扩展功能
文章信息
- 张燕, 王丹, 周敬祝, 师伟芳, 罗小龙, 孔雪雪, 余好, 管毓威, 胡勇, 梁文琴
- ZHANG Yan, WANG Dan, ZHOU Jing-zhu, SHI Wei-fang, LUO Xiao-long, KONG Xue-xue, YU Hao, GUAN Yu-wei, HU Yong, LIANG Wen-qin
- 贵阳市白纹伊蚊对3种拟除虫菊酯类杀虫剂的抗药性及击倒抗性基因研究
- Resistance to three pyrethroid insecticides and knockdown resistance gene mutations in Aedes albopictus in Guiyang, Guizhou Province, China
- 中国媒介生物学及控制杂志, 2023, 34(5): 600-606
- Chin J Vector Biol & Control, 2023, 34(5): 600-606
- 10.11853/j.issn.1003.8280.2023.05.003
-
文章历史
- 收稿日期: 2023-03-23
2 贵州省疾病预防控制中心实验中心病媒生物监测科, 贵州 贵阳 550004
2 Department of Vector Surveillance, Experimental Center, Guizhou Center for Disease Control and Prevention, Guiyang, Guizhou 550004, China
白纹伊蚊(Aedes albopictus)是登革病毒、基孔肯雅病毒和寨卡病毒等虫媒病毒的重要媒介[1]。在我国,白纹伊蚊分布范围较广,覆盖大部分省、自治区和直辖市,被认为是中国登革热传播的主要媒介[2]。要最大限度地降低人类感染此类病毒的风险,关键是成功控制白纹伊蚊的种群密度。蚊虫的控制主要依赖于杀虫剂的使用,已知拟除虫菊酯类杀虫剂具有快速且高效的杀虫活性,且对哺乳动物毒性较低,自20世纪80年代以来,此类杀虫剂被广泛用于全国防治农业节肢动物害虫和蚊媒疾病(登革热等),化学防治成为蚊虫控制的最有效控制措施之一,特别是在相关疾病暴发期间[3]。持续和广泛使用拟除虫菊酯类杀虫剂进行病媒控制的一个主要威胁是蚊虫对其产生抗药性。蚊虫可能存在一种或多种抗性机制,使其对杀虫剂的敏感性降低[4]。这些抗药性机制包括行为改变、解毒代谢增加、角质层增厚和靶点抗性。拟除虫菊酯类杀虫剂的作用靶点为电压门控钠离子通道(VGSC),作用该靶点对蚊虫具有明显的击倒效应,而VGSC基因突变可导致蚊虫对菊酯类杀虫剂产生抗性,这种抗性因此称为击倒抗性(knockdown resistance,kdr)[5-6]。自2009年Kasai等[7]首次在新加坡现场采集的白纹伊蚊中检测到kdr突变后,我国在不同现场采集的白纹伊蚊种群中均报告了kdr相关突变,主要包括有V1016G、I1532T和F1534S/L/C等突变[8]。
近年来,贵阳市仅报道了2015和2016年白纹伊蚊对常用杀虫剂的抗性生物测定结果,但其kdr基因突变情况尚不清楚,因此本研究选择采集贵阳市白纹伊蚊,检测其对3种拟除虫菊酯类杀虫剂的抗性情况,并分析其kdr基因突变情况及其与抗性表型的相关性,探索该地区白纹伊蚊的抗药性机制,为蚊虫的有效防治提供一定的数据支撑和理论依据。
1 材料与方法 1.1 试剂0.03%溴氰菊酯、0.4%氯菊酯、0.08%高效氯氰菊酯和空白对照药膜纸由中国疾病预防控制中心(疾控中心)传染病预防控制所(传染病所)媒介生物控制室提供,基因组提取试剂盒,扩增引物等购自生工生物工程(上海)股份有限公司。
1.2 方法 1.2.1 蚊虫来源与饲养2022年7-8月在贵阳市东、南、西、北、中5个方位的室外花盆、轮胎、废旧桶和水缸等小型积水容器处采集白纹伊蚊自然种群幼虫,带回实验室饲养至F1~F2代,选择Ⅲ龄末Ⅳ龄初幼虫、羽化后3~5 d的健康雌蚊分别进行幼蚊和成蚊抗药性生物测定,并筛选抗性和敏感表型蚊虫。敏感品系白纹伊蚊由中国疾控中心传染病所惠赠,已饲养多年且未接触任何杀虫剂。白纹伊蚊成蚊均用10%蔗糖溶液饲喂,温度为(26±1)℃,相对湿度为(75±5)%,光周期(L∶D)为14 h∶10 h。
1.2.2 幼虫抗药性生物测定采用幼虫浸渍法,具体方法、结果判断与抗药性级别判定均同王丹等[9]的标准,野外种群幼虫抗性级别依据抗性倍数(RR50)判定,RR50 < 3为敏感(S),3≤RR50 < 10为低抗(L),10≤RR50 < 40为中抗(M),RR50≥40为高抗(H)。
