扩展功能
文章信息
- 曾予, 刘志云, 段立科, 侯学霞, 张琳, 贺丽娟, 郝琴
- ZENG Yu, LIU Zhi-yun, DUAN Li-ke, HOU Xue-xia, ZHANG Lin, HE Li-juan, HAO Qin
- 江西省吉安市微小扇头蜱携带病原菌检测及其分型研究
- Detection and genotyping of pathogenic bacteria carried by Rhipicephalus microplus in Ji'an, Jiangxi Province, China
- 中国媒介生物学及控制杂志, 2024, 35(3): 381-388
- Chin J Vector Biol & Control, 2024, 35(3): 381-388
- 10.11853/j.issn.1003.8280.2024.03.022
-
文章历史
- 收稿日期: 2024-01-08
2 吉安市中心人民医院(上海市东方医院吉安医院)检验科, 江西 吉安 343000
2 Inspection Department of Ji'an Central People's Hospital (Ji'an Hospital of Shanghai East Hospital), Ji'an, Jiangxi 343000, China
蜱是一种专性吸血的节肢动物,属于蛛形纲(Arachnida)蜱螨亚纲(Acari)寄螨总目(Parasitiformes)蜱目(Ixodida),可分为软蜱科(Argrasidae)、硬蜱科(Ixodidae)和纳蜱科(Nuttalliellidae)[1]。我国仅发现软蜱科和硬蜱科,已鉴定并命名的蜱有9属124种[2]。蜱可携带细菌、病毒、原虫等多种病原体,可通过叮咬向人类或动物传播病原体,发挥媒介作用,流行病学意义仅次于蚊,对人类和动物健康产生巨大危害[3]。蜱传疾病的感染风险与季节、蜱种分布、蜱携带病原体的种类和丰度、人类预防保护措施和人体易感性息息相关。近年来,由于全球气候变化、经济全球化、人口增长、移民增加和动物迁徙等原因,蜱的栖息地和活动区域逐渐扩大,人感染蜱传疾病的机会增加[4-5],新发蜱传疾病也不断涌现[6],蜱传疾病逐渐成为世界上重要的公共卫生问题。
1982年以来,我国已发现多种蜱传病原体。至今,已有多个研究团队在我国各地区开展蜱及蜱携带病原体的分布调查[7-11],但部分地区仍缺乏相关研究。江西省吉安市位于中国东南部,长江中下游南岸,属亚热带温暖湿润季风气候,植被茂盛,野生动物种类繁多,适合蜱类生存。已有研究表明,江西省存在18种蜱,分属于2科6属[12]。为了解蜱在江西省吉安市的分布情况及病原菌携带情况,本研究从该地区收集了417只寄生蜱,对其进行蜱种鉴定及6种蜱传病原菌特征基因的检测,以便为当地蜱传疾病防控提供科学依据。
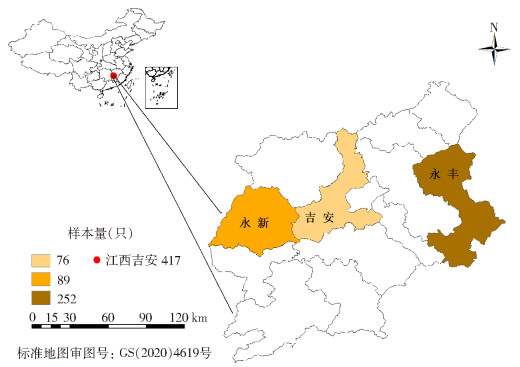
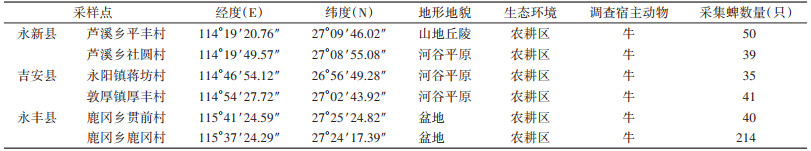
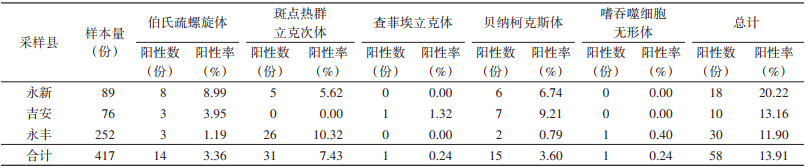
1 材料与方法 1.1 样本采集2022年6月-2023年6月,在江西省吉安市永新、永丰和吉安县共6个村的家养牛体表收集寄生蜱417只,每只牛体表收集蜱不超过15只。各县、乡镇的地理位置和采集蜱数量见图 1、表 1。
|
| 图 1 江西省吉安市3县寄生蜱的地区分布 Figure 1 Regional distribution of parasitic ticks in three counties of Ji'an, Jiangxi Province |
| |
|
DNA提取试剂盒(货号AU19014)购自无锡百泰克生物技术有限公司;用于PCR检测的2×Premix TapTM(货号RR901A)和100 bp DNA ladder(货号3422A)购自日本TaKaRa公司。
