扩展功能
文章信息
- 杨晓红, 孟浩, 齐莉莉, 刘敬泽
- YANG Xiao-hong, MENG Hao, QI Li-li, LIU Jing-ze
- 蜱唾液腺抗止血分子及研究进展
- Tick salivary gland anti-hemostatic molecules and research progress
- 中国媒介生物学及控制杂志, 2017, 28(4): 400-404
- Chin J Vector Biol & Control, 2017, 28(4): 400-404
- 10.11853/j.issn.1003.8280.2017.04.026
-
文章历史
- 收稿日期: 2017-02-24
- 网络出版时间: 2017-06-12 16:24
2 河北省动物生理生化与分子生物学重点实验室, 河北师范大学生命科学学院, 石家庄 050024
2 Key Laboratory of Animal Physiology, Biochemistry and Molecular Biology of Hebei Province, College of Life Sciences, Hebei Normal University
蜱类是专性吸血的体外寄生虫,侵入并寄生于哺乳类、爬行类、鸟类及两栖类等多种脊椎动物,在吸血寄生过程中传播多种病原体而导致人畜疾病。在虫媒传染性疾病中,由蜱传播的病原体种类较多,包括细菌、病毒、立克次体、螺旋体、原虫及毒素等,对人类健康和畜牧业发展危害极大[1]。唾液腺是蜱的重要功能器官,在蜱类吸血和传播病原体过程中,其分泌多种功能分子以应对宿主的免疫防御,同时协助病原体侵染宿主[2-3]。止血是宿主在组织损伤过程中的保护反应,主要通过血小板凝集、血管收缩和凝血作用防止组织损伤失血。与蚊、虱、蚤和螨等吸血节肢动物类似,蜱在吸血过程中分泌多种抗止血分子以应对宿主的止血反应而完成吸血[4]。与蚊、蚤等不同,蜱吸血时间较长,软蜱吸血持续数分钟甚至数小时,硬蜱可长达数天。蜱类吸血主要基于唾液腺分泌的多种抗止血反应分子,包括血小板聚集抑制分子、血管扩张分子和抗凝血分子等[5]。通过对蜱唾液腺抗止血分子的种类、抗凝血作用机制及研究现状等进行综述,为研究蜱类与宿主的相互关系及深入挖掘抗止血分子的药用价值提供基础资料。
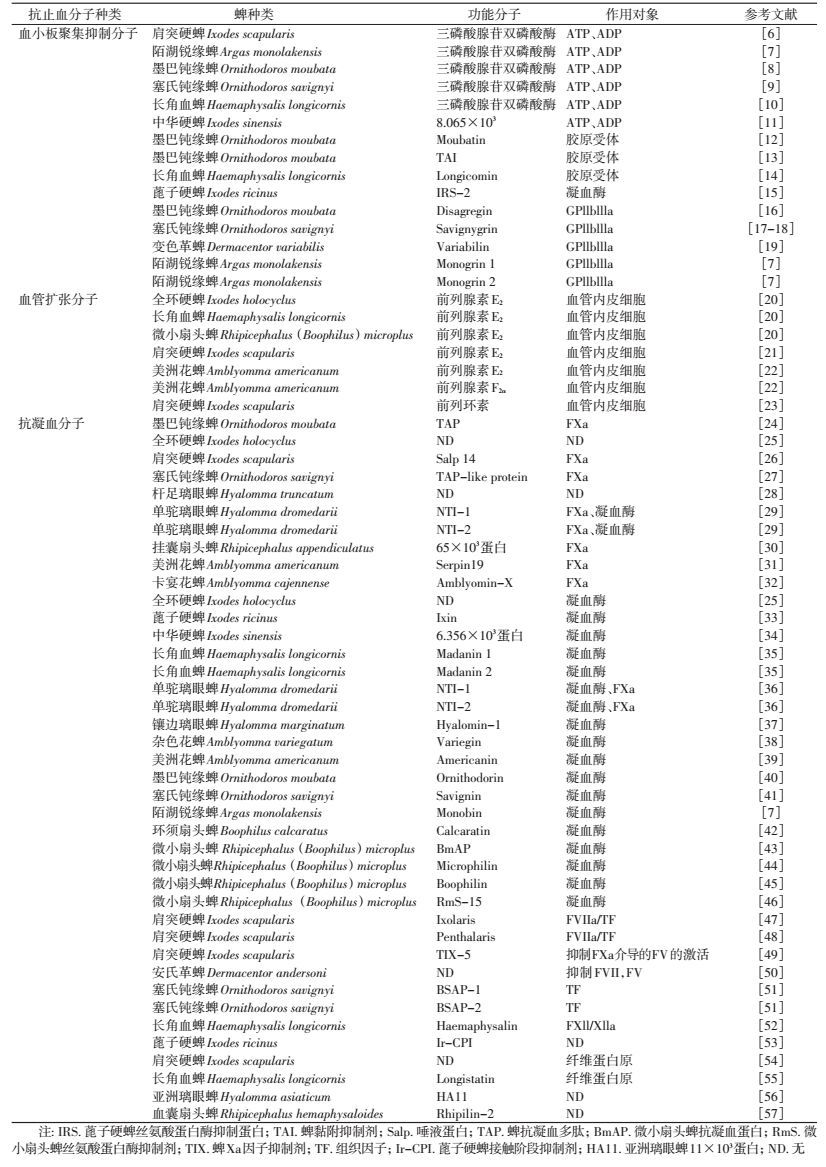
1 血小板聚集抑制分子血小板聚集是宿主应对组织损伤过程中血液流失的最初反应。二磷酸腺苷(ADP)、胶原、凝血酶和血栓素A2等多种激动剂先将血小板激活,引起血小板聚集,进而促进血液凝固和收缩血管物质释放。蜱通过多种方式抑制血小板聚集。
ADP可诱导血小板黏附和聚集,血液中ADP水平降低会抑制血小板活化和血液凝固。目前,已在肩突硬蜱(Ixodes scapularis)、长角血蜱(Haemaphysalis longicornis)、陌湖锐缘蜱(Argas monolakensis)、墨巴钝缘蜱(Ornithodoros moubata)和塞氏钝缘蜱(O. savignyi)[6-10]的唾液或唾液腺提取物中发现三磷酸腺苷双磷酸酶(Apyrase)活性(表 1)。三磷酸腺苷双磷酸酶将三磷酸腺苷(ATP)和ADP水解为磷酸腺苷(AMP)和无机磷酸盐,通过降低宿主血液ADP水平进而抑制血小板活化和血液凝固。刘勇厚等[11]首次在中华硬蜱(I. sinensis)唾液腺中分离纯化出血小板凝集抑制分子,可有效地抑制ADP诱导的血小板凝集。
蜱类唾液蛋白通过与血小板胶原受体结合,抑制宿主血液胶原与血小板结合产生血小板聚集。如墨巴钝缘蜱唾液腺蛋白中的Moubatin和TAI、长角血蜱唾液腺中的Longicormin均通过该方式抑制血小板聚集[12-14]。凝血酶可在不同位点激活血小板,因而凝血酶抑制剂能抑制血小板聚集,如在蓖子硬蜱中发现的丝氨酸蛋白酶抑制蛋白IRS-2可抑制宿主血小板凝集[15]。
血小板被多种激动剂激活后,其表面整合素IIb-IIIa(GPIIbIIIa)受体被激活,后与纤维蛋白原或血浆血管性血友病因子结合使血小板聚集,该反应是活化的血小板与大多数激动剂反应的最后步骤。如墨巴钝缘蜱唾液腺分泌的Disagregin、陌湖锐缘蜱的Monogrin 1和Monogrin 2、塞氏钝缘蜱的Savignygrin及变色革蜱的Variabilin均能与GPIIbIIIa结合,抑制宿主血液的纤维蛋白原与GPIIbIIIa结合或使其结合于错误位点[7, 16-19],阻断血小板聚集的最后步骤,抑制血小板聚集。
2 血管扩张分子蜱类口器刺入宿主皮肤损伤宿主血管,活化的血小板会释放花生四烯酸,并将其转化成血栓素A2,促进血小板聚集、脱粒和血管收缩。活化的血小板分泌血清素和血栓素A2共同参与血管收缩。蜱唾液腺通过分泌血管扩张分子应对宿主血管收缩,增加宿主血液向吸血部位的流速。目前已发现的血管扩张分子主要为前列腺素和前列环素,能促进平滑肌松弛,引起血管扩张。前列腺素E2存在于全环硬蜱、长角血蜱、微小扇头蜱、肩突硬蜱和美洲花蜱等多种蜱类的唾液中,且在美洲花蜱唾液腺中还发现前列腺素F2α[20-22]。蜱类前列腺素除具有舒张血管功能外,还具有免疫抑制和抑制血小板凝集作用[21, 58]。前列环素仅在肩突硬蜱唾液中鉴定出[23]。
