扩展功能
文章信息
- 朱彩英, 赵春春, 王纯玉, 陈建勇, 肖珊, 王文强, 孟凤霞
- ZHU Cai-ying, ZHAO Chun-chun, WANG Chun-yu, CHEN Jian-yong, XIAO Shan, WANG Wen-qiang, MENG Feng-xia
- 湖南省长沙市内五区白纹伊蚊电压门控钠离子通道基因突变研究
- A study on voltage-gated sodium channel gene mutations in Aedes albopictus in five urban districts of Changsha, Hunan Province, China
- 中国媒介生物学及控制杂志, 2024, 35(4): 405-410
- Chin J Vector Biol & Control, 2024, 35(4): 405-410
- 10.11853/j.issn.1003.8280.2024.04.004
-
文章历史
- 收稿日期: 2023-11-01
2 传染病溯源预警与智能决策全国重点实验室, 中国疾病预防控制中心传染病预防控制所媒介生物控制室, WHO媒介生物监测与管理合作中心, 北京 102206;
3 辽宁省疾病预防控制中心, 辽宁 沈阳 110000
2 Department of Vector Biology and Control, National Key Laboratory of Intelligent Tracking and Forecasting for Infectious Diseases, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, WHO Collaborating Centre for Vector Surveillance and Management, Beijing 102206, China;
3 Liaoning Center for Disease Control and Prevention, Shenyang, Liaoning 110000, China
白纹伊蚊(Aedes albopictus)是我国常见蚊种之一,侵袭力极强,可以传播登革热、寨卡病毒病和基孔肯亚热等多种重要传染病[1]。近年来,我国登革热疫情形势严峻,云南、广东、浙江等多个省份均出现登革热本地疫情暴发、流行[2-3]。使用化学杀虫剂杀灭成蚊是登革热疫情防控的有效应对措施。拟除虫菊酯类杀虫剂因其广谱、高效、低毒等优势,在防蚊灭蚊工作中被广泛应用,但随着杀虫剂的广泛、持续、大规模使用,蚊虫抗药性的出现与增强也随之凸显[4-6]。
电压门控钠离子通道(voltage-gated sodium channel,VGSC)是拟除虫菊酯类杀虫剂的主要作用靶标,由VGSC基因编码,该基因突变会导致编码的氨基酸产生变化,进而导致VGSC对杀虫剂的敏感程度大幅降低,进而产生击倒抗性(knockdown resistance,kdr)。开展VGSC基因突变研究有利于开发其做为杀虫剂抗药性分子检测的生物标记,助力抗药性快速准确诊断[7]。目前kdr研究的热点集中在VGSC基因的第Ⅱ、Ⅲ跨膜结构域,主要的有义突变位点包括V1016、I1532和F1534位点。我国多个城市已进行白纹伊蚊野外种群VGSC基因突变的研究[8-10],但长沙市尚未见报道。
随着交通、旅游业日益发展,国内外交流和人口流动进一步增强,长沙市作为中南地区重要省份的重要城市之一,面临着巨大的登革热疫情输入风险。2022年,长沙市市委市政府组织开展创建国家卫生城市活动,蚊虫密度控制作为创卫工作的重要组成部分,依赖于杀虫剂的科学有效使用。白纹伊蚊作为长沙市传病风险较高的主要蚊种,生物测定结果表明,成蚊对溴氰菊酯、氯菊酯等杀虫剂已经产生了抗药性[11]。本研究对5个白纹伊蚊野外种群的VGSC基因突变情况进行研究,初步探索其对拟除虫菊酯类杀虫剂的抗药性机制,以期为国家卫生城市创建及登革热疫情防控提供科学依据。
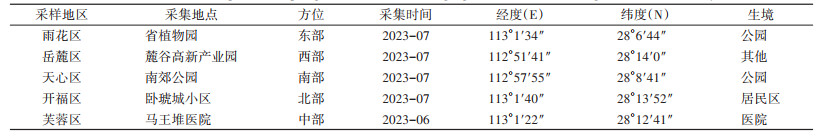
1 材料与方法 1.1 试虫来源2023年6-7月,在长沙市人口密度大的5个中心主城区(即“内五区”)东、西、南、北、中5个方位,选择有代表性的采样点,采用人诱法采集白纹伊蚊成蚊,具体采样信息见表 1。共采集蚊虫184只,麓谷高新产业园由于物业服务好,灭蚊频率高,园区积水少,所以获取的样本数量较少。
|