1.2.2 主要仪器PCR仪(德国SensoQuest公司)、电泳仪器(北京君意东方电泳设备有限公司)、凝胶成像仪(美国Bio-Rad公司)、研磨仪(北京国科融智生物技术有限公司)、全自动核酸提取仪(无锡百泰克生物技术有限公司)。
1.3 蜱研磨和DNA的提取将每只蜱用75%乙醇溶液浸泡、洗涤2次,每次5~10 min,之后用超纯水洗净残留的乙醇。将清洗后的蜱放入装有3~5颗磁珠的研磨管中,每管加入200 ml 1×磷酸盐缓冲液(PBS),放入研磨仪充分研磨。研磨程序为1 800 r/min,研磨30 s,间歇10 s,6个循环。采用DNA提取试剂盒和全自动核酸提取仪提取研磨后的蜱DNA。
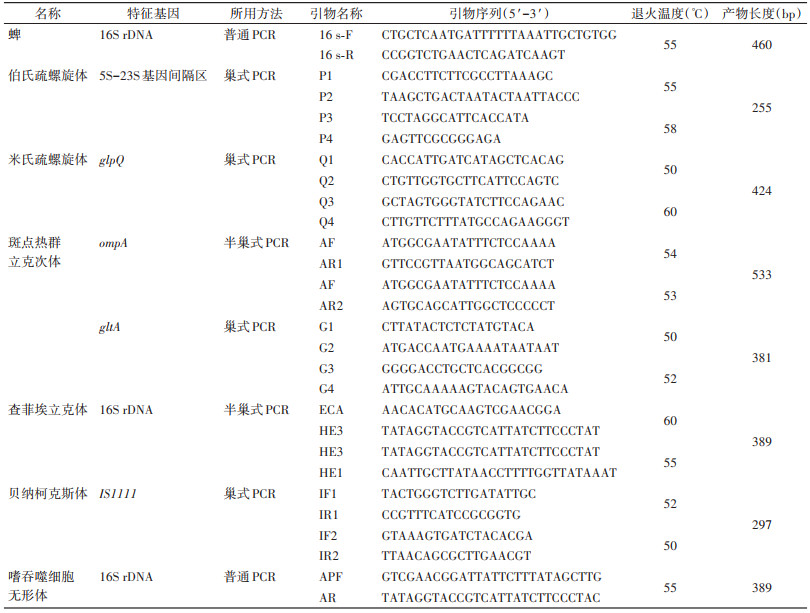
1.4 蜱种鉴定将蜱按照种、性别及生活史阶段进行初步分类[13]。采用普通PCR法扩增蜱的16S rDNA序列[14],所用引物及扩增条件见表 2。将阳性产物进行一代测序,在美国国立生物技术信息中心(NCBI)网站上利用基于局部比对算法的搜索工具(BLAST)比对产物序列和参考序列的相似性,并结合形态学特征鉴定蜱种。
|
采用巢式PCR或普通PCR法(特征基因、引物及反应条件见表 2)检测417只蜱中6类蜱传病原菌的特征基因,包括伯氏疏螺旋体(Borrelia burgdorferi,B.b)[15]、米氏疏螺旋体(B. miyamotoi,B.m)[16]、斑点热群立克次体(spotted fever group Rickettsia,SFGR)[17-18]、查菲埃立克体(Ehrlichia chaffeensis,EC)[19-20]、贝纳柯克斯体(Coxiella burnetii,C.b)[21]和嗜吞噬细胞无形体(Anaplasma phagocytophilum,AP)[22],并利用1.5%琼脂糖凝胶电泳分离PCR产物。将阳性产物进行一代测序。采用MEGA 11软件,使用Clustal W法将阳性产物序列与NCBI的参考序列进行比对,并在bootstrap值为1 000的条件下采用邻接法(neighbor- joining method,NJ法)对序列进行系统进化分析。
2 结果 2.1 蜱种鉴定经形态学和16S rDNA分子生物学鉴定(序列分析结果见图 2),417只蜱均为微小扇头蜱(Rhipicephalus microplus)。采获蜱数量、性别、发育阶段、饱血状态在各县的分布见表 3。
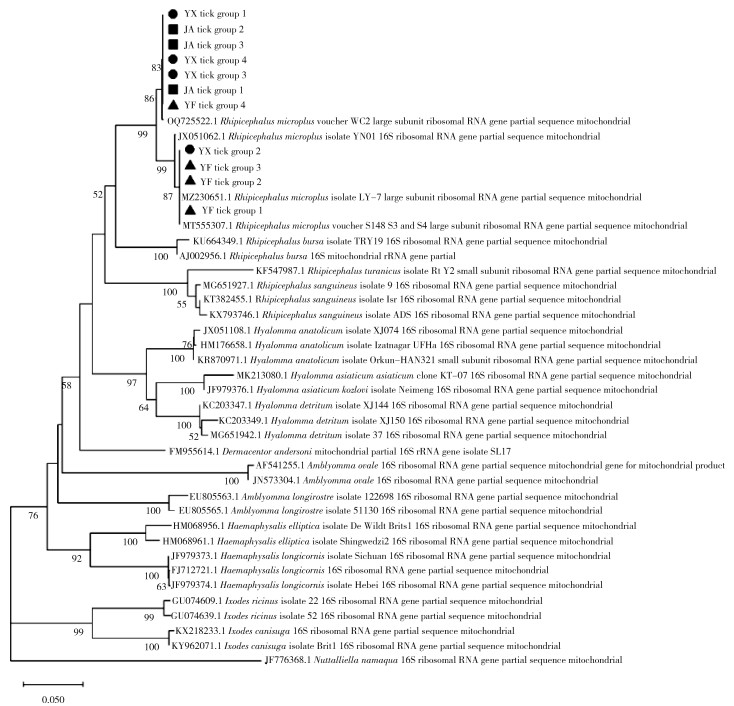
|
| 注:●代表采集自永新县的样本;■代表采集自吉安县的样本;▲代表采集自永丰县的样本。 图 2 江西省吉安市3县采获寄生蜱16S rDNA序列系统进化分析 Figure 2 Phylogenetic analysis of 16S rDNA gene sequences of parasitic ticks collected from three counties of Ji'an, Jiangxi Province |
| |
|
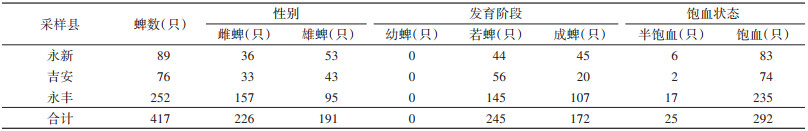
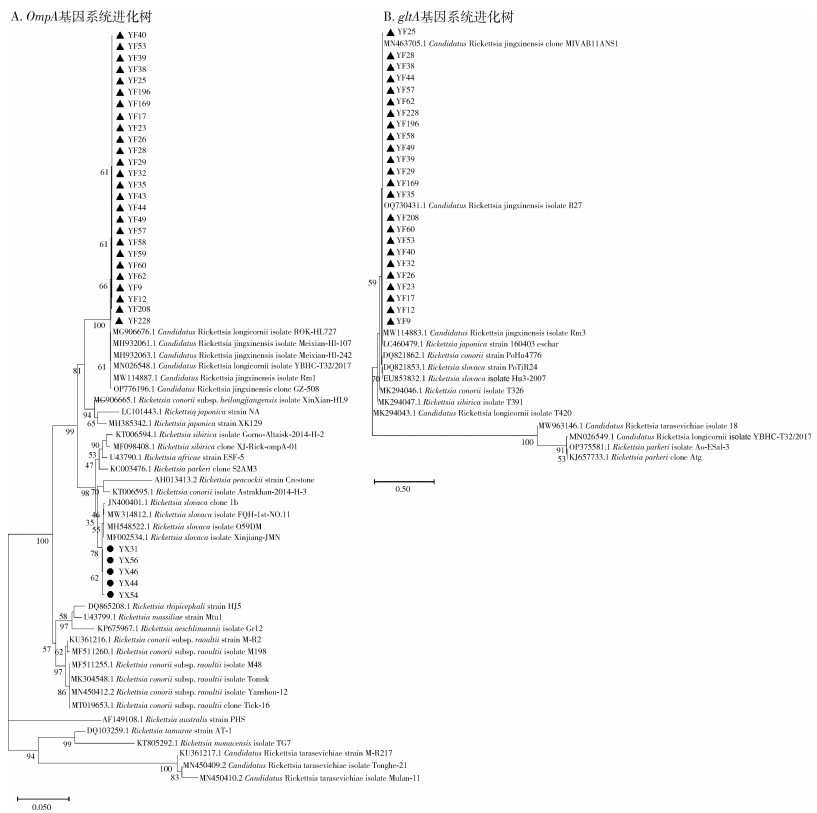
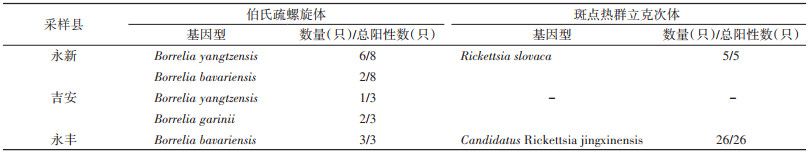
417只寄生蜱中共检出5类病原菌,分别是B.b、SFGR、EC、C.b和AP。3县各病原菌的检出情况见表 4。系统进化分析结果表明,不同县的B.b(图 3)和SFGR(图 4)基因型分布有所不同(表 5)。在永丰县的26份SFGR阳性样本中,除YF43和YF59外,其余24份样本仅以OmpA基因(图 4A)无法确定其基因型,因此进一步扩增其gltA基因(图 4B)。3县均存在蜱的复合感染,见表 6。
|
|