1.2.3 成蚊抗药性生物测定与表型筛选采用成蚊接触筒法,具体方法、结果判断与抗药性级别判定均同王丹等[9]的标准。本次杀虫剂和药膜诊断剂量为氯菊酯(0.4%)、溴氰菊酯(0.03%)、高效氯氰菊酯(0.08%)。表型判断标准:试虫完全不动或者仅四肢、翅膀等震颤并无存活的可能视为死亡,判断为敏感表型。反之视为存活,判断为抗性表型。将抗性表型和敏感表型的成蚊均单只装入含有95%乙醇溶液的1.5 ml离心管中,-80 ℃保存,待基因检测。蚊虫种群抗药性等级判定根据蚊虫死亡率进行:死亡率 < 80%为抗性种群,80%≤死亡率 < 98%为可疑抗性种群;死亡率≥98%为敏感种群。
1.2.4 kdr基因检测共选取316只白纹伊蚊(包括敏感品系成蚊20只,自然种群抗性表型成蚊247只,敏感表型成蚊49只),送生工生物工程(上海)股份有限公司提取基因并测序。以单只蚊虫基因组DNA为模板,扩增VGSC第Ⅱ、Ⅲ跨膜结构域的部分基因片段,参考文献[7, 10]合成引物,第Ⅱ结构域扩增引物为aegSCF20:5′-GAC AAT GTG GAT CGC TTC CC-3′(正向引物),aegSCR21:5′-GCA ATC TGG CTT GTT AAC TTG-3′(反向引物)。第Ⅲ结构域扩增引物为aegSCF7:5′-GAG AAC TCG CCG ATG AAC TT-3′(正向引物),aegSCR7:5′-GAC GAC GAA ATC GAA CAG GT-3′(反向引物)。PCR反应条件为:预变性95 ℃ 5 min;10个循环(94 ℃ 30 s,63 ℃ 30 s,72 ℃ 30 s);30个循环(95 ℃ 30 s,58 ℃ 30 s,72 ℃ 30 s),最后72 ℃延伸10 min,并4 ℃保存。第Ⅱ结构域测序引物为反向aegSCR22:5′-TTC ACG AAC TTG AGC GCG TTG-3′,第Ⅲ结构域测序引物为反向aegSCR8:5′-TAG CTT TCA GCG GCT TCT TC-3′。扩增产物经1%琼脂糖凝胶电泳检测后,条带清晰无拖尾的PCR产物再进行Sanger测序。
1.3 统计学分析应用SPSS 24.0软件中概率回归方法计算幼蚊对杀虫剂的半数致死浓度(LC50)和95%置信区间,毒力回归方程等。RR50=野外种群LC50/敏感品系LC50,敏感品系LC50由贵州省疾控中心病媒生物监测团队实验室人员使用上述方法获得。应用Chromas v2.6.5软件进行峰图分析,用Seqman软件对测得的序列进行比对,与美国国立生物技术信息中心(NCBI)网站公布的VGSC基因参考序列(KC152045.1和KC152046.1)比对并观察各位点突变情况,判断kdr等位基因类型和基因型,用Excel 2013软件统计和计算白纹伊蚊VGSC基因的等位基因和基因型频率,组间比较采用χ2检验,以P < 0.05为差异有统计学意义。
2 结果 2.1 白纹伊蚊幼虫抗药性测定结果贵阳市白纹伊蚊幼虫对3种拟除虫菊酯类杀虫剂均表现出抗性,其中对氯菊酯(RR50=433.33)和溴氰菊酯(RR50=46.67)产生了高抗,对高效氯氰菊酯产生了中抗(RR50=16.44)。见表 1。
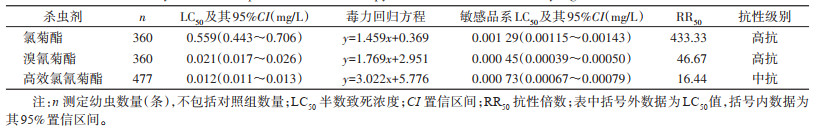
|
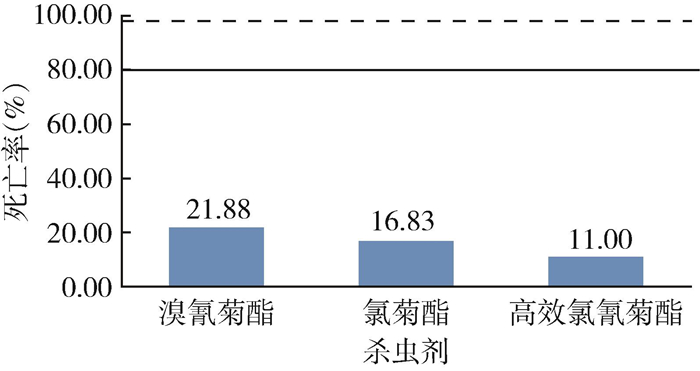
贵阳市白纹伊蚊分别接触3种拟除虫菊酯类杀虫剂诊断剂量的药膜纸,24 h后死亡率最低为11.00%,最高为21.88%,均远小于抗性判断标准死亡率界限80%,属于抗性种群(图 1)。溴氰菊酯抗性测定后筛选出敏感表型白纹伊蚊21只,抗性表型74只;氯菊酯抗性测定后筛选出敏感表型17只,抗性表型84只;高效氯氰菊酯抗性测定后筛选出敏感表型11只,抗性表型89只。
|
| 注:实线表示死亡率为80%,虚线表示死亡率为98%。 图 1 贵阳市白纹伊蚊野外种群接触3种拟除虫菊酯类杀虫剂诊断剂量药膜纸后的死亡率 Figure 1 Mortality of field populations of Aedes albopictus exposed to papers impregnated with three pyrethroid insecticides at diagnostic doses in Guiyang, Guizhou Province, China |
| |
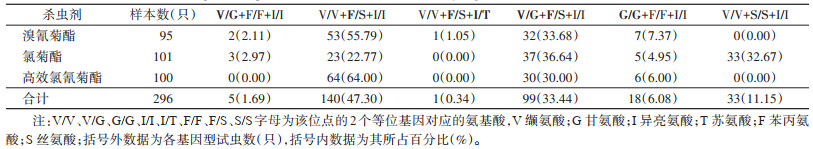