2×Taq PCR预混液购自北京全式金生物技术有限公司,动物组织DNA提取试剂盒购自无锡百泰克生物技术有限公司,引物均由北京奥科鼎盛生物科技有限公司合成。
1.3 VGSC基因型检测 1.3.1 全基因组DNA提取经形态学鉴定为白纹伊蚊的单只成蚊样本,按照试剂盒说明书提取基因组DNA。
1.3.2 基因扩增与测序以基因组DNA为模板,扩增VGSC基因的第Ⅱ、Ⅲ跨膜结构域目的片段,参照Kasai等[12-13]研究合成引物。扩增引物序列、PCR反应体系及程序见表 2。扩增产物首先经1%琼脂糖凝胶电泳,电泳条带清晰无拖尾的PCR扩增产物送至北京奥科鼎盛生物科技有限公司进行测序。第Ⅱ、Ⅲ跨膜结构域对应的测序引物分别为aegSCR22:5′-TTCACGAACTTGAGCGCGTTG-3′和aegSCR8:5′-TAGCTTTCAGCGGCTTCTTC-3′。

|
用MEGA 7.0软件对测得的序列进行比对,用SeqMan软件分析测序峰图,观察各位点突变情况,并确定等位基因类型和基因型,用Excel 2016软件统计和计算各种群VGSC基因的基因型与等位基因型出现频率(突变率)。
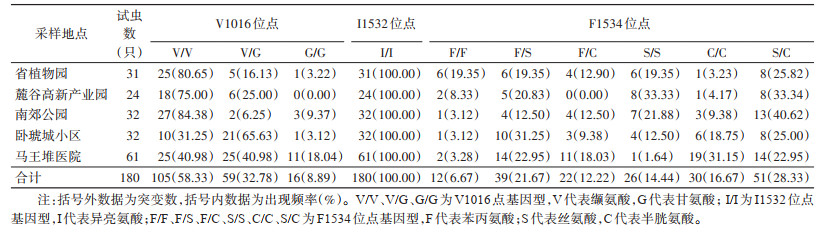
2 结果 2.1 VGSC基因各位点整体突变情况对长沙市5个中心城区的184份(只)白纹伊蚊DNA样本进行特异性扩增后反向测序,3个位点均测序成功的样本共计180份。VGSC基因第Ⅱ、Ⅲ结构域共获得360条有效序列,将有效序列置于GenBank中进行基于局部比对算法的搜索工具(BLAST)比对,结果显示,所有有效序列与白纹伊蚊VGSC基因第Ⅱ结构域(KC152045.1)、第Ⅲ结构域(KC152046.1)部分序列一致性达99.00%,显示本研究扩增产物确为白纹伊蚊目的基因片段。
对序列进一步分析发现,位于VGSC基因第Ⅱ结构域的V1016位点已经发生突变。V1016位点发现野生型等位基因GTA/V(占比74.72%,269/360)突变为GGA/G(25.28%,91/360)型突变等位基因,编码的氨基酸由缬氨酸突变成甘氨酸;3种基因型中野生型纯合子V/V频率最高(58.33%,105/180),另外2种分别是野生/突变型杂合子V/G(32.78%,59/180)和突变型纯合子G/G(8.89%,16/180)。第Ⅲ结构域只在F1534位点观察到突变,I1532位点未发现碱基突变样本。I1532位点的等位基因均为野生型ACC/I(100%,360/360);只有1种基因型,即野生型纯合子I/I(100%,180/180)。F1534位点的野生型等位基因TTC/F(23.62%,85/360)发生了2种单碱基突变,分别是TCC/S(39.44%,142/360)和TGC/C(36.94%,133/360),编码的氨基酸由苯丙氨酸突变成丝氨酸和半胱氨酸;3种等位基因共组成6种基因型,其中野生型纯合子F/F(6.67%,12/180)频率最低,另外5种分别为野生/突变型杂合子F/S(21.67%,39/180)和F/C(12.22%,22/180),突变型纯合子S/S(14.44%,26/180)和C/C(16.67%,30/180),突变型杂合子S/C(28.33%,51/180)。见表 3。
|
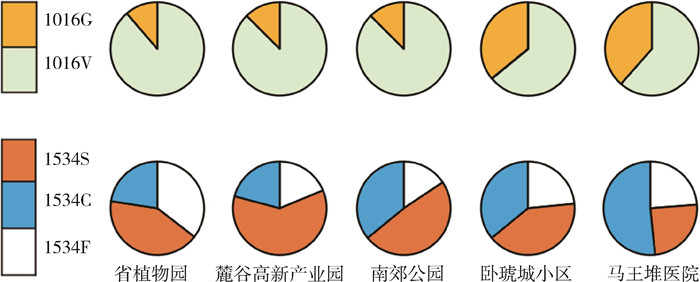
从基因型的角度分析发现,各种群均发生V1016位点突变,卧琥城小区(68.75%,22/32)和马王堆医院(59.02%,36/61)种群突变基因型频率较高,且马王堆医院种群G/G型突变纯合子最多(18.04%,11/61),其他3个种群突变基因型频率均在20.00%左右。I1532位点在各个种群中均未发现突变个体。各种群在F1534位点上发现的突变基因型均比较丰富,且频率都处于高位。除省植物园种群的突变基因型频率(80.65%,25/31)稍低外,其余4个种群频率均已超过90.00%(表 3)。从等位基因型方面来看,V1016位点中马王堆医院种群GGA/G突变等位基因型频率最高(38.52%,47/122),省植物园最低(11.29%,7/62)。F1534位点,TCC/S和TGC/C突变型等位基因在各个种群中均有发现,TCC/S型等位基因在麓谷高新产业园种群中最高(60.42%,29/48),TGC/C型等位基因在马王堆医院种群中最高(51.64%,63/122)。见图 1。
|
