扩展功能
文章信息
- 傅菁文, 刘文娟, 张忠, 张瑞玲
- FU Jing-wen, LIU Wen-juan, ZHANG Zhong, ZHANG Rui-ling
- miR-276-3p在白纹伊蚊幼虫几丁质代谢调控中的作用研究
- Role of miR-276-3p in regulation of chitin metabolism in Aedes albopictus larvae
- 中国媒介生物学及控制杂志, 2023, 34(5): 589-595
- Chin J Vector Biol & Control, 2023, 34(5): 589-595
- 10.11853/j.issn.1003.8280.2023.05.001
-
文章历史
- 收稿日期: 2023-02-13
2 山东第一医科大学(山东省医学科学院)实验动物学院(省实验动物中心), 山东 济南 250000
2 School of Laboratory Animal & Shandong Laboratory Animal Center, Shandong First Medical University & Shandong Academy of Medical Sciences, Ji'nan, Shandong 250000, China
白纹伊蚊(Aedes albopictus),又名“亚洲虎蚊”,隶属于双翅目(Diptera)长角亚目(Nematocera)蚊科(Culicidae),是登革病毒、黄热病毒、基孔肯雅病毒和寨卡病毒等多种虫媒病毒的重要传播媒介,带来严重的公共卫生问题[1]。目前蚊虫防控的主要手段仍是使用化学杀虫剂,拟除虫菊酯类杀虫剂因其低毒、高效、低滞留等优点被广泛应用于蚊虫的防制[2],但长期大量使用杀虫剂,导致蚊虫出现严重的抗药性,并对环境造成不可逆的污染[3]。因此迫切需要环保无公害的蚊虫防控方法。
microRNAs(miRNAs)是一种存在于真核生物中,由21~24个核苷酸组成的非编码RNA[4],成熟的单链miRNA可与RNA诱导沉默复合物(RNA-induced silencing complex)结合,进而诱导信使RNA(mRNA)的降解和翻译抑制[5],在基因表达调控过程中发挥着至关重要的作用[6]。作为重要的转录后调控因子,miRNA在真核生物的发育、凋亡、细胞分化、繁殖等过程中具有重要作用[7]。在多种昆虫研究中发现,miRNA在昆虫个体发育中具有重要调控作用,如miR-8可以影响埃及伊蚊(Ae. aegypti)雌蚊卵黄原蛋白的表达,从而调控其卵巢发育[8];miR-14和miR-281可通过负反馈调节蜕皮激素受体EcR-B基因的表达而分别参与调控果蝇(Drosophila)和家蚕(Bombyx mori)的变态发育[9]。
几丁质是由N-乙酰-D-葡萄糖胺聚合而成的线性多糖[10],是昆虫表皮、气管和围食膜的主要结构成分[11]。昆虫在生长过程中需要进行周期性蜕皮,该过程包括旧表皮角质层降解和新角质层合成[12]。而几丁质是角质层外骨骼的重要组成部分,对蚊虫的生长和发育至关重要[13]。对白纹伊蚊全转录组测序数据的分析发现miR-276-3p在幼虫期高表达[14],且参与几丁质代谢调控,本研究对miR-276-3p在白纹伊蚊幼虫阶段调控几丁质代谢的作用进行实验验证,以期为利用几丁质代谢相关基因开展白纹伊蚊的防控提供新靶点。
1 材料与方法 1.1 样本收集白纹伊蚊蚊株于2017年采自山东省淄博市,在实验室环境中[温度:(26±1)℃;湿度:(75±5)%;光周期(L∶D)为14 h∶10 h]饲养。幼蚊以猪肝粉喂养,成蚊饲以10%蔗糖溶液,通过吸血昆虫饲喂系统(英国Hemotek)供以小鼠血液(北京索莱宝科技有限公司)为雌蚊提供血餐,以保证雌蚊正常产卵。
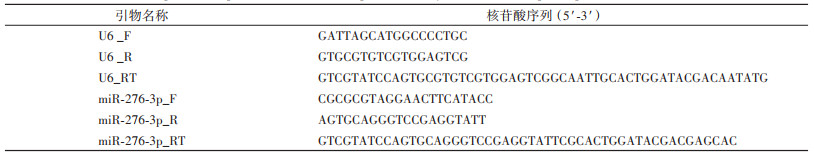
1.2 miR-276-3p特异性模拟物agomir和抑制剂antagomir合成分别设计合成miR-276-3p agomir和antagomir及其阴性对照(agomir NC和antagomir NC)(上海吉玛制药技术有限公司),序列信息见表 1。

