扩展功能
文章信息
- 孙印旗, 王勇, 姜霞, 姚娜, 钱振宇, 刘晓丽, 陈创夫, 张丽娟
- SUN Yin-qi, WANG Yong, JIANG Xia, YAO Na, QIAN Zhen-yu, LIU Xiao-li, CHEN Chuang-fu, ZHANG Li-juan
- 河北新发斑点热及人粒细胞无形体病实验室调查分析
- Molecular characteristics of emerging spotted fever group Rickettsiae and Anaplasma phagocytophilum in Hebei province, China
- 中国媒介生物学及控制杂志, 2015, 26(4): 344-348
- Chin J Vector Biol & Control, 2015, 26(4): 344-348
- 10.11853/j.issn.1003.4692.2015.04.004
-
文章历史
- 收稿日期:2015-03-10
2 中国疾病预防控制中心传染病预防控制所, 北京102206;
3 新疆石河子大学动物学院, 石河子832003;
4 新疆塔里木大学动物学院
2 National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing 102206, China;
3 College of Animal Science and Technology, Shihezi University, Shihezi 832003, Xinjiang Uygur Autonomous Region, China;
4 College of Animal Science, Tarim University
新发蜱传人兽共患立克次体病是世界范围内严重威胁人及家畜健康的急性发热性疾病[1, 2, 3]。随着全球气候变暖、全球一体化等自然因素及社会因素如旅游业发展及人们户外活动增加,该类疾病表现呈逐年增加趋势[4]。因缺乏典型临床表现及快速诊断技术,导致误诊、病情加重甚至病死率增加[5]。在我国,立克次体病诊断仍使用传统的非特异外斐氏反应,而该试验OX2及OX19抗原对应的抗体检测很难区别不同种类立克次体病[6]。近年来,河北省石家庄、保定和唐山地区立克次体病临床报告病例明显增多,但缺乏实验室依据。因此,我们与中国疾病预防控制中心(CDC)传染病预防控制所联合进行了实验室调查分析。除传统蚤传斑疹伤寒(待发表)外,我们还重点对检测到的新发蜱传斑点热群立克次体及人粒细胞无形体进行了分子遗传特征分析。 1 材料与方法 1.1 病例定义与标本采集
本次报告的新发蜱传立克次体是2009-2012年对河北省进行斑疹伤寒主动监测中发现的。该调查是根据以往河北省斑疹伤寒流行地区,按原卫生部2008年公布的中华人民共和国《流行性和地方性斑疹伤寒诊断标准》(WS215-2008)进行病例定义及收集。对病例个案调查登记,信息包括患者基本信息,如年龄、性别、职业、住所、野外作业及虫媒叮咬史以及主要临床信息。同时对患者进行标本采集,包括急性期和恢复期(间隔至少2~3周)非抗凝血各2 ml,分离血清与血块置低温-20 ℃或-40 ℃冰箱保存,后送中国CDC传染病预防控制所进行实验室检测。 1.2 实验室检测分析 1.2.1 血清学检测
抗体检测采用WHO立克次体协作中心推荐的间接免疫荧光方法(IFA)[7]。同一时间、同批次检测发热期及恢复期血清IgM和IgG抗体。试验抗原包括我国河北省沧州市人粒细胞无形体CZ-HGA-2菌株,山东省莱州市人粒细胞无形体菌株LZ-HGA-3[8]、斑疹伤寒群立克次体-普氏立克次体(Rickettsiae prowazekii)、斑疹伤寒群立克次体-莫氏立克次体(R. typhi Wilmington)、蚤传斑点热立克次体(R. felis)、蚤传横赛巴尔通体(Bartonellahenselae) 、螨传恙虫病东方体(Orientiatsutsugamushi)、蜱传黑龙江立克次体(R. heilongjiangensis)、西伯利亚立克次体(R. sibirica)、海南蜱传斑点热群立克次体(Hainan-1)、人单核细胞埃立克体(Ehrlichiachaffeensis)及Q 热病原体-贝氏考克斯体(Coxiellaburnetii)等。IFA操作简述:血清用3%脱脂奶粉PBS按1∶40(IgM)和1∶80(IgG)临界值稀释后,加适量到抗原孔内,37 ℃作用45 min,PBS吐温洗涤2次,每次10 min,再水洗5 min,烘干。每孔加适量伊文斯蓝稀释抗人IgM或IgG(美国KPL公司)抗体,37 ℃作用45 min后,按前述方法洗涤烘干,加缓冲甘油密片后,荧光显微镜下镜检。混合正常人血清及上述8种立克次体免疫兔血清分别做阴性及阳性对照。阳性标本进一步倍比稀释进行半定量试验。 1.2.2 特异基因扩增与序列分析