| 注:1016G、1016V、1534S、1534C、1534F为等位基因型,数字代表位点,G代表甘氨酸,V代表缬氨酸,S代表丝氨酸,C代表半胱氨酸,F代表苯丙氨酸。 图 1 湖南省长沙市白纹伊蚊电压门控钠离子通道基因突变等位基因型及频率 Figure 1 Mutant alleles and frequencies of voltage-gated sodium channel gene of Aedes albopictus in Changsha, Hunan Province, China |
| |
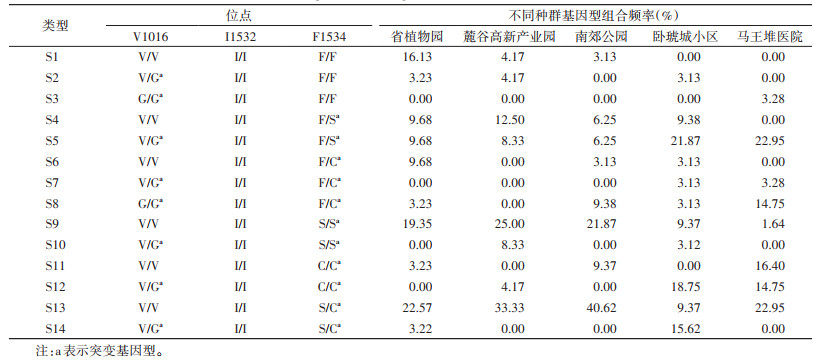
180只白纹伊蚊样本中,检测到14种突变基因型组合,编号S1~S14。3个位点均为野生纯合子的S1型,仅在省植物园、麓谷高新产业园和南郊公园种群少部分样本中检测到。S5、S9和S13型在5个种群中广泛分布,且S13型在14种突变基因型组合中频率最高。S3型仅在马王堆医院种群中发现,本研究未发现3个位点同时发生有义突变的个体。见表 4。
|
Kasai等[13]对意大利和越南白纹伊蚊种群研究时发现,1016G型等位基因与氯菊酯抗药性表型相关,且较1534S和1534C型等位基因突变效应更强,而1532T型等位基因与氯菊酯抗药性无关联。Gao等[4]的研究中发现1532T型等位基因与氯菊酯抗药性亦无关联,但与溴氰菊酯抗药性呈负相关;1534S型等位基因与溴氰菊酯和氯菊酯抗药性呈正相关,王晓花等[14]和Xu等[15]的研究也支持这一结论。本研究发现,长沙市内五区白纹伊蚊VGSC基因V1016、F1534位点发生有义突变,突变等位基因型包括1016G、1534S和1534C型3种。各种群F1534位点基因突变率均超过80.00%,且1534C型突变等位基因频率较高,与谭爱等[16]近年研究结果相似。值得注意的是,我国白纹伊蚊种群之前以1534S型突变等位基因为主,而1534C型等位基因型频率大都处于较低水平[14, 17-21],说明1534C型突变等位基因近年呈扩散趋势。由此我们推测,在杀虫剂的选择压力与频繁人流、物流的迁移动力双重作用下,我国白纹伊蚊VGSC基因F1534位点氨基酸正在发生苯丙氨酸F—丝氨酸S—半胱氨酸C的适应性进化。这体现了白纹伊蚊对环境超强的适应能力,提示我们应加强对VGSC基因的动态连续监测,并加强对各突变型等位基因的抗药性效应研究。另外,本研究未发现1534L型等位基因,该型等位基因在意大利和广东省中山市等种群中曾有报导[15, 22-24]。V1016位点基因突变率总体而言较F1534位点低,但卧琥城小区和马王堆医院2个种群基因突变率已在60.00%左右,这可能与这2个种群所属的生境有关,居民区和医院生境人口密度大,防蚊需求高,杀虫剂使用频繁,形成较强选择压力,种群抗药性靶标基因随之产生适应性进化。2020年8月,本课题组采用成蚊接触筒法对芙蓉区马王堆医院的白纹伊蚊成蚊开展抗药性测定,结果表明该种群对溴氰菊酯、氯菊酯、高效氯氰菊酯和高效氯氟氰菊酯4种拟除虫菊酯类杀虫剂已经产生了抗药性[11],这与本次研究结果互相印证,说明击倒抗性是长沙市白纹伊蚊对拟除虫菊酯类杀虫剂产生抗药性的机制之一。I1532位点暂未发现有义突变,这与湖南省永州和张家界市[25]、广西壮族自治区南宁市[8]等种群的研究结果相似,而湖南省衡阳和娄底市[8]、北京市[26]、河南省禹州地区[27]及山东省济宁市[28]等多个野外种群已在该位点发现有义突变。在埃及伊蚊(Ae. aegypti)中,研究证实VGSC基因多位点联合突变对拟除虫菊酯类杀虫剂抗药性有协同作用[1]。经过统计,本研究共发现14种组合基因型,V1016和F1534 2个位点同时发生突变的有6种,以V/G+I/I+F/S型为主(38.89%,70/180),这可能是杀虫剂长期作用的结果。以上研究结果表明,长沙市主城区白纹伊蚊对常用拟除虫菊酯类杀虫剂已经产生抗药性,提示宜减少拟除虫菊酯类杀虫剂的使用,以延缓抗药性发展,同时应加强对蚊虫抗药性动态连续监测,以指导科学防蚊灭蚊,适时调整用药策略。
本研究通过调查白纹伊蚊VGSC基因突变情况对抗药性机制进行初步探索,存在一定的局限性,如未结合杀虫剂使用情况和生物测定结果进行综合评估、个别采样点样本数量略少等。课题组下一步将同步展开生物测定及杀虫剂使用情况调查,以便更加全面深入地了解长沙市白纹伊蚊抗药性现状。
利益冲突无
| [1] |
Smith LB, Kasai S, Scott JG. Pyrethroid resistance in Aedes aegypti and Aedes albopictus: Important mosquito vectors of human diseases[J]. Pestic Biochem Physiol, 2016, 133: 1-12. DOI:10.1016/j.pestbp.2016.03.005 |