|
将0.1 g壳聚糖加入到100 ml NaAc-HAc缓冲液(0.1 mol/L,pH=4.0)中以配制0.1%壳聚糖缓冲液;取1.5 ml EP管,分别加入32 μg miR-276-3p agomir、miR-276-3p antagomir[15]、agomir NC和antagomir NC,各组(管)均重复制备2份(共8份);随后每份样品中均加入1 μl硫酸钠溶液(2.5 mol/L),用无菌无酶水补足至100 μl,混匀后再加入100 μl壳聚糖溶液;随后将混合物置于55 ℃水浴箱静置1 min,迅速转至高速涡旋仪振荡30 s;在4 ℃条件下以13 000×g离心10 min,所得沉淀即为miRNA壳聚糖纳米颗粒,在室温下空气干燥约10 min。
取0.5 g琼脂粉(北京索莱宝科技有限公司),加入25 ml 1×TAE缓冲液,加热溶解,放入55 ℃水浴锅待用;待miRNA壳聚糖纳米颗粒干燥后,加入6 mg猪肝粉(100目筛过滤),用灭菌牙签混合;将40 μl 2%的预熔化琼脂糖凝胶溶液加入到猪肝粉与miRNA壳聚糖纳米颗粒混合物中,在55 ℃下再次用牙签混合,低速振荡涡旋混合均匀。凝胶凝固后立即使用或者4 ℃保存。
每组分别挑取90只白纹伊蚊2日龄幼虫,均放置于盛有50 ml去氯自来水的烧杯中。分别将上述4种壳聚糖纳米颗粒与琼脂糖凝胶的混合物用电子天平称重,随后根据其质量用无菌牙签将8份混合物每份均分成2等份,共得到16份等重混合物。分别取上述均分的混合物加到对应烧杯中饲喂幼蚊,每24 h等量饲喂1次,连续饲喂4 d;180只饲喂miR-276-3p agomir或miR-276-3p antagomir壳聚糖纳米颗粒的幼蚊为处理组,180只饲喂agomir NC或antagomir NC的幼蚊为对照组。
1.4 miR-276-3p表达水平检测分别收集饲喂miR-276-3p agomir和antagomir壳聚糖纳米颗粒后第1、3、5、7和9天的幼蚊样品,样品收集后立即置于液氮中冷冻,然后储存于-80 ℃冰箱备用。
使用miRNA Design软件(http://www.vazyme.com/companyfile/7/)设计miR-276-3p和内参基因U6的反转录实时荧光定量PCR(RT-qPCR)引物,引物序列交由北京六合华大基因科技有限公司合成,引物序列见表 2。依据RNA isolater Total RNA Extraction Reagent(南京诺唯赞生物科技股份有限公司,以下简称诺唯赞)说明书提取RNA;以提取的总RNA为模板,利用miRNA 1st-Strand cDNA Synthesis Kit(by stem-loop)试剂盒(诺唯赞)反转录生成cDNA;获得cDNA后,使用miRNA Universal SYBR qPCR Master Mix试剂盒(诺唯赞)进行RT-qPCR扩增,20 μl反应体系如下:2×miRNA Universal SYBR qPCR Master Mix 10 μl,正、反向引物各0.4 μl,cDNA模板2 μl,无酶无菌水7.2 μl,反应条件为:95 ℃预变性5 min;40个循环(95 ℃变性10 s,60 ℃退火30 s);溶解曲线采集(95 ℃ 15 s,60 ℃ 60 s,95 ℃ 15 s)。
|
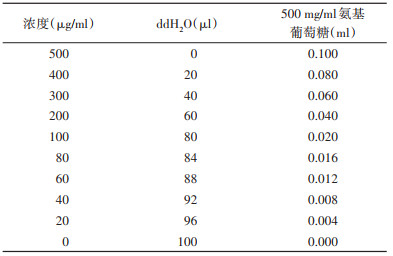