| 注:●代表采集自永新县的样本;▲代表采集自永丰县的样本。 图 4 江西省吉安市3县采获寄生蜱中斑点热群立克次体特征基因系统进化分析 Figure 4 Phylogeny analysis of specific gene sequences of spotted fever group Rickettsia of parasitic tick collected from three counties of Ji'an, Jiangxi Province |
| |
|

|
近年来,蜱传疾病感染逐渐成为全球关注的话题。江西省吉安市位于我国华东地区,属亚热带湿润性气候,野生动物种类繁多,植被茂盛,适合蜱类生存和繁殖,具蜱传疾病流行的风险。为了解蜱在吉安市的分布情况及病原体携带情况,本研究从吉安市永新、吉安和永丰县的家养牛体表采集了417只寄生蜱,并对其进行蜱种鉴定和6类蜱传病原菌特异基因检测。结果表明,417只寄生蜱均为微小扇头蜱,其中有58只蜱携带蜱传病原菌,阳性率为13.91%。检出的病原菌分别为B.b、C.b、SFGR、EC和AP。
结果表明,不同县寄生蜱携带的病原菌种类各不相同。永新县优势病原菌为B.b,阳性率为8.99%;吉安县为C.b,阳性率为9.21%;永丰县为SFGR,阳性率为10.32%。此外,EC仅在吉安县寄生蜱中检出,而AP仅在永丰县寄生蜱中检出。
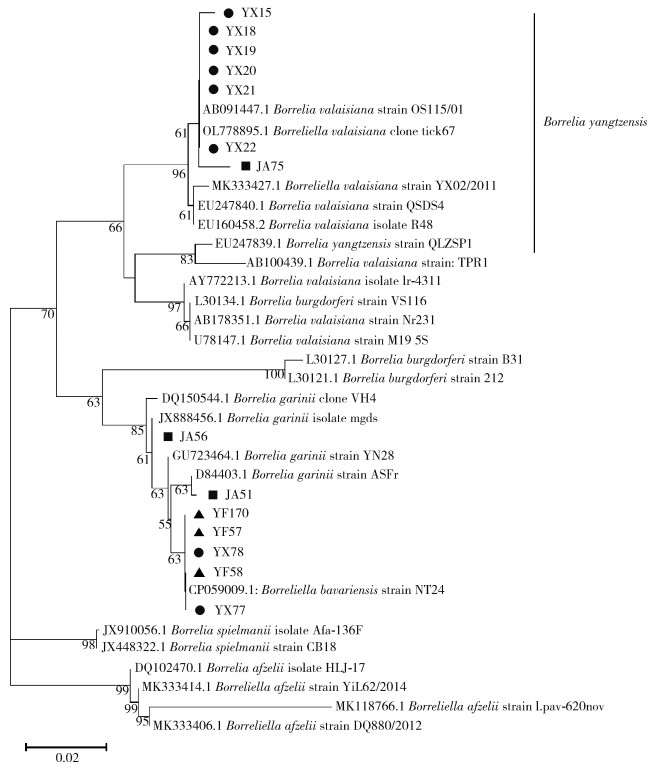
B.b是引起莱姆病的病原体,该病为多器官、多系统疾病,临床症状复杂多样,可表现为皮肤损伤、神经系统损伤、关节炎等[26]。该病原体在永新、吉安和永丰县均检出,但优势基因型存在差异。永新县的优势基因型为B. yangtzensis(图 3中为B. valasiana,该种已被认定为B. yangzensis的同物异名[23-25]);吉安县的优势基因型为B. garinii;永丰县的优势基因型为B. bavariensis。B. bavariensis曾属于B. garinii,于2009年被提议独立并命名[27]。两者的区别在于宿主动物不同,B. garinii主要为鸟类,而B. bavariensis主要为啮齿动物[27-28]。两者对人均致病,而B. yangtzensis目前未被证明有明确致病性[28],但曾有研究团队在病人体内检测出B. yangtzensis基因片段[29-30],这提示其具有潜在致病力。以上结果表明吉安市存在莱姆病流行的风险。
除B.b外,SFGR在不同县的优势基因型也存在差异。该病原体是引起斑点热的一类立克次体,患者可出现发热、皮疹和其他症状[31]。本研究中,永新和永丰县检出SFGR,永新县的优势基因型为R. slovaca型,该型曾在我国新疆维吾尔自治区、甘肃省等地区的蜱内检出[32-33]。该型已被证实具有明确致病性,患者可出现头皮焦痂和淋巴结肿大等症状[34-35]。永丰县的优势基因型为Candidatus Rickettsia jingxinensis型,该型曾在我国上海市、江苏省等地区的蜱内检出[36-37]。目前尚未报道该型具有致病性,其致病能力还需进一步研究调查。吉安市检出SFGR的阳性率较高,后续应关注斑点热在当地的流行情况,开展深入调查,做好防控。
本研究在吉安市3个县的寄生蜱中均发现病原菌的复合感染现象,阳性率为0.96%(4/417)。且4例复合感染均表现为伯氏疏螺旋体与其他蜱传病原菌的混合感染,表明伯氏疏螺旋体在吉安地区的蜱中广泛存在,应予以重视。
综合以上结果,吉安市寄生蜱携带病原菌种类多样,情况复杂。应进一步开展蜱传疾病调查和监测工作,全面了解当地蜱传疾病的媒介、宿主及人群的感染状况,为有效防控蜱传疾病提供科学依据。
利益冲突 无
| [1] |
Barker SC, Murrell A. Systematics and evolution of ticks with a list of valid genus and species names[J]. Parasitology, 2004, 129(Suppl 1): S15-36. DOI:10.1017/S0031182004005207 |
| [2] |