| [2] |
Yue YJ, Liu XB, Ren DS, et al. Spatial dynamics of dengue fever in Chinese mainland, 2019[J]. Int J Environ Res Public Health, 2021, 18(6): 2855. DOI:10.3390/ijerph18062855 |
| [3] |
Yue YJ, Liu XB, Xu M, et al. Epidemiological dynamics of dengue fever in Chinese mainland, 2014-2018[J]. Int J Environ Res Public Health, 2019, 86: 82-93. DOI:10.1016/j.ijid.2019.06.015 |
| [4] |
Gao JP, Chen HM, Shi H, et al. Correlation between adult pyrethroid resistance and knockdown resistance (kdr) mutations in Aedes albopictus (Diptera: Culicidae) field populations in China[J]. Infect Dis Poverty, 2018, 7(1): 86. DOI:10.1186/s40249-018-0471-y |
| [5] |
Moyes CL, Vontas J, Martins AJ, et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans[J]. PLoS Negl Trop Dis, 2017, 11(7): e0005625. DOI:10.1371/journal.pntd.0005625 |
| [6] |
Li YJ, Zhou GF, Zhong DB, et al. Widespread multiple insecticide resistance in the major dengue vector Aedes albopictus in Hainan Province, China[J]. Pest Manag Sci, 2021, 77(4): 1945-1953. DOI:10.1002/ps.6222 |
| [7] |
宋晓, 黄晓丹, 杨琳琳, 等. 山东省济宁市白纹伊蚊对溴氰菊酯抗性与kdr等位基因突变观察[J]. 中华卫生杀虫药械, 2019, 25(5): 403-406. Song X, Huang XD, Yang LL, et al. Resistance of Aedes albopictus to deltamethrin and kdr allele mutation in Jining of Shandong Province[J]. Chin J Hyg Insect Equip, 2019, 25(5): 403-406. DOI:10.19821/j.1671-2781.2019.05.003 |
| [8] |
Zhao CC, Zhou XX, Xue CZ, et al. Knockdown resistance mutations distribution and characteristics of Aedes albopictus field populations within eleven dengue local epidemic provinces in China[J]. Front Cell Infect Microbiol, 2023, 12: 981702. DOI:10.3389/fcimb.2022.981702 |
| [9] |
刘洁楠, 易井萍, 李科峰, 等. 舟山市白纹伊蚊对拟除虫菊酯类杀虫剂的抗性表型和kdr基因突变分析[J]. 预防医学, 2020, 32(12): 1253-1256. Liu JN, Yi JP, Li KF, et al. Pyrethroid insecticide resistance phenotype and kdr gene mutation in Aedes albopictus in Zhoushan[J]. Prev Med, 2020, 32(12): 1253-1256. DOI:10.19485/j.cnki.issn2096-5087.2020.12.016 |