使用生化分析方法测定饲喂处理后的幼虫与蛹体内几丁质含量。分别收集饲喂miR-276-3p agomir/antagomir壳聚糖纳米颗粒后第9天的幼虫与化蛹后24 h的蚊蛹(每组、每个时间点取5只幼虫或3只蛹,设3个生物学重复)参考Arakane等[16]描述的方法测定几丁质含量。将收集样本65 ℃烘干1 h并称重;加入1 ml灭菌ddH2O充分研磨后将液体转移至EP管中;1 800×g离心15 min后往沉淀中加入400 μl十二烷基硫酸钠(SDS)溶液(3%),旋涡振荡使沉淀悬浮;悬浮液100 ℃水浴15 min,冷却后1 800×g离心10 min;弃上清,500 μl灭菌ddH2O洗涤沉淀后再次1 800×g离心10 min;再次弃上清并往沉淀中加入300 μl KOH溶液(120%),振荡使沉淀悬浮;随后140 ℃孵育40 min;冷却后加入800 μl冰乙醇(75%),冰上孵育15 min;加入30 μl硅藻土悬浮液并混匀后,4 ℃ 1 800×g离心5 min;沉淀分别用500 μl乙醇(40%)和500 μl灭菌ddH2O依次洗涤,加入100 μl灭菌ddH2O使沉淀悬浮后作为处理组,同时取100 μl灭菌ddH2O为对照组;2组均加入50 μl NaNO2溶液(10%)和50 μl KHSO4溶液(10%),室温孵育15 min,4 ℃ 1 800×g离心15 min;随后建立盐酸氨基葡萄糖(D-glucosamine hydrochloride)浓度测定标准曲线,将500 μg/ml的盐酸氨基葡萄糖溶液以不同的浓度梯度(表 3)加入96孔板中,并用灭菌ddH2O补至100 μl;分别吸取2组上清液与盐酸氨基葡萄糖溶液60 μl加入0.2 ml八连管中,加入20 μl NH4SO3NH2溶液(12.5%)剧烈振荡5 min;加入20 μl 3-甲基-2-苯并噻唑酮腙溶液(MBTH),离心至管底后于99 ℃孵育3 min,冷却后加入20 μl FeCl3溶液(0.83%);室温静置25 min后使用iMark酶标仪在630 nm波长下读取吸光度值。几丁质含量计算具体公式为:几丁质质量(mg)/组织质量(g)=氨基葡萄糖浓度(mg/ml)/组织质量(g)。
|
每组挑取30只2日龄幼蚊,共4组,每组设置3个生物学重复,从饲喂壳聚糖纳米颗粒1 d后每隔24 h统计其存活率。
分别选取饲喂agomir或antagomir后生存状态良好的幼蚊用于统计化蛹率,每隔24 h观察表型变化并统计化蛹数量,直至所有幼蚊均化蛹。
待所有幼蚊均化蛹后,分别选取饲喂agomir或antagomir壳聚糖纳米颗粒且化蛹后12 h的蚊蛹进行羽化率统计分析,每隔24 h观察表型变化并统计羽化数量,直至所有蚊蛹均羽化。
1.7 统计学分析采用2-∆∆Ct法计算miR-276-3p的相对表达水平;采用单因素方差分析的Tukey多重比较检验进行差异显著性分析,统计数据以平均值±标准差表示;采用乘积极限法(Kaplan-Meier method)的log-rank检验和Mantel-Cox检验估计生存率。
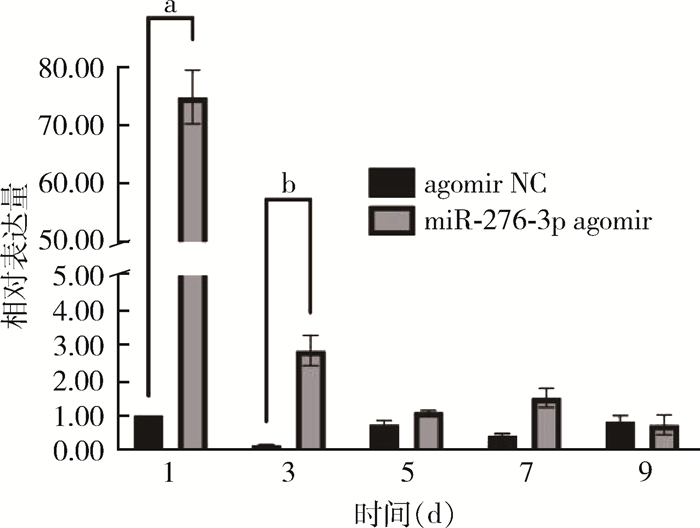
2 结果 2.1 miR-276-3p的表达量RT-qPCR检测结果显示,饲喂miR-276-3p agomir壳聚糖纳米颗粒后第1和3天的幼虫中miR-276-3p的表达量分别较对照组上调73.96%和16.95%,差异有统计学意义(F=932.401,P<0.001;F=114.551,P=0.047);miR-276-3p的表达量在第5、7和9天的幼蚊中与对照组相比差异无统计学意义(F=15.821,P=0.999;F=43.092,P=0.723;F=0.195,P=0.948)(图 1)。饲喂miR-276-3p antagomir壳聚糖纳米颗粒的幼虫中miR-276-3p的表达量在第1和5天分别较对照组下调13.42%(F=293.632,P<0.001)和1.49%(F=69.542,P=0.014);第3、7和9天与对照组相比差异均无统计学意义(F=2.206,P=0.972;F=17.900,P=0.982;F=25.614,P=0.863)。见图 2。
|
| 注:agomir NC 阴性对照组;miR-276-3p agomir 处理组;a P<0.001;b P<0.05。 图 1 饲喂agomir壳聚糖纳米颗粒后白纹伊蚊幼虫miR-276-3p的相对表达量 Figure 1 Relative expression of miR-276-3p in Aedes albopictus larvae after feeding with agomir chitosan nanoparticles |