患者急性期血块DNA提取使用德国QIAGEN公司试剂盒(Cat. No.69506),按说明书操作。本室保存的人粒细胞无形体和西伯利亚立克次体培养物及无菌水同步操作作为PCR阳性及阴性对照。用2套巢氏PCR分别对斑点热群立克次体热休克蛋白基因(groEL)[9]及人粒细胞无形体16S rRNA[10]进行扩增。groEL 扩增外引物对Gro-1:5′- AAG AAG GA/CGT GAT AAC-3′;Gro-2:5′-ACT TCA/CGT AGC ACC-3′;内引物对SF-1:5′-GAT AGA AGA AAA GCA ATG ATG -3′;SR-2:5′-CAG CTA TTT GAG ATT TAA TTTG-3′。第一轮反应条件:94 ℃预变性5 min,39次循环:94 ℃变性40 s,45 ℃退火40 s,72 ℃延伸40 s。72 ℃总延伸4 min。第二轮反应条件:94 ℃预变性5 min,39次循环:94 ℃变性35 s,56 ℃退火35 s,72 ℃延伸35 s,72 ℃总延伸4 min。预期片段217 bp。人粒细胞无形体16S rRNA 扩增外引物对:Eh-out(AF414399):5′- TTG AGA GTT TGA TCC TGGCTC AGA ACG-3′和5′-CAC CTC TAC ACT AGGAAT TCC GCT-3′。内引物对:HGA1:5′-GTC GAACGG ATT CTT TAT AGC TTG-3′和HGA2:5′-TATAGG TAC CGT CAT TAT CTT CCC TAC-3′。第一轮与第二轮PCR反应条件相同:94 ℃预变性5 min,39次循环:94 ℃变性45 s,55 ℃退火45 s,72 ℃延伸1 min,最后72 ℃总延伸5 min。预期片段389 bp。PCR产物送北京擎科新业生物技术有限公司双向测序。Blast同源比较后,对差异碱基人工核实,必要时送第二家测序公司测序核实。选择不同地区,不同宿主来源序列,用DNAStar,MegAlign进行同源分析。 2 结果 2.1 标本收集及血清抗体检测
2009-2012年间收集斑疹伤寒临床诊断病例877例,采集101份急性期血清,17份恢复期血清。除12例发生斑疹伤寒群莫氏立克次体IgG抗体4倍转换(升高或降低)外,3例IgG抗体对我国河北省沧州地区人粒细胞无形体分离株CZ-HGA-2有4倍升高。101份急性期血清CZ-HGA-2分离株IgM抗体阳性率为11.9%(12/101)。无形体抗体阳性标本除了对山东省莱州地区分离株有弱交叉反应(1∶80)外,对其他鉴别诊断抗原无交叉反应。尽管PCR检测到其他斑点热群立克次体包括新发病原体groEL 基因,但因无相应病原体抗原,故无法进行血清抗体检测。斑点热群立克次体PCR阳性血清对我国常见的黑龙江立克次体、西伯利亚立克次体、蚤传斑点热立克次体及海南蜱传斑点热群立克次体(Hainan-1)无交叉反应。 2.2 PCR扩增及序列分析
101份急性期血液DNA标本,10.9%(11/101)病例斑点热群立克次体groEL扩增阳性。同源分析测序成功的7份标本(207 bp)分为2个序列型(3个碱基差异),以HB-TY-1为代表的序列有5 例(5/7),该型不仅与R. rickettsii str.Morgan、R. slovaca str. D-CNPP 100%同源,还与新发斑点热群立克次体R. massiliae str. AZT80、R. raoultii及R. philipii str. 365 100%同源(图 1)。以HB-LZY-2为代表的另一序列有2 例(2/7),与R. africae str.ESF-5、R. conorii str. Malshi7、R. sibirica 100% 同源。此外,该序列还与新发斑点热群立克次体R. massiliae str. MTU5、R. peacockii str. Rustic 100%同源(图 1)。人粒细胞无形体16S rRNA扩增阳性率为8.9%(9/101),PCR阳性患者急性期血清IgM抗体全部阳性,其中3份急性期与恢复期血清IgG抗体发生转换。测序成功的9个序列100%(341 bp)同源,且与该地区无形体病例分离株CZ-HGA-2 100%同源(图 2)。
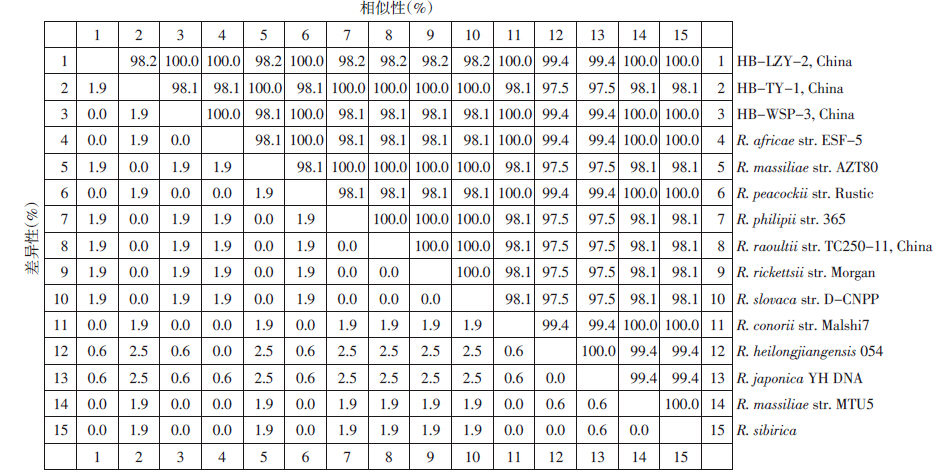
|
| 图 1 斑点热群立克次体groEL基因(207 bp)同源性比较分析 Figure 1 Sequence distances of spotted fever group rickettsiae based on the groEL genes |

|
| 图 2 人粒细胞无形体16S rRNA基因同源性比较分析 Figure 2 Sequence distances of A. phagocytophilum based on the 16S rRNA genes |
立克次体病是一类缺乏典型临床表现的急性发热性疾病。主要表现为急性发热、头痛、肌肉痛、关节痛、皮疹等。缺乏实验室诊断技术时,很难鉴别。除了传统立克次体病如斑疹伤寒、恙虫病、斑点热、Q热等外,近年大量的流行病学资料显示,我国存在多种新发蜱传立克次体病[11, 12, 13, 14, 15]。西伯利亚立克次体、黑龙江立克次体、内蒙古立克次体及海南斑点热群立克次体是我国常见的斑点热病原体[16]。随着流行病学调查的广泛深入,许多新发斑点热群立克次体被发现,如近期R. raoultii 在我国新疆、甘肃等地被检测证实[17, 18]。这一病原体的致病性在国内外均已经得到证实[19, 20]。因本试验检测到基因扩增片段大小有限,还无法进行“种”的分类,但血清学结果证实与我国常见黑龙江立克次体、西伯利亚立克次体、海南蜱传斑点热群立克次体(Hainan-1)不存在抗原及抗体反应,提示很可能为新发斑点热群立克次体。进一步病原学研究对当地新发斑点热群立克次体流行病学及防治具有重要意义。
