扩展功能
文章信息
- 霍锡元, 张晔, 刘国军, 苑森梅, 韩雪峰, 赵春春, 孟凤霞
- HUO Xi-yuan, ZHANG Ye, LIU Guo-jun, YUAN Sen-mei, HAN Xue-feng, ZHAO Chun-chun, MENG Feng-xia
- 山东省潍坊市2022年白纹伊蚊击倒抗性基因型分布研究
- Distribution of knockdown resistance genotypes in Aedes albopictus in Weifang, Shandong Province, China, 2022
- 中国媒介生物学及控制杂志, 2024, 35(2): 156-160
- Chin J Vector Biol & Control, 2024, 35(2): 156-160
- 10.11853/j.issn.1003.8280.2024.02.005
-
文章历史
- 收稿日期: 2023-09-07
2 潍坊市妇幼保健院, 山东 潍坊 261061;
3 传染病溯源预警与智能决策全国重点实验室, 中国疾病预防控制中心传染病预防控制所, 世界卫生组织媒介生物监测与管理合作中心, 北京 102206
2 Weifang Maternal and Child Health Hospital, Weifang, Shandong 261061, China;
3 National Key Laboratory of Intelligent Tracking and Forecasting for Infectious Diseases, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, WHO Collaborating Centre for Vector Surveillance and Management, Beijing 102206, China
近年来,登革热流行日趋严重,范围不断扩大。白纹伊蚊(Aedes albopictus)作为我国登革热传播的主要媒介之一,在登革热的传播过程中发挥了关键作用。同时,白纹伊蚊是潍坊市的优势蚊种,在全市分布范围广、密度高。化学杀虫剂见效快、使用方便,能够快速降低成蚊密度,成为控制蚊虫特别是虫媒传染病疫情防控时媒介生物控制的重要手段。但随着卫生杀虫剂的广泛、大量使用,白纹伊蚊对多种卫生杀虫剂都已经有了不同程度的抗药性[1-3]。
电压门控钠离子通道(voltage-gated sodium channel,VGSC)为拟除虫菊酯类杀虫剂作用的主要靶点,该通道编码基因突变可导致蚊虫对该类卫生杀虫剂的亲和力降低,因此产生抗性,称为击倒抗性(knockdown resistance,kdr)[4]。近年来,潍坊市疾病预防控制中心对潍坊市白纹伊蚊的密度和抗药性进行了监测,但并未对该地区白纹伊蚊击倒抗性基因进行研究。本研究通过采集山东省潍坊市高新区、寒亭区、高密市、安丘市和临朐县的白纹伊蚊并检测其VGSC基因1016、1532、1534位点突变情况,为生物测定法抗药性监测提供关联性证据,从而更加科学评估潍坊市白纹伊蚊抗药性水平和指导卫生杀虫剂使用。
1 材料与方法 1.1 蚊虫采集2022年7月采用勺捕法在潍坊市高新区张营南埠村、寒亭区西杨家埠村、高密市大庄村、安丘市关王村和临朐县南流村的农村居民区采集伊蚊幼虫,在实验室集中饲养至羽化,羽化1 d后经形态学鉴定,挑选白纹伊蚊作为试虫(其中高新区19只,寒亭区22只,高密市23只,安丘市16只,临朐县16只),放入75%乙醇溶液中-20 ℃保存备用。
1.2 白纹伊蚊核酸提取、基因扩增和测序将单只白纹伊蚊研磨后,采用磁珠法按照组织基因组DNA提取试剂盒说明书(百泰克生物技术有限公司)提取基因组DNA。参照Kasai等[5-6]文献中的引物和方法,对白纹伊蚊VGSC基因的Domain Ⅱ和Domain Ⅲ2个片段进行扩增。扩增产物送北京德奥平生物公司进行测序。
1.3 序列比对及统计学分析利用Seqman和MEGA 7.0软件对测序结果进行序列比对和峰图分析,对各个位点突变情况进行观察,确定等位基因类型与基因型。统计分析各个样本在1016、1532和1534位点的突变情况,计算潍坊市5个县(市、区)白纹伊蚊VGSC基因的等位基因型和突变率(突变率=突变数/观察数×100%)。
2 结果 2.1 VGSC基因的等位基因和基因型96只白纹伊蚊扩增产物经测序共获得192条DNA序列,测序片段长度约400 bp。测序结果经比对分析发现,有3个位点存在突变,分别为1016、1532和1534位点(图 1)。
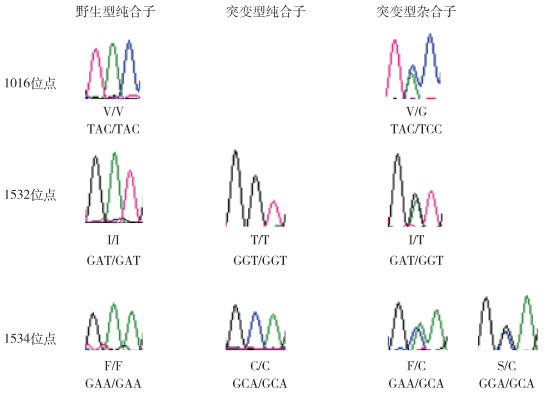
|