| |

|
| 注:antagomir NC 阴性对照组;miR-276-3p antagomir 处理组;a P<0.001;b P<0.05。 图 2 饲喂antagomir壳聚糖纳米颗粒后白纹伊蚊幼虫miR-276-3p的相对表达量 Figure 2 Relative expression of miR-276-3p in Aedes albopictus larvae after feeding with antagomir chitosan nanoparticles |
| |
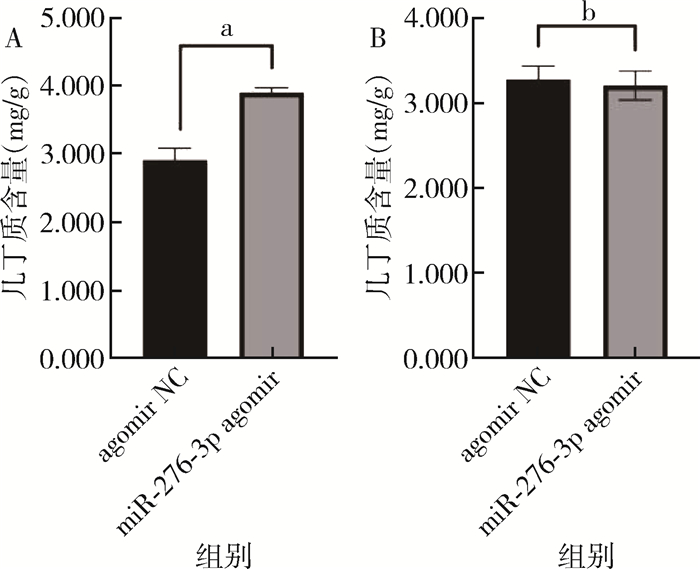
饲喂miR-276-3p agomir壳聚糖纳米颗粒后,幼虫的几丁质含量较对照组升高了25.34%(t=85.838,P=0.019),其中处理组样品的几丁质平均含量为3.950 mg/g,对照组为2.949 mg/g;处理组和对照组蚊蛹的几丁质含量差异无统计学意义(t=0.313,P=0.606)。见图 3。
|
| 注:agomir NC 阴性对照组;agomir-276-3p 处理组;a P<0.05;b P > 0.05。 图 3 饲喂miR-276-3p agomir壳聚糖纳米颗粒后白纹伊蚊幼虫(A)和蛹(B)的几丁质含量 Figure 3 Chitin content of Aedes albopictus larvae (A) and pupae (B) after feeding with miR-276-3p agomir chitosan nanoparticles |
| |
饲喂miR-276-3p antagomir壳聚糖纳米颗粒的幼虫与蛹的几丁质含量均显著下降,处理组幼虫与蛹的几丁质平均含量分别为5.229和2.615 mg/g,阴性对照组分别为8.724和4.917 mg/g;与阴性对照组比,处理组幼虫的几丁质含量下降了40.06%(t=71.811,P=0.028);处理组蛹的几丁质含量下降了46.82%(t=92.147,P=0.011)。见图 4。

|
| 注:antagomir NC 阴性对照组;antagomir-276-3p 处理组;a P<0.05。 图 4 饲喂miR-276-3p antagomir壳聚糖纳米颗粒后白纹伊蚊幼虫(A)和蛹(B)的几丁质含量 Figure 4 Chitin content of Aedes albopictus larvae (A) and pupae (B) after feeding with miR-276-3p antagomir chitosan nanoparticles |
| |
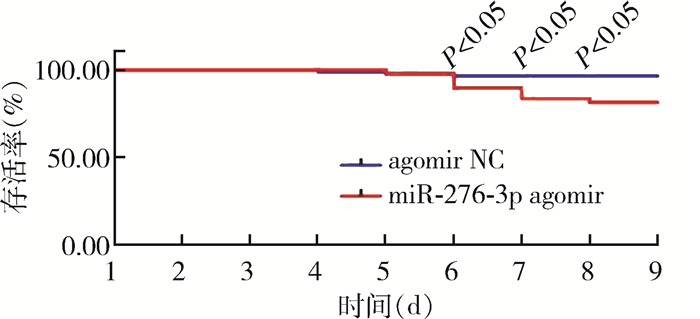
饲喂miR-276-3p agomir壳聚糖纳米颗粒后,白纹伊蚊幼虫存活率降低(表 4)。饲喂后第8天效果最为明显,处理组的存活率降低至80.00%,较对照组显著下降,差异有统计学意义(χ2=77.490,P=0.020)。见图 5。
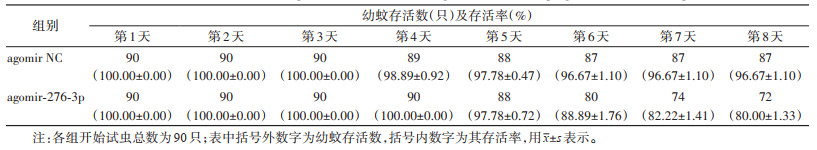
|
|
| 图 5 饲喂miR-276-3p agomir壳聚糖纳米颗粒后白纹伊蚊幼虫存活率 Figure 5 Survival rate of Aedes albopictus larvae after feeding with miR-276-3p agomir chitosan nanoparticles |
| |
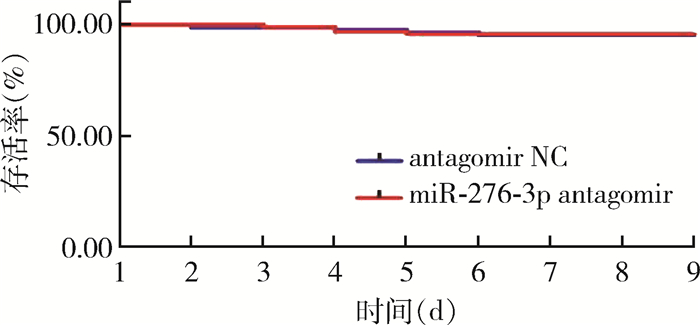
饲喂miR-276-3p antagomir壳聚糖纳米颗粒后,白纹伊蚊幼虫的存活率在第3天时为98.89%,第4天时是96.67%,第5天和第6天时均为95.56%,较阴性对照组差异无统计学意义(χ2=1.592,P=0.879)。见表 5、图 6。
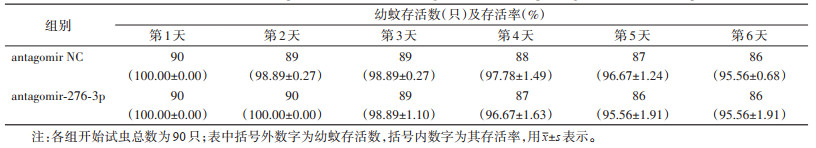
|
|