共检测316只白纹伊蚊的VGSC第Ⅱ结构域(1016位点)和第Ⅲ结构域(1532和1534位点)部分序列基因突变,20只敏感品系白纹伊蚊检测结果显示3个位点均无非同义突变(未分析同义突变),296只自然种群白纹伊蚊检测结果表明3个位点均存在非同义突变。1016位点有2种等位基因,一是编码缬氨酸的野生型GTA(V),占比76.35%(452/592),另一种是编码甘氨酸的突变型GGA(G),占比23.65%(140/592),其敏感表型与抗性表型对3种拟除虫菊酯类杀虫剂抗性突变基因频率差异无统计学意义(χ2=1.810,P=0.178);有3种基因型,包括野生型纯合子V/V,占比58.78%(174/296),野生/突变型杂合子V/G,占比35.14%(104/296),突变纯合子G/G,占比6.08%(18/296)。1532位点有2种等位基因,一是编码异亮氨酸的野生型ATC(I),占比99.83%(591/592),另一种是编码苏氨酸的突变型ACC(T),占比0.17%(1/592);有2种基因型,一是野生型纯合子I/I,占比99.66%(295/296),另一种是野生/突变型杂合子I/T,占比0.34%(1/296)。1534位点有2种等位基因,一是编码苯丙氨酸的野生型TTC(F),占比48.48%(287/592),另一种是编码丝氨酸的突变型TCC(S),占比51.52%(305/592),其敏感表型与抗性表型对3种拟除虫菊酯类杀虫剂抗性突变基因频率差异无统计学意义(χ2=0.603,P=0.437);有3种基因型,一是野生型纯合子F/F,占比8.11%(24/296),二是野生/突变型杂合子F/S,占比80.74%(239/296),另一种是突变型纯合子S/S,占比11.15%(33/296)。见表 2、3,图 2。
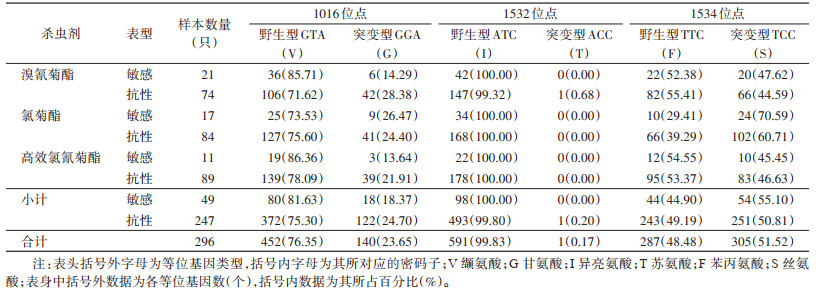
|
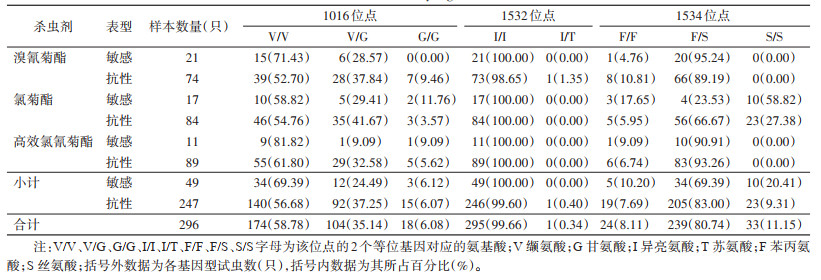
|

|
| 注:括号内为等位基因对应的基因型,/前后为等位基因对应的氨基酸;V 缬氨酸;G 甘氨酸;I 异亮氨酸;T 苏氨酸;F 苯丙氨酸;S 丝氨酸。 图 2 贵阳市3种杀虫剂处理的白纹伊蚊对击倒抗性突变测序峰图 Figure 2 A sequencing chromatogram of knockdown resistance gene mutations in Aedes albopictus populations exposed to three insecticides in Guiyang, Guizhou province, China |
| |
此次对白纹伊蚊3种拟除虫菊酯类杀虫剂抗性基因检测中仅发现1个样本在1532位点发生杂合突变(I/T),此样本同时是1534位点的杂合突变(F/S),占总样本数的0.34%;此外还发现了1016和1534位点同时突变的个体,占总样本数的33.44%。未发现1532纯合突变,仅发现1016和1534纯合突变,且F1534纯合突变仅发生在氯菊酯处理的测定样本中。见表 4。
|
白纹伊蚊是我国登革热传播的主要媒介。贵阳市居住人口多,大部分地区人口密集,白纹伊蚊孳生条件适宜。使用低毒且有效的拟除虫菊酯类杀虫剂(喷雾等)是控制白纹伊蚊最常用的手段,已在家庭、公园和绿地等人居环境广泛使用多年,杀虫剂抗药性的出现和发展是控制病媒传播疾病的最大挑战[11]。梁文琴等[12]曾对贵阳市白纹伊蚊的抗药性进行监测,发现其对拟除虫菊酯类杀虫剂已产生抗性。本次研究中,幼虫的抗药性结果表明,其对溴氰菊酯、氯菊酯和高效氯氰菊酯的抗性仍属于中高抗性,但LC50明显高于2016年的蚊虫监测数据[12],高效氯氰菊酯LC50约是2016年的2倍,溴氰菊酯LC50约是2016年的30倍,氯菊酯LC50约是2016年的18倍,可能与本次蚊虫收集的场所较多位于居民区附近,经常使用杀虫剂防治蚊虫有关。在这些地区,幼虫和成蚊阶段均可能持续暴露于不同浓度的拟除虫菊酯类杀虫剂下,因此产生了严重的抗药性。
此次研究中,贵阳市白纹伊蚊成蚊对3种拟除虫菊酯类杀虫剂均表现出抗性,与幼虫抗药性结果相似。此监测结果与伍思翰等[13]在同诊断剂量下对福建省厦门市的成蚊监测数据相比,白纹伊蚊成蚊对溴氰菊酯(21.88%)、氯菊酯(16.83%)和高效氯氰菊酯(11.00%)的死亡率明显低于厦门市的死亡率(分别为84.33%、72.60%和26.91%)。近年来由于诊断剂量的不统一和不确定性,无法准确判断贵阳市成蚊的抗药性变化,因此更加迫切需要从分子水平对抗药性进行抗性判断。