| 注:红色峰形代表T碱基,绿色峰形代表A碱基,蓝色峰形代表C碱基,黑色峰形代表G碱基;/前后为该个体的2个等位基因对应的氨基酸;TAC、TCC、GAT、GGT、GAA、GCA和GGA为氨基酸密码子,分别编码缬氨酸(V)、甘氨酸(G)、异亮氨酸(I)、苏氨酸(T)、苯丙氨酸(F)、半胱氨酸(C)和丝氨酸(S)。 图 1 山东省潍坊市白纹伊蚊击倒抗性突变位点不同基因型测序峰图 Figure 1 Sequencing chromatogram of genotypes at knockdown resistance gene mutation loci in Aedes albopictus in Weifang, Shandong Province |
| |
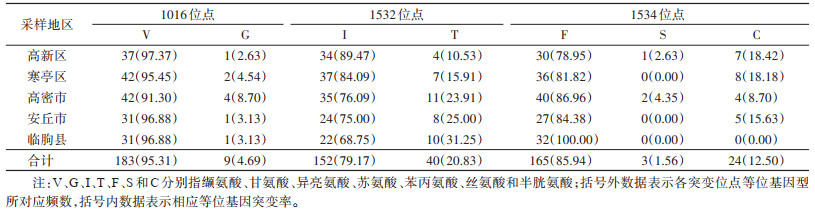
1016位点存在2种等位基因,分别是野生型1016V(95.31%)和突变型1016G(4.69%)。1532位点存在2种等位基因,即野生型1532I(79.17%)和突变型1532T(20.83%)。1534位点存在3种等位基因,即野生型1534F(85.94%)、突变型1534S(1.56%)和1534C(12.50%)。突变型等位基因1016G和1532T在5个县(市、区)中均有发现,1534S只在高新区和高密市有发现,而1534C除临朐县外均有发现。1532位点等位基因突变率最高(20.83%),其次是1534位点(14.06%),而1016位点等位基因突变率最低(4.69%)。见表 1。
|
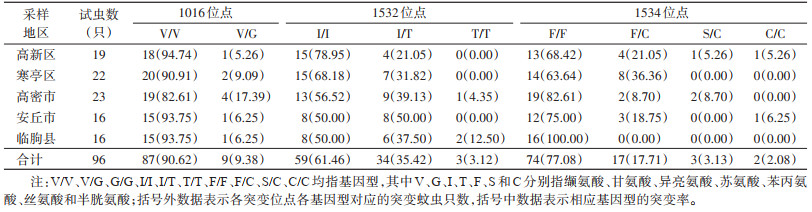
3个突变位点均以野生型纯合子为主,野生/突变型杂合子次之,突变型纯合子所占比例最低。1016位点存在2种基因型,分别是野生型纯合子V/V[87(突变蚊虫数),90.62%(突变率)]和野生/突变型杂合子V/G(9,9.38%);在1532位点发现3种基因型,分别是野生型纯合子I/I(59,61.46%)、野生/突变型杂合子I/T(34,35.42%)和突变型纯合子T/T(3,3.12%);1534位点较1016和1532位点突变类型更加复杂,包括1种野生型纯合子F/F(74,77.08%)、1种野生/突变型杂合子F/C(17,17.71%)、1种突变型杂合子S/C(3,3.13%)以及1种突变型纯合子C/C(2,2.08%)。见表 2。
|
此次检测的5个县(市、区)的白纹伊蚊中,除临朐县白纹伊蚊的1534位点未发现突变外,其余县(市、区)的白纹伊蚊3个位点均出现突变。在1016位点,只有1种突变等位基因1016G,高密市1016V/G突变率最高(17.39%)。在1532位点,也只有1种突变等位基因1532T,安丘市1532I/T突变率最高(50.00%),临朐县1532T/T突变率最高(12.50%)。在1534位点,有2种突变等位基因1534S和1534C,寒亭区1534F/C突变率最高(36.36%),高密市1534S/C突变率最高(8.70%),安丘市1534C/C突变率最高(6.25%)。见表 2。
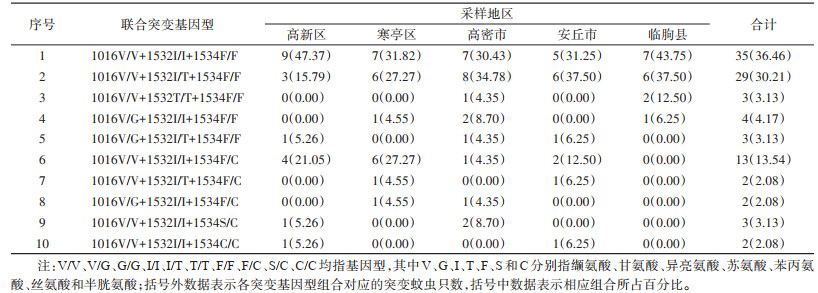
2.2 1016、1532和1534位点联合突变共检测到10种突变基因型组合,未检测到3位点同时突变的基因型。本次检测到的10种基因型组合中,3个位点均为野生型纯合子的样本所占比例最高,为36.46%;其余9种突变基因组合中,V/V+I/T+F/F所占比例最高,为30.21%。见表 3。
|
近年来,国内外登革热的发病呈上升趋势,2017年,在山东省济宁市发生了登革热本地流行。潍坊市近几年有登革热散发输入病例报告,虽未引起本地流行,但应引起重视。目前,由于缺乏特效的登革热治疗药物和预防疫苗,对于登革热疫情的防控主要依靠化学防治降低媒介伊蚊的数量。拟除虫菊酯类杀虫剂因其广谱、高效及低毒等特点被广泛使用。近年来,随着卫生城市创建的举措,以及登革热疫情的预防,化学杀虫剂在潍坊市被大量使用,使得白纹伊蚊对多种杀虫剂产生了不同程度的抗药性,尤其是拟除虫菊酯类。潍坊市在2020-2022年对辖区的卫生杀虫剂使用情况进行调查,结果显示,拟除虫菊酯类杀虫剂使用最多,特别是氯氰菊酯;并且2020-2022年潍坊市白纹伊蚊成蚊对0.08%高效氯氰菊酯、0.4%氯菊酯、0.07%高效氯氟氰菊酯和0.03%溴氰菊酯均产生抗性[7]。拟除虫菊酯类杀虫剂能够结合开放状态的神经突触钠离子通道,维持通道的开放状态,使得钠离子内流时间延长,膜电位持续去极化,引起重复的神经放电和后放,导致昆虫麻痹死亡。神经突触钠离子通道氨基酸序列的变异引起昆虫的击倒抗性。目前多个研究表明,白纹伊蚊VGSC基因的1016、1532和1534位点的突变与拟除虫菊酯类杀虫剂抗药性具有相关性[8-10]。潍坊市以往的抗药性监测仅限于生物测定方法,缺乏对白纹伊蚊抗药性分子作用机制方面的研究和监测。