| 图 6 饲喂miR-276-3p antagomir壳聚糖纳米颗粒后幼虫存活率 Figure 6 Survival rate of Aedes albopictus larvae after feeding with miR-276-3p antagomir chitosan nanoparticles |
| |
饲喂miR-276-3p agomir壳聚糖纳米颗粒后幼虫化蛹率为70.00%,阴性对照组化蛹率为90.00%,且2组蚊蛹全部羽化。
饲喂miR-276-3p antagomir壳聚糖纳米颗粒后,处理组和对照组的化蛹率均为86.67%,且2组蚊蛹全部羽化。
3 讨论白纹伊蚊是世界上重要的虫媒病毒传播媒介之一。为解决化学杀虫剂造成其产生抗药性这一难题,通过筛选、验证调控蚊虫生长发育的关键miRNA作为靶标,研发RNA杀虫剂或生长抑制剂,进而降低蚊虫种群数量,或许是一种新型的蚊虫防控方法。
壳聚糖纳米颗粒作为载体用于递送活性成分可以增强活性成分的稳定性,促进其吸收,具有良好的生物相融性[17]。饲喂法可以更方便地达到基因表达调控的目的[18],且不会对幼虫造成损伤,因此本研究制作了miR-276-3p agomir/antagomir壳聚糖纳米颗粒,并通过饲喂的方式调控miR-276-3p的表达。结果显示在饲喂miR-276-3p agomir壳聚糖纳米颗粒后,白纹伊蚊幼虫体内miR-276-3p的表达量在第1、3、5和7天均有不同程度的上升,以第1天上升最为显著;饲喂miR-276-3p antagomir壳聚糖纳米颗粒后,miR-276-3p的表达量在第1和第5天均显著降低。
几丁质是自然界含量最丰富的有机化合物之一,普遍存在于多种真菌和节肢动物中[19]。在昆虫中,几丁质主要分布在表皮、围食膜和气管等组织中,在维持虫体形状和保护昆虫免受外界损害方面起着至关重要的作用[20]。Zhang等[21]通过对冈比亚按蚊(Anopheles gambiae)幼虫饲喂几丁质合成酶基因(AgCHS1和AgCHS2)的dsRNA纳米颗粒成功抑制了其表达;miR-8-5p和miR-2a-3p通过控制褐飞虱(Nilaparvata lugens)体内的20-羟基二酮(20E)从而调节几丁质代谢过程[22];miR-2703可以靶向调节褐飞虱的CHS1a,从而控制20-羟基蜕皮素信号通路以达到调节几丁质合成途径的目的[23];miR-71和miR-263可调控飞蝗(Locusta migratoria)的几丁质合成酶和几丁质酶进而控制其几丁质代谢[24]。在本研究中,用miR-276-3p agomir壳聚糖纳米颗粒饲喂白纹伊蚊幼虫后,几丁质含量测定结果表明处理组幼虫的几丁质含量较对照组显著增加,蛹期的几丁质含量则无显著变化,由此推测过表达miR-276-3p主要影响幼虫期的几丁质含量。此外,过表达miR-276-3p后白纹伊蚊幼虫存活率及化蛹率均降低,其中以第8天的存活率下降最明显,这可能是由于过表达miR-276-3p导致幼虫体内几丁质代谢紊乱,影响幼虫的生长发育,造成存活率降低。
本研究通过饲喂miR-276-3p agomir/antagomir壳聚糖纳米颗粒,以达到调控白纹伊蚊几丁质代谢相关基因miR-276-3p的目的,为利用白纹伊蚊幼虫几丁质代谢相关基因进行蚊虫防控提供了基础资料。
利益冲突 无
| [1] |
朱伟, 刘曜, 刘翔宇, 等. 白纹伊蚊对高效氯氰菊酯的抗性变化趋势与用药策略的初步研究[J]. 上海预防医学, 2022, 34(7): 699-704. Zhu W, Liu Y, Liu XY, et al. Trend in resistance to beta-cypermethrin in Aedes albopictus andpreliminary strategies of beta-cypermethrin use in Xuhui district, Shanghai[J]. Shanghai J Prev Med, 2022, 34(7): 699-704. DOI:10.19428/j.cnki.sjpm.2022.21659 |
| [2] |