VGSC基因中的kdr突变是蚊虫对拟除虫菊酯类杀虫剂产生抗性的一个指标。此前在意大利、希腊和中国多地区的白纹伊蚊中报道过V1016G、I1532T和F1534S/L/C突变[10, 14-18]。本次研究中,在贵阳市白纹伊蚊野外种群中仅发现了V1016G、I1532T和F1534S突变,以F1534S突变为主,与我国多地区检测结果一致[13, 19-20];此外V1016G的突变频率(23.65%)也较高,与我国云南省景洪市检测到的突变频率(25.32%)相近[19],但在江苏省(0)[18]、福建省厦门市(12.28%)[13]和北京市(2.50%)[21]的突变频率相对较低,可能与种群间地理差异有关;此次仅发现1例I1532T突变(0.17%),且为杂合突变,未发现纯合突变,与伍思翰等[13]的研究结果一致。也有研究发现kdr基因突变与白纹伊蚊对拟除虫菊酯类杀虫剂所致的抗性表型相关。在我国,Liu等[22]研究证明了I1532T和F1534S突变与溴氰菊酯的抗性表现呈正相关,1016的突变频率普遍较低,但Kasai等[10]对白纹伊蚊的研究显示,V1016G与F1534S突变类型相比较具有更高的拟除虫菊酯抗性水平。本次研究发现敏感品系白纹伊蚊3个位点均未发生突变,自然种群敏感表型和抗性表型均存在一定程度的突变,并且存在2个位点同时突变的现象,但与拟除虫菊酯类杀虫剂抗性表型的抗性突变基因频率差异无统计学意义,可能与药膜诊断浓度未能准确区分抗性表型和敏感表型个体有关,也可能是存在多种抗性机制(代谢抗性等)的结果。也有研究认为,高频率使用各种类型的杀虫产品,是导致蚊虫种群多个位点同时突变的原因[23]。贵阳市白纹伊蚊的抗药性基因突变也可能是还存在其他类杀虫剂诱导所致,今后工作中可增加对其他类杀虫剂的抗药性检测。
在中国许多地区,白纹伊蚊幼虫和成虫已对拟除虫菊酯类杀虫剂产生抗性[24-26],本次贵阳市发现多击倒抗性基因突变,为延缓抗药性的产生和发展,应充分结合抗药性监测结果,正确选择杀虫剂并科学合理的使用。其次应持续加强抗药性监测,不断探索本地区蚊虫抗药性机制,为研究制定更加快速有效的抗药性检测方法、蚊虫控制的有效方案以及蚊媒传染病的防控提供一定的研究基础。
利益冲突 无
| [1] |
孟凤霞, 王义冠, 冯磊, 等. 我国登革热疫情防控与媒介伊蚊的综合治理[J]. 中国媒介生物学及控制杂志, 2015, 26(1): 4-10. Meng FX, Wang YG, Feng L, et al. Review on dengue prevention and control and integrated mosquito management in China[J]. Chin J Vector Biol Control, 2015, 26(1): 4-10. DOI:10.11853/j.issn.1003.4692.2015.01.002 |
| [2] |
Huang XY, Ma HX, Wang HF, et al. Outbreak of dengue fever in central China, 2013[J]. Biomed Environ Sci, 2014, 27(11): 894-897. DOI:10.3967/bes2014.125 |
| [3] |
Lin HX, Wang XT, Li ZG, et al. Epidemiological characteristics of dengue in mainland China from 1990 to 2019: A descriptive analysis[J]. Medicine (Baltimore), 2020, 99(36): e21982. DOI:10.1097/MD.0000000000021982 |
| [4] |
Djiappi-Tchamen B, Nana-Ndjangwo MS, Mavridis K, et al. Analyses of insecticide resistance genes in Aedes aegypti and Ae. albopictus mosquito populations from Cameroon[J]. Genes (Basel), 2021, 12(6): 828. DOI:10.3390/genes12060828 |
| [5] |
Silva JJ, Scott JG. Conservation of the voltage-sensitive sodium channel protein within the Insecta[J]. Insect Mol Biol, 2020, 29(1): 9-18. DOI:10.1111/imb.12605 |
| [6] |