本次研究潍坊市白纹伊蚊VGSC基因检测到1016位点有2种等位基因,即野生型1016V和突变型1016G,与2021年周小洁等[11]报道的北京市白纹伊蚊1016位点基因型一致,均未检测到突变型纯合子,潍坊市白纹伊蚊1016位点的突变率(4.69%)与北京市白纹伊蚊1016位点的突变率(5.00%)接近,低于2022年李雪等[12]报道的南宁市白纹伊蚊1016位点的突变率(21.72%)。1532位点有2种等位基因,即野生型1532I和突变型1532T,与母群征等[13]报道的河南省禹州市1532位点基因型一致,但潍坊市白纹伊蚊1532位点的突变率(20.83%)比河南省禹州市1532位点的突变率(15.00%)高。1534位点等位基因多样,本次研究结果等位基因突变型为1534S(1.56%)和1534C(12.50%),1534S和1534C与白纹伊蚊的抗药性密切相关已有研究报道[14-15]。本次检测获得的白纹伊蚊1534位点突变率和突变类型与国内其他地区有所不同,广西壮族自治区南宁市以1534C为主[13],云南省景洪市以1534S为主[16],广东省中山市以1534L为主[17],造成这种情况出现的原因可能与当地使用卫生杀虫剂种类、强度和频次以及蚊虫遗传因素、选择压力有关[12]。
本研究首次在潍坊地区进行白纹伊蚊VGSC基因突变调查,VGSC基因突变的多样性及较高的突变率提示当地白纹伊蚊对拟除虫菊酯类抗药性的严峻性,在日后的蚊虫防控活动中应减少拟除虫菊酯类杀虫剂的使用。今后将扩大研究地区,与邻近地区进行比较,对白纹伊蚊VGSC基因分布和基因交流做进一步的研究,结合近年的杀虫剂抗药性监测结果,更加科学合理地指导卫生杀虫剂的使用。
利益冲突 无
| [1] |
邹亚明, 兰策介, 刘蕴华, 等. 江苏省无锡市2015-2019年白纹伊蚊对5种常用卫生杀虫剂的抗药性发展趋势[J]. 中国媒介生物学及控制杂志, 2021, 32(1): 74-77. Zou YM, Lan CJ, Liu YH, et al. Resistance development trend of Aedes albopictus to five commonly used insecticides in Wuxi, Jiangsu Province, China, 2015-2019[J]. Chin J Vector Biol Control, 2021, 32(1): 74-77. DOI:10.11853/j.issn.1003.8280.2021.01.015 |
| [2] |
赵春春, 朱彩英, 贾清臣, 等. 2017-2018年我国不同区域白纹伊蚊对常用杀虫剂的抗药性[J]. 中国媒介生物学及控制杂志, 2020, 31(2): 126-132. Zhao CC, Zhu CY, Jia QC, et al. Resistance of Aedes albopictus to commonly used insecticides in different areas of China, 2017-2018[J]. Chin J Vector Biol Control, 2020, 31(2): 126-132. DOI:10.11853/j.issn.1003.8280.2020.02.002 |
| [3] |
刘洪霞, 冷培恩, 刘曜, 等. 上海地区2015-2019年白纹伊蚊和家蝇的抗药性发展动态[J]. 中国媒介生物学及控制杂志, 2020, 31(2): 137-142. Liu HX, Leng PE, Liu Y, et al. Insecticide resistance tendency of Aedes albopictus and Musca domestica in Shanghai, China from 2015-2019[J]. Chin J Vector Biol Control, 2020, 31(2): 137-142. DOI:10.11853/j.issn.1003.8280.2020.02.004 |
| [4] |
Itokawa K, Sekizuka T, Maekawa Y, et al. High-throughput genotyping of a full voltage-gated sodium channel gene via genomic DNA using target capture sequencing and analytical pipeline MoNaS to discover novel insecticide resistance mutations[J]. PLoS Negl Trop Dis, 2019, 13(11): e0007818. DOI:10.1371/journal.pntd.0007818 |