陈泽, 杨晓军 . 蜱的系统分类学[M]. 北京: 科学出版社, 2021: 126. Chen Z, Yang XJ. Systematic and taxonomy of Ixodida[M]. Beijing: Science Press, 2021: 126. |
| [3] |
Dantas-Torres F, Chomel BB, Otranto D. Ticks and tick-borne diseases: A One Health perspective[J]. Trends Parasitol, 2012, 28(10): 437-446. DOI:10.1016/j.pt.2012.07.003 |
| [4] |
Rochlin I, Benach JL, Furie MB, et al. Rapid invasion and expansion of the Asian longhorned tick (Haemaphysalis longicornis) into a new area on Long Island, New York, USA[J]. Ticks Tick Borne Dis, 2023, 14(2): 102088. DOI:10.1016/j.ttbdis.2022.102088 |
| [5] |
de Vries DH, Kinsman J, Cremers AL, et al. Public health preparedness and response synergies between institutional authorities and the community: A qualitative case study of emerging tick-borne diseases in Spain and the Netherlands[J]. BMC Public Health, 2021, 21(1): 1882. DOI:10.1186/s12889-021-11925-z |
| [6] |
刘玮. 中国新发蜱媒病原体概况[J]. 传染病信息, 2017, 30(1): 11-14. Liu W. Emerging tick borne agents in China[J]. Infect Dis Info, 2017, 30(1): 11-14. DOI:10.3969/j.issn.1007-8134.2017.01.005 |
| [7] |
张群芝, 窦会娟, 郭嘉林, 等. 豫南地区蜱类分布及蜱媒病原体调查与分析[J]. 现代预防医学, 2015, 42(19): 3578-3580. Zhang QZ, Dou HJ, Guo JL, et al. Investigation and analysis of tick distribution and tick vector in Yunan District[J]. Mod Prev Med, 2015, 42(19): 3578-3580. |
| [8] |
孙响, 张桂林, 刘晓明, 等. 新疆和硕地区主要蜱类及蜱媒病原体调查[J]. 中国媒介生物学及控制杂志, 2013, 24(1): 5-7, 10. Sun X, Zhang GL, Liu XM, et al. Investigation of tick species and tick-borne pathogens in Hoxud County of Xinjiang Uygur Autonomous Region, China[J]. Chin J Vector Biol Control, 2013, 24(1): 5-7, 10. |
| [9] |
吴彤宇, 王伟, 陈树斌, 等. 天津市蜱及蜱媒病原体的初步调查[J]. 中国媒介生物学及控制杂志, 2013, 24(3): 246-248. Wu TY, Wang W, Chen SB, et al. Preliminary survey of ticks and tick-borne pathogens in Tianjin, China[J]. Chin J Vector Biol Control, 2013, 24(3): 246-248. |
