫病杂志, 2017, 35(2): 120-124. |
| [15] |
Chi H, Liu H. Two new methods for the study of insect population ecology[J]. Bull Inst Zool Acad Sin, 1985, 24(2): 225-240. |
| [16] |
Chi H. Life-table analysis incorporating both sexes and variable development rates among individuals[J]. Environ Entomol, 1988, 17(1): 26-34. DOI:10.1093/ee/17.1.26 |
| [17] |
Gupta AK, Nayduch D, Verma P, et al. Phylogenetic characterization of bacteria in the gut of house flies (Musca domestica L.)[J]. FEMS Microbiol Ecol, 2012, 79(3): 581-593. DOI:10.1111/j.1574-6941.2011.01248.x |
| [18] |
Chang JT, Wang MY. Nutritional requirements of the common housefly, Musca domestica vicina macq[J]. Nature, 1958, 181(4608): 566. DOI:10.1038/181566a0 |
| [19] |
Schmidtmann ET, Martin PAW. Relationship between selected bacteria and the growth of immature house flies, Musca domestica, in an axenic test system[J]. J Med Entomol, 1992, 29(2): 232-235. DOI:10.1093/jmedent/29.2.232 |
| [20] |
苏志坚, 庞义, 李广宏, 等. 麦麸饲料中细菌对家蝇幼虫生长发育的影响[J]. 环境昆虫学报, 2010, 32(1): 25-35. DOI:10.3969/j.issn.1674-0858.2010.01.005 |
| [21] |
Rochon K, Lysyk TJ, Selinger LB. Persistence of Escherichia coli in immature house fly and stable fly (Diptera:Muscidae) in relation to larval growth and survival[J]. J Med Entomol, 2004, 41(6): 1082-1089. DOI:10.1603/0022-2585-41.6.1082 |
| [22] |
Zurek L, Schal C, Watson DW. Diversity and contribution of the intestinal bacterial community to the development of Musca domestica (Diptera:Muscidae) larvae[J]. J Med Entomol, 2000, 37(6): 924-928. DOI:10.1603/0022-2585-37.6.924 |
| [23] |
Moon RD, Hinton JL, O'Rourke SD, et al. Nutritional value of fresh and composted poultry manure for house fly (Diptera:Muscidae) larvae[J]. J Econ Entomol, 2001, 94(5): 1308-1317. DOI:10.1603/0022-0493-94.5.1308 |
 2019, Vol. 30
2019, Vol. 30