| [5] |
Kasai S, Caputo B, Tsunoda T, et al. First detection of a Vssc allele V1016G conferring a high level of insecticide resistance in Aedes albopictus collected from Europe (Italy) and Asia (Vietnam), 2016: A new emerging threat to controlling arboviral diseases[J]. Euro Surveill, 2019, 24(5): 1700847. DOI:10.2807/1560-7917.ES.2019.24.5.1700847 |
| [6] |
Kasai S, Ng LC, Lam-Phua SG, et al. First detection of a putative knockdown resistance gene in major mosquito vector, Aedes albopictus[J]. Jpn J Infect Dis, 2011, 64(3): 217-221. DOI:10.7883/yoken.64.217 |
| [7] |
霍锡元, 丁树刚, 李东英, 等. 山东省潍坊市白纹伊蚊对常用卫生杀虫剂抗性调查[J]. 中国媒介生物学及控制杂志, 2023, 34(5): 682-685. Huo XY, Ding SG, Li DY, et al. A survey of the resistance of Aedes albopictus to commonly used insecticides in Weifang, Shandong Province, China[J]. Chin J Vector Biol Control, 2023, 34(5): 682-685. DOI:10.11853/j.issn.1003.8280.2023.05.017 |
| [8] |
Ismail BA, Kafy HT, Sulieman JE, et al. Temporal and spatial trends in insecticide resistance in Anopheles arabiensis in Sudan: Outcomes from an evaluation of implications of insecticide resistance for malaria vector control[J]. Parasit Vectors, 2018, 11(1): 122. DOI:10.1186/s13071-018-2732-9 |
| [9] |
Zoh DD, Ahoua Alou LP, Toure M, et al. The current insecticide resistance status of Anopheles gambiae (s. l.) (Culicidae) in rural and urban areas of Bouaké, Côte d'Ivoire[J]. Parasit Vectors, 2018, 11(1): 118. DOI:10.1186/s13071-018-2702-2 |
| [10] |
赵春春, 朱彩英, 开文龙, 等. 海口市2018年白纹伊蚊击倒抗性基因型分布研究[J]. 中国媒介生物学及控制杂志, 2019, 30(1): 7-11. Zhao CC, Zhu CY, Kai WL, et al. Genotypes of knockdown resistance gene and their distribution in Aedes albopictus in Haikou, China, in 2018[J]. Chin J Vector Biol Control, 2019, 30(1): 7-11. DOI:10.11853/j.issn.1003.8280.2019.01.002 |
| [11] |