| [10] |
包子豪, 张琳, 侯学霞, 等. 新疆维吾尔自治区喀什地区亚洲璃眼蜱携带病原菌研究[J]. 中国人兽共患病学报, 2023, 39(1): 10-19. Bao ZH, Zhang L, Hou XX, et al. Hyalomma asiaticum asiaticum tick-borne pathogenic bacteria in Kashgar Prefecture, Xinjiang Uygur Autonomous Region[J]. Chin J Zoonoses, 2023, 39(1): 10-19. DOI:10.3969/j.issn.1002-2694.2022.00.178 |
| [11] |
李伊娜, 陈宇飞, 云托娅, 等. 内蒙古自治区中西部口岸地区2020年蜱类优势种及其病原携带情况的调查研究[J]. 中国媒介生物学及控制杂志, 2022, 33(2): 216-220. Li YN, Chen YF, Yun TY, et al. Dominant species of ticks and tick-borne pathogens in the central and western ports areas of the Inner Mongolia Autonomous Region, China, 2020[J]. Chin J Vector Biol Control, 2022, 33(2): 216-220. DOI:10.11853/j.issn.1003.8280.2022.02.009 |
| [12] |
Tian JH, Li K, Zhang SZ, et al. Tick (Acari: Ixodoidea) fauna and zoogeographic division of Jiangxi Province, China[J]. Ticks Tick Borne Dis, 2023, 14(2): 102099. DOI:10.1016/j.ttbdis.2022.102099 |
| [13] |
邓国藩, 姜在阶 . 中国经济昆虫志 第39册 蜱螨亚纲·硬蜱科[M]. 北京: 科学出版社, 1991: 1-359. Deng GF, Jiang ZJ. Economic insect fauna of China. fasc 39 Acari: Ixodidae[M]. Beijing: Science Press, 1991: 1-359. |
| [14] |
Wang YZ, Mu LM, Zhang K, et al. A broad-range survey of ticks from livestock in northern Xinjiang: Changes in tick distribution and the isolation of Borrelia burgdorferi sensu stricto[J]. Parasit Vectors, 2015, 8: 449. DOI:10.1186/s13071-015-1021-0 |
| [15] |
张琳, 苗广青, 侯学霞, 等. 巢式PCR和实时荧光定量PCR在莱姆病宿主动物监测中的应用评价[J]. 中国媒介生物学及控制杂志, 2018, 29(5): 425-427. Zhang L, Miao GQ, Hou XX, et al. Evaluation of nested PCR and real-time PCR in host surveillance of Lyme disease[J]. Chin J Vector Biol Control, 2018, 29(5): 425-427. DOI:10.11853/j.issn.1003.8280.2018.05.001 |