自2006年我国安徽省某医院人粒细胞无形体院内感染暴发以来[21],大量流行病学调查尤其是病原学调查均证实人粒细胞无形体在我国广泛存在[8, 11, 12]。病原学研究发现我国无形体病临床重症表现与病原体重要毒力基因密切相关[8]。
河北是我国中原大省,以往资料显示该地区是斑疹伤寒重要流行地区,而且,近年临床报告可疑病例明显增加。通过对2009-2012年主动监测和实验室调查发现,除蚤传斑疹伤寒外,还发现了本文重点研究的新发蜱传斑点热群立克次体及人粒细胞无形体。近期当地一项媒介调查发现长角血蜱(Heamaphysalis longicornis)及草原革蜱(Dermacentornutalli)人粒细胞无形体携带率分别为14.6%(39/267,蜱池)和30.8%(4/13,蜱池)[22],进一步支持人粒细胞无形体的存在。然而令人担忧的是尽管流行病学资料显示我国蜱传立克次体病广泛存在,但临床上却很少有主动诊断病例,缺乏快速诊断技术及对该病认识不足是导致这一现象的主要原因。值得注意的是,误诊的直接后果是患者多器官受累,病死率升高。因此,我国立克次体病防治的最大挑战是早期快速诊断和鉴别诊断。在目前临床广泛缺乏实验室检测技术的情况下,建议按美国CDC立克次体病诊治标准,在不排除其他感染性疾病的情况下,及时应用抗立克次体药物。
| [1] | Chapman AS,Bakken JS,Folk SM, et al. Diagnosis and management of tick-borne rickettsial diseases: rocky mountain spotted fever,ehrlichioses,and anaplasmosis-United States:a practical guide for physicians and other health-care and public health professional[J]. MMWR Recomm Rep,2006,55(RR04): 1-27. |
| [2] | Walker DH,Paddock CD,Dumler JS. Emerging and re-emerging tick - transmitted rickettsial and ehrlichial infections[J]. Med Clin North Am,2008,92(6):1345-1361. |
| [3] | Parola P,Paddock CD,Socolovschi C,et al. Update on tick-borne rickettsioses around the world: a geographic approach[J]. Clin Microbiol Rev,2013,26(4):657-702. |
| [4] | Parola P,Socolovschi C,Jeanjean L,et al. Warmer weather linked to tick attack and emergence of severe rickettsioses[J]. PLoS Negl Trop Dis,2008,2(11):e338. |
| [5] | Thomas RJ,Dumler JS,Carlyon JA. Current management of human granulocytic anaplasmosis, human monocytic ehrlichiosis and Ehrlichia ewingii ehrlichiosis[J]. Expert Rev Anti Infect Ther,2009,7(6):709-722. |
| [6] | 栾明春,俞东征,唐立,等. 立克次体病实验室诊断现状[J]. 中国热带医学,2007,7(3):443-446. |
| [7] | Eremeeva ME,Balayeva NM,Raoult D. Serological response of patients suffering from primary and recrudescent typhus comparison of complement fixation reaction, Weil - Felix test, microimmunofluorescence,and immunoblotting[J]. Clin Diagn Lab Immunol,1994,1(3):318-324. |
| [8] | Zhang LJ,Wang GQ,Liu QH,et al. Molecular analysis of Anaplasma phagocytophilum isolated from patients with febrile diseases of unknown etiology in China[J]. PLoS One,2013,8(2):e57155. |