3 抗凝血分子血液凝固分为凝血酶原激活物形成、凝血酶形成及纤维蛋白形成3个过程。血液凝固由2个不同途径激活,即内源性激活途径和外源性激活途径(组织因子途径)。两种途径均需形成凝血因子X(FX),后被激活成凝血因子Xa(FXa),再将凝血酶原变成凝血酶,然后将纤维蛋白原酶解为纤维蛋白。纤维蛋白再聚合,最终导致血液凝固,防止血液从受损组织流失。在蜱的吸血过程中,唾液腺分泌多种抗凝血分子抑制或延迟凝血反应。FXa和凝血酶是大多数抗凝血分子的作用靶向。
3.1 凝血因子Xa(FXa)抑制分子蜱类唾液腺中的抗凝血物质最早发现于1898年。Waxman等[24]于1990年从墨巴钝缘蜱体内分离鉴定出第一个抗凝血分子TAP,对凝血因子Xa有高度的选择性抑制作用。随后在硬蜱属、钝缘蜱属、璃眼蜱属(Hyalomma)和扇头蜱属等鉴定到多种唾液腺蛋白,直接与FXa结合,抑制凝血酶原的激活[25-30](表 1)。近几年新发现美洲花蜱唾液中的Kunitz型丝氨酸蛋白酶抑制分子serpin19和卡宴花蜱蛋白Amblyomin-X 2种抗凝血分子,可抑制FXa活性,具有抗凝血作用[31-32]。
3.2 凝血酶抑制分子凝血酶是一种丝氨酸蛋白酶,在凝血过程中起关键作用。有些蜱种分泌唾液腺蛋白,与凝血酶结合抑制其酶解纤维蛋白原,从而抑制凝血。目前,发现很多蜱的唾液腺蛋白有抑制凝血酶活性作用,如硬蜱属[25, 33-34]、血蜱属[35]、璃眼蜱属[36-37]、花蜱属[38-39]、钝缘蜱属[40-41]、锐缘蜱属[7]和扇头蜱属[42-46](表 1)。在微小扇头蜱唾液腺先后发现了BmAP、Microphilin、Boophilin和RmS-15四种抗凝血酶分子,说明蜱类能分泌多种抗凝血分子,且其抗凝血功能相似。
3.3 其他分子蜱唾液中的抗凝血分子也可作用于内源性激活途径和外源性激活途径。肩突硬蜱[47-49]、安氏革蜱[50]和塞氏钝缘蜱[51]分泌的唾液蛋白可抑制外源性激活途径(表 1)。肩突硬蜱唾液腺蛋白Ixolaris和Penthalaris以结合FXa或FX抑制FVIIa/TF复合物,进而抑制外源性激活途径。虽然2种蛋白结构差异很大,但能协同发挥抗凝血作用[48]。肩突硬蜱唾液腺蛋白TIX-5可通过抑制FXa介导的FV的激活抑制宿主凝血[49]。长角血蜱唾液中的Haemaphysalin和蓖子硬蜱唾液腺功能分子Ir-CPI可抑制内源性激活途径[52-53]。
蜱类抗凝血分子除通过作用于FXa和凝血酶抑制凝血外,还可通过其他方式,如肩突硬蜱唾液蛋白组分和长角血蜱唾液腺蛋白Longistatin通过水解纤维蛋白原抑制凝血[54-55]。亚洲璃眼蜱唾液腺蛋白HA11和血囊扇头蜱的Rhipilin-2均具有抑制凝血作用,但其分子机制尚不明确[56-57]。
4 研究进展及医疗应用通常以分离纯化的方法获得蜱唾液腺抗止血蛋白。近年来,基因组学、转录组学和蛋白质组学在蜱类研究中的应用较多,使更多的功能蛋白被发现,促进了蜱类唾液腺蛋白的研究。Kunitz蛋白家族在蜱类抗凝血过程中发挥非常重要的作用,如微小扇头蜱Boophilin和RmS-15、蓖子硬蜱Ir-CPI、美洲花蜱Serpin 19、血囊扇头蜱Rhipilin-2、卡宴花蜱Amblyomin-X和亚洲璃眼蜱HA11等均属于Kunitz蛋白家族。目前,仅对部分已发现的抗止血分子进行了相对深入的研究,如TAP蛋白,但大多数抗止血分子作用机制还有待明确。
蜱类抗凝分子的抗凝血活性可用于医疗临床应用研究,以期发现新的治疗人类疾病的药物。目前,已对TAP、Ixolaris和Amblyomin-X开展药物研发相关研究。TAP有望成为新型抗血栓药物,目前已完成动物模型试验,还未进行临床试验[59]。Ixolaris和Amblyomin-X有望研发为新型抗癌药物,其中Ixolaris具有抑制癌细胞增殖的作用,其生物制剂在鼠体内完成了初步的动物模型试验,临床试验还有待开展[60]。Amblyomin-X为新发现的抗凝血蛋白,具有诱导癌细胞凋亡的作用,关于抗癌药物的研究刚刚开始[61]。
5 结语在生物进化过程中,蜱唾液腺可分泌多种抗止血分子抑制宿主止血反应,分子种类多,作用机制复杂。部分蜱种可分泌多种不同功能的抗止血分子,来源于不同蜱种的抗止血分子有相似的功能。随着分子生物学研究方法的发展,新的蜱抗止血分子不断被发现,其作用机制研究也逐渐深入。根据分子功能和作用机制进行药物研发的研究相对较少且进展较慢,目前还没有应用于临床的相关药物,有待进一步研究。
| [1] |
Sonenshine DE. Biology of ticks[M]. Oxford: Oxford University Press, 1993, 320-332.
|
| [2] |