| [16] |
Schwan TG, Schrumpf ME, Hinnebusch BJ, et al. GlpQ: An antigen for serological discrimination between relapsing fever and Lyme borreliosis[J]. J Clin Microbiol, 1996, 34(10): 2483-2492. DOI:10.1128/jcm.34.10.2483-2492.1996 |
| [17] |
Eremeeva M, Yu X, Raoult D. Differentiation among spotted fever group rickettsiae species by analysis of restriction fragment length polymorphism of PCR-amplified DNA[J]. J Clin Microbiol, 1994, 32(3): 803-810. DOI:10.1128/jcm.32.3.803-810.1994 |
| [18] |
田冰. 发热伴血小板减少综合征疫源地中斑点热群立克次体感染的研究[D]. 沈阳: 中国医科大学, 2020. DOI: 10.27652/d.cnki.gzyku.2020.001498. Tian B. Investigation of spotted fever group Rickettsia in the epidemic region of severe fever with thrombocytopenia syndrome[D]. Shenyang: China Medical University, 2020. DOI: 10.27652/d.cnki.gzyku.2020.001498.(inChinese) |
| [19] |
Dawson JE, Stallknecht DE, Howerth EW, et al. Susceptibility of white-tailed deer (Odocoileus virginianus) to infection with Ehrlichia chaffeensis, the etiologic agent of human ehrlichiosis[J]. J Clin Microbiol, 1994, 32(11): 2725-2728. DOI:10.1128/jcm.32.11.2725-2728.1994 |
| [20] |
Anderson BE, Sumner JW, Dawson JE, et al. Detection of the etiologic agent of human ehrlichiosis by polymerase chain reaction[J]. J Clin Microbiol, 1992, 30(4): 775-780. DOI:10.1128/jcm.30.4.775-780.1992 |
| [21] |
Fenollar F, Fournier PE, Raoult D. Molecular detection of Coxiella burnetii in the sera of patients with Q fever endocarditis or vascular infection[J]. J Clin Microbiol, 2004, 42(11): 4919-4924. DOI:10.1128/JCM.42.11.4919-4924.2004 |
| [22] |