Scott JG. Life and death at the voltage-sensitive sodium channel: Evolution in response to insecticide use[J]. Annu Rev Entomol, 2019, 64: 243-257. DOI:10.1146/annurev-ento-011118-112420 |
| [7] |
Kasai S, Ng LC, Lam-Phua SG, et al. First detection of a putative knockdown resistance gene in major mosquito vector, Aedes albopictus[J]. Jpn J Infect Dis, 2011, 64(3): 217-221. DOI:10.7883/yoken.64.217 |
| [8] |
Auteri M, La Russa F, Blanda V, et al. Insecticide resistance associated with kdr mutations in Aedes albopictus: An update on worldwide evidences[J]. Biomed Res Int, 2018, 2018: 3098575. DOI:10.1155/2018/3098575 |
| [9] |
王丹, 史鹏, 赵文平, 等. 贵州省兴义及赤水市白纹伊蚊抗药性监测分析[J]. 中国媒介生物学及控制杂志, 2021, 32(3): 302-306. Wang D, Shi P, Zhao WP, et al. Monitoring and analysis of insecticide resistance of Aedes albopictus in Xingyi and Chishui cities of Guizhou province, China[J]. Chin J Vector Biol Control, 2021, 32(3): 302-306. DOI:10.11853/j.issn.1003.8280.2021.03.009 |
| [10] |
Kasai S, Caputo B, Tsunoda T, et al. First detection of a Vssc allele V1016G conferring a high level of insecticide resistance in Aedes albopictus collected from Europe (Italy) and Asia (Vietnam), 2016: A new emerging threat to controlling arboviral diseases[J]. Euro Surveill, 2019, 24(5): 1700847. DOI:10.2807/1560-7917.ES.2019.24.5.1700847 |
| [11] |
Wei Y, Zheng XL, He S, et al. Insecticide susceptibility status and knockdown resistance (kdr) mutation in Aedes albopictus in China[J]. Parasit Vectors, 2021, 14(1): 609. DOI:10.1186/s13071-021-05095-5 |
| [12] |
梁文琴, 林懿, 黎红, 等. 贵阳市白纹伊蚊对常用杀虫剂的抗性研究[J]. 中华卫生杀虫药械, 2018, 24(4): 348-351. Liang WQ, Lin Y, Li H, et al. Resistance of Aedes albopictus to commonly used insecticides in Guiyang city of China[J]. Chin J Hyg Insect Equip, 2018, 24(4): 348-351. DOI:10.19821/j.1671-2781.2018.04.009 |
| [13] |
伍思翰, 周欣欣, 柯雪梅, 等. 福建省厦门市2020年白纹伊蚊对拟除虫菊酯类杀虫剂敏感性及击倒抗性基因研究[J]. 中国媒介生物学及控制杂志, 2022, 33(2): 177-182. Wu SH, Zhou XX, Ke XM, et al. Study on sensitivity and knockdown resistance genes of Aedes albopictus to pyrethroid insecticides in Xiamen, Fujian province, China, 2020[J]. Chin J Vector Biol Control, 2022, 33(2): 177-182. DOI:10.11853/j.issn.1003.8280.2022.02.003 |