Gillespie RD, Mbow ML, Titus RG. The immunomodulatory factors of bloodfeeding arthropod saliva[J]. Parasite Immunol, 2000, 22(7): 319-331. DOI:10.1046/j.1365-3024.2000.00309.x |
| [3] |
Chmelar J, Calvo E, Pedra JHF, et al. Tick salivary secretion as a source of antihemostatics[J]. J Proteomics, 2012, 75(13): 3842-3854. DOI:10.1016/j.jprot.2012.04.026 |
| [4] |
Champagne DE. Antihemostatic molecules from saliva of blood-feeding arthropods[J]. Pathophysiol Haemost Thromb, 2005, 34(4/5): 221-227. |
| [5] |
Francischetti IM, Sa-Nunes A, Mans BJ, et al. The role of saliva in tick feeding[J]. Front Biosci, 2009, 14: 2051-2088. |
| [6] |
Ribeiro JM, Makoul GT, Levine J, et al. Antihemostatic, antiinflammatory, and immunosuppressive properties of the saliva of a tick, Ixodes dammini[J]. J Exp Med, 1985, 161(2): 332-344. DOI:10.1084/jem.161.2.332 |
| [7] |
Mans BJ, Andersen JF, Schwan TG, et al. Characterization of anti-hemostatic factors in the argasid, Argas monolakensis:Implications for the evolution of blood-feeding in the soft tick family[J]. Insect Biochem Mol Biol, 2008, 38(1): 22-41. DOI:10.1016/j.ibmb.2007.09.002 |
| [8] |
Ribeiro JM, Endris TM, Endris R. Saliva of the soft tick, Ornithodoros moubata, contains anti-platelet and apyrase activities[J]. Comp Biochem Physiol A Physiol, 1991, 100(1): 109-112. DOI:10.1016/0300-9629(91)90190-N |
| [9] |
Mans BJ, Gaspar ARMD, Louw AI, et al. Apyrase activity and platelet aggregation inhibitors in the tick Ornithodoros savignyi (Acari:Argasidae)[J]. Exp Appl Acarol, 1998, 22(6): 353-366. DOI:10.1023/A:1024517209621 |
| [10] |
程远国, 吴厚永, 李德昌, 等. 长角血蜱唾液腺Apyrase的生物学特性和功能的研究[J]. 寄生虫与医学昆虫学报, 2000, 7(3): 175-182. |
| [11] |
刘勇厚, 徐春花, 刘至刚, 等. 中华硬蜱血小板集聚抑制剂的分离纯化与活性研究[J]. 中国寄生虫学与寄生虫病杂志, 2005, 23(6): 424-427. |
| [12] |
Waxman L, Connolly TM. Isolation of an inhibitor selective for collagen-stimulated platelet aggregation from the soft tick Ornithodoros moubata[J]. J Biol Chem, 1993, 268(8): 5445-5449. |
| [13] |
Karczewski J, Waxman L, Endris RG, et al. An inhibitor from the argasid tick Ornithodoros moubata of cell adhesion to collagen[J]. Biochem Biophys Res Commun, 1995, 208(2): 532-541. DOI:10.1006/bbrc.1995.1371 |
| [14] |