杨苹, 王鑫炜. 几种拟除虫菊酯杀虫剂灭蚊效果观察[J]. 中华卫生杀虫药械, 2002, 8(4): 34-36. Yang P, Wang XW. Observation on the killing efficacy of the several pyrethroid insecticides against mosquitoes[J]. Chin J Hyg Insect Equip, 2002, 8(4): 34-36. DOI:10.3969/j.issn.1671-2781.2002.04.013 |
| [3] |
范苏云, 石向辉, 陈建, 等. 蚊浓核病毒亚致死剂量感染对白纹伊蚊溴氰菊酯抗药性的影响研究[J]. 中国媒介生物学及控制杂志, 2022, 33(4): 499-502. Fan SY, Shi XH, Chen J, et al. Effect of sublethal dose of mosquito densovirus infection on deltamethrin resistance of Aedes albopictus[J]. Chin J Vector Biol Control, 2022, 33(4): 499-502. DOI:10.11853/j.issn.1003.8280.2022.04.011 |
| [4] |
Lü J, Liu JH, Chen L, et al. Screening of brown planthopper resistant miRNAs in rice and their roles in regulation of brown planthopper fecundity[J]. Rice Sci, 2022, 29(6): 559-568. DOI:10.1016/j.rsci.2022.05.003 |
| [5] |
Stavast CJ, Erkeland SJ. The non-canonical aspects of microRNAs: Many roads to gene regulation[J]. Cells, 2019, 8(11): 1465. DOI:10.3390/cells8111465 |
| [6] |
Neshat SY, Tzeng SY, Green JJ. Gene delivery for immunoengineering[J]. Curr Opin Biotechnol, 2020, 66: 1-10. DOI:10.1016/j.copbio.2020.05.008 |
| [7] |
Moran Y, Agron M, Praher D, et al. The evolutionary origin of plant and animal microRNAs[J]. Nat Ecol Evol, 2017, 1(3): 27. DOI:10.1038/s41559-016-0027 |
| [8] |
Lucas KJ, Roy S, Ha JS, et al. MicroRNA-8 targets the wingless signaling pathway in the female mosquito fat body to regulate reproductive processes[J]. Proc Natl Acad Sci USA, 2015, 112(5): 1440-1445. DOI:10.1073/pnas.1424408112 |
| [9] |
Varghese J, Cohen SM. MicroRNA miR-14 acts to modulate a positive autoregulatory loop controlling steroid hormone signaling in Drosophila[J]. Genes Dev, 2007, 21(18): 2277-2282. DOI:10.1101/gad.439807 |
| [10] |