| [10] |
吴丽群, 周欣欣, 周良才, 等. 湖北省武汉市居民区白纹伊蚊电压门控钠离子通道基因突变分析[J]. 中国媒介生物学及控制杂志, 2023, 34(2): 212-217. Wu LQ, Zhou XX, Zhou LC, et al. Analysis of voltage-gated sodium channel gene mutations in Aedes albopictus in the residential area of Wuhan, Hubei Province, China[J]. Chin J Vector Biol Control, 2023, 34(2): 212-217. DOI:10.11853/j.issn.1003.8280.2023.02.011 |
| [11] |
林斌, 彭莱, 肖珊, 等. 长沙市2020-2021年白纹伊蚊和家蝇抗药性监测[J]. 医学动物防制, 2023, 39(9): 843-845. Lin B, Peng L, Xiao S, et al. Insecticide resistance surveillance of Aedes albopictus and Musca domestica in Changsha from 2020 to 2021[J]. J Med Pest Control, 2023, 39(9): 843-845. DOI:10.7629/yxdwfz202309006 |
| [12] |
Kasai S, Ng LC, Lam-Phua SG, et al. First detection of a putative knockdown resistance gene in major mosquito vector, Aedes albopictus[J]. Jpn J Infect Dis, 2011, 64(3): 217-221. DOI:10.7883/yoken.64.217 |
| [13] |
Kasai S, Caputo B, Tsunoda T, et al. First detection of a Vssc allele V1016G conferring a high level of insecticide resistance in Aedes albopictus collected from Europe (Italy) and Asia (Vietnam), 2016: A new emerging threat to controlling arboviral diseases[J]. Euro Surveill, 2019, 24(5): 1700847. DOI:10.2807/1560-7917.ES.2019.24.5.1700847 |
| [14] |
王晓花, 陈辉莹, 杨新艳, 等. 海口市白纹伊蚊对菊酯类杀虫剂的抗药性及击倒抗性基因突变分析[J]. 第二军医大学学报, 2015, 36(8): 832-838. Wang XH, Chen HY, Yang XY, et al. Resistance to pyrethroid insecticides and analysis of knockdown resistance (kdr) gene mutations in Aedes albopictus from Haikou city[J]. Acad J Second Mil Med Univ, 2015, 36(8): 832-838. DOI:10.3724/SP.J.1008.2015.00832 |
| [15] |
Xu JB, Bonizzoni M, Zhong DB, et al. Multi-country survey revealed prevalent and novel F1534S mutation in voltage-gated sodium channel (VGSC) gene in Aedes albopictus[J]. PLoS Negl Trop Dis, 2016, 10(5): e0004696. DOI:10.1371/journal.pntd.0004696 |
| [16] |