Cheng YG, Wu HY, Li DC. An inhibitor selective for collagen-stimulated platelet aggregation from the salivary glands of hard tick Haemaphysalis longicornis and its mechanism of action[J]. Sci China Ser C Life Sci, 1999, 42(5): 457-464. DOI:10.1007/BF02881768 |
| [15] |
Chmelar J, Oliveira CJ, Rezacova P, et al. A tick salivary protein targets cathepsin G and chymase and inhibits host inflammation and platelet aggregation[J]. Blood, 2011, 117(2): 736-744. DOI:10.1182/blood-2010-06-293241 |
| [16] |
Karczewski J, Endris R, Connolly TM. Disagregin is a fibrinogen receptor antagonist lacking the Arg-Gly-Asp sequence from the tick, Ornithodoros moubata[J]. J Biol Chem, 1994, 269(9): 6702-6708. |
| [17] |
Mans BJ, Louw AI, Neitz AWH. Disaggregation of aggregated platelets by savignygrin, a αⅡbβ3 antagonist from Ornithodoros savignyi[J]. Exp Appl Acarol, 2002, 27(3): 231-239. DOI:10.1023/A:1021613001297 |
| [18] |
Mans BJ, Louw AI, Neitz AWH. Savignygrin, a platelet aggregation inhibitor from the soft tick Ornithodoros savignyi, presents the RGD integrin recognition motif on the Kunitz-BPTI fold[J]. J Biol Chem, 2002, 277(24): 21371-21378. DOI:10.1074/jbc.M112060200 |
| [19] |
Wang XN, Coons LB, Taylor DB, et al. Variabilin, a novel RGD-containing antagonist of glycoprotein Ⅱb-Ⅲa and platelet aggregation inhibitor from the hard tick Dermacentor variabilis[J]. J Biol Chem, 1996, 271(30): 17785-17790. DOI:10.1074/jbc.271.30.17785 |
| [20] |
Inokuma H, Kemp DH, Willadsen P. Comparison of prostaglandin E2(PGE2) in salivary gland of Boophilus microplus, Haemaphysalis longicornis and Ixodes holocyclus, and quantification of PGE2 in saliva, hemolymph, ovary and gut of B. microplus[J]. J Vet Med Sci, 1994, 56(6): 1217-1218. |
| [21] |
Sá-Nunes A, Bafica A, Lucas DA, et al. Prostaglandin E2 is a major inhibitor of dendritic cell maturation and function in Ixodes scapularis saliva[J]. J Immunol, 2007, 179(3): 1497-1505. DOI:10.4049/jimmunol.179.3.1497 |
| [22] |
Ribeiro JM, Evans PM, MacSwain JL, et al. Amblyomma americanum:characterization of salivary prostaglandins E2 and F2α by RP-HPLC/bioassay and gas chromatography-mass spectrometry[J]. Exp Parasitol, 1992, 74(1): 112-116. DOI:10.1016/0014-4894(92)90145-Z |
| [23] |