周小洁, 赵宇晗, 刘念, 等. 北京市两个白纹伊蚊种群kdr抗性突变检测分析[J]. 中华卫生杀虫药械, 2021, 27(4): 304-307. Zhou XJ, Zhao YH, Liu N, et al. Detection and analysis of kdr resistance mutations in two populations of Aedes albopictus in Beijing[J]. Chin J Hyg Insect Equip, 2021, 27(4): 304-307. DOI:10.19821/j.1671-2781.2021.04.003 |
| [12] |
李雪, 凌峰, 韦舒琳, 等. 南宁市2022年白纹伊蚊击倒抗性基因型分布研究[J]. 中国媒介生物学及控制杂志, 2023, 34(4): 480-484. Li X, Ling F, Wei SL, et al. Distribution of knockdown resistance genotypes in Aedes albopictus in Nanning, Guangxi Zhuang Autonomous Region, China, 2022[J]. Chin J Vector Biol Control, 2023, 34(4): 480-484. DOI:10.11853/j.issn.1003.8280.2023.04.007 |
| [13] |
母群征, 华栋栋, 李文玉, 等. 河南省禹州地区白纹伊蚊击倒抗性基因突变检测[J]. 中国媒介生物学及控制杂志, 2023, 34(3): 303-307, 335. Mu QZ, Hua DD, Li WY, et al. Detection of knockdown resistance gene mutations in Aedes albopictus in Yuzhou, Henan Province, China[J]. Chin J Vector Biol Control, 2023, 34(3): 303-307, 335. DOI:10.11853/j.issn.1003.8280.2023.03.004 |
| [14] |
Auteri M, La Russa F, Blanda V, et al. Insecticide resistance associated with kdr mutations in Aedes albopictus: An update on worldwide evidences[J]. Biomed Res Int, 2018, 2018: 3098575. DOI:10.1155/2018/3098575 |
| [15] |
Xu JB, Bonizzoni M, Zhong DB, et al. Multi-country survey revealed prevalent and novel F1534S mutation in voltage-gated sodium channel (VGSC) gene in Aedes albopictus[J]. PLoS Negl Trop Dis, 2016, 10(5): e0004696. DOI:10.1371/journal.pntd.0004696 |
| [16] |
朱彩英, 赵春春, 伦辛畅, 等. 云南省景洪市2018-2019年白纹伊蚊击倒抗性基因型分布研究[J]. 中国媒介生物学及控制杂志, 2020, 31(1): 7-11. Zhu CY, Zhao CC, Lun XC, et al. Distribution of knockdown resistance genotypes in Aedes albopictus in Jinghong, Yunnan Province, China, 2018-2019[J]. Chin J Vector Biol Control, 2020, 31(1): 7-11. DOI:10.11853/j.issn.1003.8280.2020.01.002 |
| [17] |
杨罗菊, 刘德星, 陈健, 等. 中山市白纹伊蚊现场种群击倒抗性基因检测分析[J]. 中国人兽共患病学报, 2021, 37(2): 171-175. Yang LJ, Liu DX, Chen J, et al. Detection and analysis of the knockdown resistance gene in the field populations of Aedes albopictus in Zhongshan[J]. Chin J Zoonoses, 2021, 37(2): 171-175. DOI:10.3969/j.issn.1002-2694.2021.00.005 |
 2024, Vol. 35
2024, Vol. 35