谭爱, 刘鹃, 王雅伟, 等. 四川省内江市白纹伊蚊野外群体电压门控钠离子通道基因突变检测分析[J]. 中国媒介生物学及控制杂志, 2023, 34(3): 314-318. Tan A, Liu J, Wang YW, et al. Detection of mutations in the voltage-gated sodium channel genes of field Aedes albopictus populations in Neijiang, Sichuan Province, China[J]. Chin J Vector Biol Control, 2023, 34(3): 314-318. DOI:10.11853/j.issn.1003.8280.2023.03.006 |
| [17] |
Chen HY, Li KL, Wang XH, et al. First identification of kdr allele F1534S in VGSC gene and its association with resistance to pyrethroid insecticides in Aedes albopictus populations from Haikou city, Hainan Island, China[J]. Infect Dis Poverty, 2016, 5(1): 31. DOI:10.1186/s40249-016-0125-x |
| [18] |
兰学梅, 徐家宝, 姜进勇. 云南省瑞丽市白纹伊蚊对拟除虫菊酯类杀虫剂抗性种群的电压门控钠离子通道基因突变分析[J]. 中国媒介生物学及控制杂志, 2019, 30(2): 158-162. Lan XM, Xu JB, Jiang JY. An analysis of voltage-gated sodium channel gene mutation in Aedes albopictus resistant populations against pyrethroid insecticides in Ruili, Yunnan Province, China[J]. Chin J Vector Biol Control, 2019, 30(2): 158-162. DOI:10.11853/j.issn.1003.8280.2019.02.010 |
| [19] |
李玉伟, 黄婧雯, 章灿明, 等. 福建省福州市和莆田市2020年白纹伊蚊击倒抗性基因突变分析[J]. 中国热带医学, 2021, 21(10): 952-955, 969. Li YW, Huang JW, Zhang CM, et al. Knockdown resistance gene mutations of Aedes albopictus from Fuzhou and Putian, Fujian, 2020[J]. China Trop Med, 2021, 21(10): 952-955, 969. DOI:10.13604/j.cnki.46-1064/r.2021.10.08 |
| [20] |
赵春春. 我国白纹伊蚊抗药性及kdr基因分布研究[D]. 北京: 中国疾病预防控制中心, 2019. Zhao CC. Study on insecticides resistance and kdr gene distribution of Aedes albopictus in China[D]. Beijing: Chinese Center for Disease Control and Prevention, 2019. (in Chinese) |
| [21] |
Zhu CY, Zhao CC, Wang YG, et al. Establishment of an innovative and sustainable PCR technique for 1534 locus mutation of the knockdown resistance (kdr) gene in the dengue vector Aedes albopictus[J]. Parasit Vectors, 2019, 12(1): 603. DOI:10.1186/s13071-019-3829-5 |
| [22] |
杨罗菊, 刘德星, 陈健, 等. 中山市白纹伊蚊现场种群击倒抗性基因检测分析[J]. 中国人兽共患病学报, 2021, 37(2): 171-175. Yang LJ, Liu DX, Chen J, et al. Detection and analysis of the knockdown resistance gene in the field populations of Aedes albopictus in Zhongshan[J]. Chin J Zoonoses, 2021, 37(2): 171-175. DOI:10.3969/j.issn.1002-2694.2021.00.005 |