Ribeiro JMC, Makoul GT, Robinson DR. Ixodes dammini:evidence for salivary prostacyclin secretion[J]. J Parasitol, 1988, 74(6): 1068-1069. DOI:10.2307/3282240 |
| [24] |
Waxman L, Smith DE, Arcuri KE, et al. Tick anticoagulant peptide (TAP) is a novel inhibitor of blood coagulation factor Xa[J]. Science, 1990, 248(4955): 593-596. DOI:10.1126/science.2333510 |
| [25] |
Anastopoulos P, Thurn MJ, Broady KW. Anticoagulant in the tick Ixodes holocyclus[J]. Aust Vet J, 1991, 68(11): 366-367. DOI:10.1111/j.1751-0813.1991.tb00740.x |
| [26] |
Narasimhan S, Koski RA, Beaulieu B, et al. A novel family of anticoagulants from the saliva of Ixodes scapularis[J]. Insect Mol Biol, 2002, 11(6): 641-650. DOI:10.1046/j.1365-2583.2002.00375.x |
| [27] |
Joubert AM, Louw AI, Joubert F, et al. Cloning, nucleotide sequence and expression of the gene encoding factor Xa inhibitor from the salivary glands of the tick, Ornithodoros savignyi[J]. Exp Appl Acarol, 1998, 22(10): 603-619. DOI:10.1023/A:1006198713791 |
| [28] |
Joubert AM, Crause JC, Gaspar ARM, et al. Isolation and characterization of an anticoagulant present in the salivary glands of the bont-legged tick, Hyalomma truncatum[J]. Exp Appl Acarol, 1995, 19(2): 79-92. DOI:10.1007/BF00052548 |
| [29] |
Ibrahim MA, Ghazy AH, Maharem TM, et al. Factor Xa (FXa) inhibitor from the nymphs of the camel tick Hyalomma dromedarii[J]. Comp Biochem Physiol B Biochem Mol Biol, 2001, 130(4): 501-512. DOI:10.1016/S1096-4959(01)00459-6 |
| [30] |
Limo MK, Voigt WP, Tumbo-Oeri AG, et al. Purification and characterization of an anticoagulant from the salivary glands of the ixodid tick Rhipicephalus appendiculatus[J]. Exp Parasitol, 1991, 72(4): 418-429. DOI:10.1016/0014-4894(91)90088-E |
| [31] |
Kim TK, Tirloni L, Radulovic Z, et al. Conserved Amblyomma americanum tick Serpin19, an inhibitor of blood clotting factors Xa and XIa, trypsin and plasmin, has anti-haemostatic functions[J]. Int J Parasitol, 2015, 45(9/10): 613-627. |
| [32] |
Batista IFC, Ramos OHP, Ventura JS, et al. A new Factor Xa inhibitor from Amblyomma cajennense with a unique domain composition[J]. Arch Biochem Biophys, 2010, 493(2): 151-156. DOI:10.1016/j.abb.2009.10.009 |
| [33] |