Liu XJ, Zhang HH, Li S, et al. Characterization of a midgut-specific chitin synthase gene (LmCHS2) responsible for biosynthesis of chitin of peritrophic matrix in Locusta migratoria[J]. Insect Biochem Mol Biol, 2012, 42(12): 902-910. DOI:10.1016/j.ibmb.2012.09.002 |
| [11] |
赵文静, 刘萍, 吴慧, 等. 致倦库蚊三个几丁质酶基因CqCht5-1, CqCht5-2和CqCht5-3的分析和表达[J]. 基因组学与应用生物学, 2021, 40(2): 563-568. Zhao WJ, Liu P, Wu H, et al. Characterization and expression analysis of three chitinase genes, CqCht5-1, CqCht5-2, and CqCht5-3, in Culex quinquefasciatus[J]. Genomics Appl Biol, 2021, 40(2): 563-568. DOI:10.13417/j.gab.040.000563 |
| [12] |
Liu XJ, Cooper AMW, Zhang JZ, et al. Biosynthesis, modifications and degradation of chitin in the formation and turnover of peritrophic matrix in insects[J]. J Insect Physiol, 2019, 114: 109-115. DOI:10.1016/j.jinsphys.2019.03.006 |
| [13] |
Merzendorfer H, Zimoch L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases[J]. J Exp Biol, 2003, 206(24): 4393-4412. DOI:10.1242/jeb.00709 |
| [14] |
Liu WJ, An S, Cheng P, et al. Whole-transcriptome profiling across different developmental stages of Aedes albopictus (Diptera: Culicidae) provides insights into chitin-related non-coding RNA and competing endogenous RNA networks[J]. Parasit Vectors, 2023, 16(1): 33. DOI:10.1186/s13071-022-05648-2 |
| [15] |
Zhang Q, Hua G, Adang MJ. Chitosan/DsiRNA nanoparticle targeting identifies AgCad1 cadherin in Anopheles gambiae larvae as an in vivo receptor of Cry11Ba toxin of Bacillus thuringiensis subsp. jegathesan[J]. Insect Biochem Mol Biol, 2015, 60: 33-38. DOI:10.1016/j.ibmb.2015.03.001 |
| [16] |
Arakane Y, Muthukrishnan S, Kramer KJ, et al. The Tribolium chitin synthase genes TcCHS1 and TcCHS2 are specialized for synthesis of epidermal cuticle and midgut peritrophic matrix[J]. Insect Mol Biol, 2005, 14(5): 453-463. DOI:10.1111/j.1365-2583.2005.00576.x |