| [23] |
Li YJ, Xu JB, Zhong DB, et al. Evidence for multiple-insecticide resistance in urban Aedes albopictus populations in southern China[J]. Parasit Vectors, 2018, 11(1): 4. DOI:10.1186/s13071-017-2581-y |
| [24] |
伍思翰, 周欣欣, 柯雪梅, 等. 福建省厦门市2020年白纹伊蚊对拟除虫菊酯类杀虫剂敏感性及击倒抗性基因研究[J]. 中国媒介生物学及控制杂志, 2022, 33(2): 177-182. Wu SH, Zhou XX, Ke XM, et al. Study on sensitivity and knockdown resistance genes of Aedes albopictus to pyrethroid insecticides in Xiamen, Fujian Province, China, 2020[J]. Chin J Vector Biol Control, 2022, 33(2): 177-182. DOI:10.11853/j.issn.1003.8280.2022.02.003 |
| [25] |
朱彩英. 中国白纹伊蚊击倒抗性基因突变及检测技术研究[D]. 北京: 中国疾病预防控制中心, 2020. DOI: 10.27511/d.cnki.gzyyy.2020.000116. Zhu CY. Study on knockdown resistance gene mutation and detection technique of Aedes albopictus in China[D]. Beijing: Chinese Center for Disease Control and Prevention, 2020. DOI: 10.27511/d.cnki.gzyyy.2020.000116.(inChinese) |
| [26] |
Zhou XJ, Yang C, Liu N, et al. Knockdown resistance (kdr) mutations within seventeen field populations of Aedes albopictus from Beijing China: First report of a novel V1016G mutation and evolutionary origins of kdr haplotypes[J]. Parasit Vectors, 2019, 12(1): 180. DOI:10.1186/s13071-019-3423-x |
| [27] |
母群征, 华栋栋, 李文玉, 等. 河南省禹州地区白纹伊蚊击倒抗性基因突变检测[J]. 中国媒介生物学及控制杂志, 2023, 34(3): 303-307, 335. Mu QZ, Hua DD, Li WY, et al. Detection of knockdown resistance gene mutations in Aedes albopictus in Yuzhou, Henan Province, China[J]. Chin J Vector Biol Control, 2023, 34(3): 303-307, 335. DOI:10.11853/j.issn.1003.8280.2023.03.004 |
| [28] |
刘鲁宏. 济宁市白纹伊蚊kdr基因突变检测[J]. 中华卫生杀虫药械, 2022, 28(3): 267-269. Liu LH. Detection of knockdown resistance gene mutation in Aedes albopictus of Jining City[J]. Chin J Hyg Insect Equip, 2022, 28(3): 267-269. DOI:10.19821/j.1671-2781.2022.03.020 |
 2024, Vol. 35
2024, Vol. 35