| [9] | Luan MC,Yu DZ,Tang L,et al. Identification of Orientia tsutsugamushi,spotted fever group and typhus group Rickettsia by duplex and nested PCR methods[J]. Asian Pac J Trop Med, 2008,1(4):1-8. |
| [10] | Wen BH,Jian R,Zhang YZ,et al. Simultaneous detection of Anaplasma marginale and a new Ehrlichia species closely related to Ehrlichia chaffeensis by sequence analyses of 16S ribosomal DNA in Boophilus microplus ticks from Tibet[J]. J Clin Microbiol,2002,40(9):3286-3290. |
| [11] | Zhang LJ,Liu H,Xu BL,et al. Anaplasma phagocytophilum infection in domestic animals in ten Provinces/Cities of China[J]. Am J Trop Med Hyg,2012,87(1):185-189. |
| [12] | Zhang LJ,Liu H,Xu BL,et al. Rural residents in China are at increased risk of exposure to tick-borne pathogens Anaplasma phagocytophilum and Ehrlichia chaffeensis[J]. Bio Med Res Int, 2014,2014:Article ID 313867. |
| [13] | Zhang JL,Lu GW,Kelly P,et al. First report of Rickettsia felis in China[J]. BMC Infect Dis,2014,14(1):682. |
| [14] | Tian ZC,Liu GY,Shen H,et al. First report on the occurrence of Rickettsia slovaca and Rickettsia raoultii in Demacentor silvarum in China[J]. Parasit Vectors,2012,5:19. |
| [15] | Zhang XC,Zhang LX,Li WH,et al. Ehrlichiosis and zoonotic anaplasmosis in suburban areas of Beijing,China[J]. Vector Borne Zoonotic Dis,2012,12(11):932-937. |
| [16] | 张丽娟. 立克次体与立克次体病的再认识[J]. 传染病信息杂,2006,19(2):53-54. |
| [17] | Wang YF,Liu ZJ,Yang JF,et al. Rickettsia raoultii-like bacteria in Dermacentor spp. ticks,Tibet,China[J]. Emerg Infect Dis,2012,18(9):1532-1534. |
| [18] | 孙响,张桂林,刘然,等. 新疆地区Rickettsia raoultii 分子流行学研究[J]. 中华流行病学杂志,2013,34(7):756-757. |
| [19] | Jia N,Zheng YC,Ma L,et al. Human infections with Rickettsia raoultii,China[J]. Emerg Infect Dis,2014,20(5):866-868. |
| [20] | Switaj K,Chmielewski T,Borkowski P,et al. Spotted fever rickettsiosis caused by Rickettsia raoultii-case report[J]. Przegl Epidemiol,2012,66(2):347-350. |
| [21] | Zhang LJ,Liu Y,Ni DX,et al. Nosocomial transmission of human granulocytic anaplasmosis in China[J]. JAMA,2008, 300(19):2263-2270. |
| [22] | Zou YX,Jin HT,Wang QY,et al. Molecular detection of Anaplasma phagocytophilum in ixodid ticks in Hebei province, China[J]. Vector Borne Zoonotic Dis,2011,11(10) : 1323-1327. |
 2015, Vol. 26
2015, Vol. 26