| [14] |
Balaska S, Fotakis EA, Kioulos I, et al. Bioassay and molecular monitoring of insecticide resistance status in Aedes albopictus populations from Greece, to support evidence-based vector control[J]. Parasit Vectors, 2020, 13(1): 328. DOI:10.1186/s13071-020-04204-0 |
| [15] |
Wu YY, Liu QM, Qi YP, et al. Knockdown resistance (kdr) mutations I1532T and F1534S were identified in Aedes albopictus field populations in Zhejiang province, central China[J]. Front Cell Infect Microbiol, 2021, 11: 702081. DOI:10.3389/fcimb.2021.702081 |
| [16] |
Chen HY, Li KL, Wang XH, et al. First identification of kdr allele F1534S in VGSC gene and its association with resistance to pyrethroid insecticides in Aedes albopictus populations from Haikou city, Hainan Island, China[J]. Infect Dis Poverty, 2016, 5: 31. DOI:10.1186/s40249-016-0125-x |
| [17] |
杨罗菊, 刘德星, 陈健, 等. 中山市白纹伊蚊现场种群击倒抗性基因检测分析[J]. 中国人兽共患病学报, 2021, 37(2): 171-175. Yang LJ, Liu DX, Chen J, et al. Detection and analysis of the knockdown resistance gene in the field populations of Aedes albopictus in Zhongshan[J]. Chin J Zoonoses, 2021, 37(2): 171-175. DOI:10.3969/j.issn.1002-2694.2021.00.005 |
| [18] |
王冠熙, 李雅姝, 李月月, 等. 江苏省白纹伊蚊对溴氰菊酯抗性及击倒抗性突变分析[J]. 中国寄生虫学与寄生虫病杂志, 2022, 40(4): 468-474, 480. Wang GX, Li YS, Li YY, et al. Resistance to deltamethrin and knockdown resistance mutation in Aedes albopictus from Jiangsu province[J]. Chin J Parasitol Parasit Dis, 2022, 40(4): 468-474, 480. DOI:10.12140/j.issn.1000-7423.2022.04.008 |
| [19] |
朱彩英, 赵春春, 伦辛畅, 等. 云南省景洪市2018-2019年白纹伊蚊击倒抗性基因型分布研究[J]. 中国媒介生物学及控制杂志, 2020, 31(1): 7-11. Zhu CY, Zhao CC, Lun XC, et al. Distribution of knockdown resistance genotypes in Aedes albopictus in Jinghong, Yunnan province, China, 2018-2019[J]. Chin J Vector Biol Control, 2020, 31(1): 7-11. DOI:10.11853/j.issn.1003.8280.2020.01.002 |
| [20] |