Overzier E, Pfister K, Thiel C, et al. Anaplasma phagocytophilum in questing Ixodes ricinus ticks: Comparison of prevalences and partial 16S rRNA gene variants in urban, pasture, and natural habitats[J]. Appl Environ Microbiol, 2013, 79(5): 1730-1734. DOI:10.1128/AEM.03300-12 |
| [23] |
Masuzawa T, Hashimoto N, Kudeken M, et al. New genomospecies related to Borrelia valaisiana, isolated from mammals in Okinawa archipelago, Japan[J]. J Med Microbiol, 2004, 53(5): 421-426. DOI:10.1099/jmm.0.05337-0 |
| [24] |
Chu CY, Liu W, Jiang BG, et al. Novel genospecies of Borrelia burgdorferi sensu lato from rodents and ticks in southwestern China[J]. J Clin Microbiol, 2008, 46(9): 3130-3133. DOI:10.1128/JCM.01195-08 |
| [25] |
Margos G, Chu CY, Takano A, et al. Borrelia yangtzensis sp. nov., a rodent-associated species in Asia, is related to Borrelia valaisiana[J]. Int J Syst Evol Microbiol, 2015, 65(11): 3836-3840. DOI:10.1099/ijsem.0.000491 |
| [26] |
Steere AC, Strle F, Wormser GP, et al. Lyme borreliosis[J]. Nat Rev Dis Primers, 2016, 2: 16090. DOI:10.1038/nrdp.2016.90 |
| [27] |
Margos G, Vollmer SA, Cornet M, et al. A new Borrelia species defined by multilocus sequence analysis of housekeeping genes[J]. Appl Environ Microbiol, 2009, 75(16): 5410-5416. DOI:10.1128/AEM.00116-09 |
| [28] |
Wolcott KA, Margos G, Fingerle V, et al. Host association of Borrelia burgdorferi sensu lato: A review[J]. Ticks Tick Borne Dis, 2021, 12(5): 101766. DOI:10.1016/j.ttbdis.2021.101766 |
| [29] |
Kim CM, Yun NR, Kim DM. Case report: The first Borrelia yangtzensis infection in a human in Korea[J]. Am J Trop Med Hyg, 2022, 106(1): 45-46. DOI:10.4269/ajtmh.21-0052 |
| [30] |
Ni XB, Jia N, Jiang BG, et al. Lyme borreliosis caused by diverse genospecies of Borrelia burgdorferi sensu lato in northeastern China[J]. Clin Microbiol Infect, 2014, 20(8): 808-814. DOI:10.1111/1469-0691.12532 |