Hoffmann A, Walsmann P, Riesener G, et al. Isolation and characterization of a thrombin inhibitor from the tick Ixodes ricinus[J]. Pharmazie, 1991, 46(3): 209-212. |
| [34] |
王利菊, 高国富, 柏士平, 等. 中华硬蜱凝血酶抑制剂的分离纯化与活性[J]. 动物学研究, 2005, 26(3): 328-331. |
| [35] |
Iwanaga S, Okada M, Isawa H, et al. Identification and characterization of novel salivary thrombin inhibitors from the ixodidae tick, Haemaphysalis longicornis[J]. Eur J Biochem, 2003, 270(9): 1926-1934. DOI:10.1046/j.1432-1033.2003.03560.x |
| [36] |
Ibrahim MA, Ghazy AH, Maharem T, et al. Isolation and properties of two forms of thrombin inhibitor from the nymphs of the camel tick Hyalomma dromedarii (Acari:Ixodidae)[J]. Exp Appl Acarol, 2001, 25(8): 675-698. DOI:10.1023/A:1016136207308 |
| [37] |
Jablonka W, Kotsyfakis M, Mizurini DM, et al. Identification and mechanistic analysis of a novel tick-derived inhibitor of thrombin[J]. PLoS One, 2015, 10(8): e0133991. DOI:10.1371/journal.pone.0133991 |
| [38] |
Koh CY, Kazimirova M, Trimnell A, et al. Variegin, a novel fast and tight binding thrombin inhibitor from the tropical bont tick[J]. J Biol Chem, 2007, 282(40): 29101-29113. DOI:10.1074/jbc.M705600200 |
| [39] |
Zhu KC, Bowman AS, Brigham DL, et al. Isolation and characterization of americanin, a specific inhibitor of thrombin, from the salivary glands of the lone star tick Amblyomma americanum (L.)[J]. Exp Parasitol, 1997, 87(1): 30-38. |
| [40] |
van de Locht A, Stubbs MT, Bode W, et al. The ornithodorin-thrombin crystal structure, a key to the TAP enigma?[J]. EMBO J, 1996, 15(22): 6011-6017. |
| [41] |
Nienaber J, Gaspar ARM, Neitz AWH. Savignin, a potent thrombin inhibitor isolated from the salivary glands of the tick Ornithodoros savignyi (Acari:Argasidae)[J]. Exp Parasitol, 1999, 93(2): 82-91. DOI:10.1006/expr.1999.4448 |
| [42] |
Motoyashiki T, Tu AT, Azimov DA, et al. Isolation of anticoagulant from the venom of tick, Boophilus calcaratus, from Uzbekistan[J]. Thromb Res, 2003, 110(4): 235-241. DOI:10.1016/S0049-3848(03)00409-2 |
| [43] |
Horn F, dos Santos PC, Termignoni C. Boophilus microplus anticoagulant protein:an antithrombin inhibitor isolated from the cattle tick saliva[J]. Arch Biochem Biophys, 2000, 384(1): 68-73. DOI:10.1006/abbi.2000.2076 |