李玉伟, 黄婧雯, 章灿明, 等. 福建省福州市和莆田市2020年白纹伊蚊击倒抗性基因突变分析[J]. 中国热带医学, 2021, 21(10): 952-955, 969. Li YW, Huang JW, Zhang CM, et al. Knockdown resistance gene mutations of Aedes albopictus from Fuzhou and Putian, Fujian, 2020[J]. China Trop Med, 2021, 21(10): 952-955, 969. DOI:10.13604/j.cnki.46-1064/r.2021.10.08 |
| [21] |
周小洁, 赵宇晗, 刘念, 等. 北京市两个白纹伊蚊种群kdr抗性突变检测分析[J]. 中华卫生杀虫药械, 2021, 27(4): 304-307. Zhou XJ, Zhao YH, Liu N, et al. Detection and analysis of kdr resistance mutations in two populations of Aedes albopictus in Beijing[J]. Chin J Hyg Insect Equip, 2021, 27(4): 304-307. DOI:10.19821/j.1671-2781.2021.04.003 |
| [22] |
Liu HM, Liu LH, Cheng P, et al. Bionomics and insecticide resistance of Aedes albopictus in Shandong, a high latitude and high-risk dengue transmission area in China[J]. Parasit Vectors, 2020, 13(1): 11. DOI:10.1186/s13071-020-3880-2 |
| [23] |
陈翰明, 高景鹏, 姜进勇, 等. 我国白纹伊蚊现场群体击倒抗性基因I1532和F1534突变检测及I1532T突变等位基因报告[J]. 中国媒介生物学及控制杂志, 2018, 29(2): 120-125. Chen HM, Gao JP, Jiang JY, et al. Detection of the I1532 and F1534 kdr mutations and a novel mutant allele I1532T in VGSC gene in the field populations of Aedes albopictus from China[J]. Chin J Vector Biol Control, 2018, 29(2): 120-125. DOI:10.11853/j.issn.1003.8280.2018.02.002 |
| [24] |
Li YJ, Zhou GF, Zhong DB, et al. Widespread multiple insecticide resistance in the major dengue vector Aedes albopictus in Hainan province, China[J]. Pest Manag Sci, 2021, 77(4): 1945-1953. DOI:10.1002/ps.6222 |
| [25] |
赵春春, 朱彩英, 贾清臣, 等. 2017-2018年我国不同区域白纹伊蚊对常用杀虫剂的抗药性[J]. 中国媒介生物学及控制杂志, 2020, 31(2): 126-132. Zhao CC, Zhu CY, Jia QC, et al. Resistance of Aedes albopictus to commonly used insecticides in different areas of China, 2017-2018[J]. Chin J Vector Biol Control, 2020, 31(2): 126-132. DOI:10.11853/j.issn.1003.8280.2020.02.002 |
| [26] |
Hou J, Liu QM, Wang JN, et al. Insecticide resistance of Aedes albopictus in Zhejiang province, China[J]. Biosci Trends, 2020, 14(4): 248-254. DOI:10.5582/bst.2020.03194 |
 2023, Vol. 34
2023, Vol. 34