| [31] |
叶晓东, 郑寿贵, 孙毅, 等. 蜱传斑点热群立克次体研究进展[J]. 中国人兽共患病学报, 2008, 24(4): 368-371. Ye XD, Zheng SG, Sun Y, et al. Advances in the research of tick-borne spotted fever group rickettsiae[J]. Chin J Zoonoses, 2008, 24(4): 368-371. DOI:10.3969/j.issn.1002-2694.2008.04.021 |
| [32] |
Tian ZC, Liu GY, Shen H, et al. First report on the occurrence of Rickettsia slovaca and Rickettsia raoultii in Dermacentor silvarum in China[J]. Parasit Vectors, 2012, 5: 19. DOI:10.1186/1756-3305-5-19 |
| [33] |
Cao XQ, Gu XL, Zhang L, et al. Molecular detection of Rickettsia, Anaplasma, and Bartonella in ticks from free-ranging sheep in Gansu Province, China[J]. Ticks Tick Borne Dis, 2023, 14(3): 102137. DOI:10.1016/j.ttbdis.2023.102137 |
| [34] |
Raoult D, Berbis P, Roux V, et al. A new tick-transmitted disease due to Rickettsia slovaca[J]. Lancet, 1997, 350(9071): 112-113. DOI:10.1016/S0140-6736(05)61814-4 |
| [35] |
马兰. 人感染新斑点热群立克次体的发现及自然疫源地调查研究[D]. 北京: 中国人民解放军军事医学科学院, 2015. Ma L. Discovery of human infections with novel spotted fever group rickettsiae and investigations on their natural foci[D]. Beijing: Academy of Military Medical Sciences, PLA, 2015. (in Chinese) |
| [36] |
Qi Y, Ai LL, Jiao J, et al. High prevalence of Rickettsia spp. in ticks from wild hedgehogs rather than domestic bovine in Jiangsu Province, eastern China[J]. Front Cell Infect Microbiol, 2022, 12: 954785. DOI:10.3389/fcimb.2022.954785 |
| [37] |
Zeng WB, Li ZQ, Jiang TG, et al. Identification of bacterial communities and tick-borne pathogens in Haemaphysalis spp. collected from Shanghai, China[J]. Trop Med Infect Dis, 2022, 7(12): 413. DOI:10.3390/tropicalmed7120413 |
 2024, Vol. 35
2024, Vol. 35