| [44] |
Ciprandi A, de Oliveira SK, Masuda A, et al. Boophilus microplus:its saliva contains microphilin, a small thrombin inhibitor[J]. Exp Parasitol, 2006, 114(1): 40-46. DOI:10.1016/j.exppara.2006.02.010 |
| [45] |
Macedo-Ribeiro S, Almeida C, Calisto BM, et al. Isolation, cloning and structural characterisation of boophilin, a multifunctional Kunitz-type proteinase inhibitor from the cattle tick[J]. PLoS One, 2008, 3(2): e1624. DOI:10.1371/journal.pone.0001624 |
| [46] |
Xu T, Lew-Tabor A, Rodriguez-Valle M. Effective inhibition of thrombin by Rhipicephalus microplus serpin-15(RmS-15) obtained in the yeast Pichia pastoris[J]. Ticks Tick Borne Dis, 2016, 7(1): 180-187. DOI:10.1016/j.ttbdis.2015.09.007 |
| [47] |
Francischetti IM, Valenzuela JG, Andersen JF, et al. Ixolaris, a novel recombinant tissue factor pathway inhibitor (TFPI) from the salivary gland of the tick, Ixodes scapularis:identification of factor X and factor Xa as scaffolds for the inhibition of factor Ⅶa/tissue factor complex[J]. Blood, 2002, 99(10): 3602-3612. DOI:10.1182/blood-2001-12-0237 |
| [48] |
Francischetti IM, Mather TN, Ribeiro JM. Penthalaris, a novel recombinant five-Kunitz tissue factor pathway inhibitor (TFPI) from the salivary gland of the tick vector of Lyme disease, Ixodes scapularis[J]. Thromb Haemost, 2004, 91(5): 886-898. |
| [49] |
Schuijt TJ, Bakhtiari K, Daffre S, et al. Factor Xa activation of factor Ⅴ is of paramount importance in initiating the coagulation system:lessons from a tick salivary protein[J]. Circulation, 2013, 128(3): 254-266. DOI:10.1161/CIRCULATIONAHA.113.003191 |
| [50] |
Gordon JR, Allen JR. Factors Ⅴ and Ⅶ anticoagulant activities in the salivary glands of feeding Dermacentor andersoni ticks[J]. J Parasitol, 1991, 77(1): 167-170. DOI:10.2307/3282577 |
| [51] |
Ehebauer MT, Mans BJ, Gaspar ARM, et al. Identification of extrinsic blood coagulation pathway inhibitors from the tick Ornithodoros savignyi (Acari:Argasidae)[J]. Exp Parasitol, 2002, 101(2/3): 138-148. |
| [52] |
Kato N, Iwanaga S, Okayama T, et al. Identification and characterization of the plasma kallikrein-kinin system inhibitor, haemaphysalin, from hard tick, Haemaphysalis longicornis[J]. Thromb Haemost, 2005, 93(2): 359-367. |
| [53] |
Decrem Y, Rath G, Blasioli V, et al. Ir-CPI, a coagulation contact phase inhibitor from the tick Ixodes ricinus, inhibits thrombus formation without impairing hemostasis[J]. J Exp Med, 2009, 206(11): 2381-2395. DOI:10.1084/jem.20091007 |
| [54] |
Francischetti IMB, Mather TN, Ribeiro JMC. Cloning of a salivary gland metalloprotease and characterization of gelatinase and fibrin(ogen)lytic activities in the saliva of the Lyme disease tick vector Ixodes scapularis[J]. Biochem Biophys Res Commun, 2003, 305(4): 869-875. DOI:10.1016/S0006-291X(03)00857-X |
| [55] |
Anisuzzaman, Islam MK, Alim MA, et al. Longistatin, a plasminogen activator, is key to the availability of blood-meals for ixodid ticks[J]. PLoS Pathog, 2011, 7(3): e1001312. DOI:10.1371/journal.ppat.1001312 |
| [56] |
Zhang HS, Qiao RQ, Gong HY, et al. Identification and anticoagulant activity of a novel Kunitz-type protein HA11 from the salivary gland of the tick Hyalomma asiaticum[J]. Exp Appl Acarol, 2017, 71(1): 71-85. DOI:10.1007/s10493-017-0106-1 |
| [57] |
Cao J, Shi L, Zhou YZ, et al. Characterization of a new Kunitz-type serine protease inhibitor from the hard tick Rhipicephalus hemaphysaloides[J]. Arch Insect Biochem Physiol, 2013, 84(2): 104-113. DOI:10.1002/arch.v84.2 |
| [58] |
Poole NM, Mamidanna G, Smith RA, et al. Prostaglandin E2 in tick saliva regulates macrophage cell migration and cytokine profile[J]. Parasit Vectors, 2013, 6(1): 261. DOI:10.1186/1756-3305-6-261 |
| [59] |
房兆飞, 胡厚源, 龚丽莎, 等. 抗炎抗凝双效融合蛋白TAP-SSL5的药理学分析[J]. 中国生物制品学杂志, 2014, 27(3): 375-377. |
| [60] |
Barboza T, Gomes T, Mizurini DM, et al. 99mTc-ixolaris targets glioblastoma-associated tissue factor:in vitro and pre-clinical applications[J]. Thromb Res, 2015, 136(2): 432-439. DOI:10.1016/j.thromres.2015.05.032 |
| [61] |
Chudzinski-Tavassi AM, Morais KL, Pacheco MT, et al. Tick salivary gland as potential natural source for the discovery of promising antitumor drug candidates[J]. Biomed Pharmacother, 2016, 77: 14-19. DOI:10.1016/j.biopha.2015.11.003 |
 2017, Vol. 28
2017, Vol. 28