| [17] |
陈文彬, 严文静, 徐幸莲, 等. α-生育酚壳聚糖纳米粒的制备、表征及体外缓释抗氧化性能[J]. 食品科学, 2017, 38(22): 216-223. Chen WB, Yan WJ, Xu XL, et al. Preparation, characterization and in vitro sustained antioxidant activity of α-tocopherol-loaded chitosan nanoparticles[J]. Food Sci, 2017, 38(22): 216-223. DOI:10.7506/spkx1002-6630-201722033 |
| [18] |
Tariq K, Metzendorf C, Peng W, et al. miR-8-3p regulates mitoferrin in the testes of Bactrocera dorsalis to ensure normal spermatogenesis[J]. Sci Rep, 2016, 6: 22565. DOI:10.1038/srep22565 |
| [19] |
Kramer KJ, Koga D. Insect chitin: Physical state, synthesis, degradation and metabolic regulation[J]. Insect Biochem, 1986, 16(6): 851-877. DOI:10.1016/0020-1790(86)90059-4 |
| [20] |
Moussian B. Recent advances in understanding mechanisms of insect cuticle differentiation[J]. Insect Biochem Mol Biol, 2010, 40(5): 363-375. DOI:10.1016/j.ibmb.2010.03.003 |
| [21] |
Zhang X, Zhang J, Zhu KY. Chitosan/double-stranded RNA nanoparticle-mediated RNA interference to silence chitin synthase genes through larval feeding in the African malaria mosquito (Anopheles gambiae)[J]. Insect Mol Biol, 2010, 19(5): 683-693. DOI:10.1111/j.1365-2583.2010.01029.x |
| [22] |
Chen J, Liang ZK, Liang YK, et al. Conserved microRNAs miR-8-5p and miR-2a-3p modulate chitin biosynthesis in response to 20-hydroxyecdysone signaling in the brown planthopper, Nilaparvata lugens[J]. Insect Biochem Mol Biol, 2013, 43(9): 839-848. DOI:10.1016/j.ibmb.2013.06.002 |
| [23] |
Chen J, Li T, Pang R. miR‐2703 regulates the chitin biosynthesis pathway by targeting Chitin synthase 1a in Nilaparvata lugens[J]. Insect Mol Biol, 2020, 29(1): 38-47. DOI:10.1111/imb.12606 |
| [24] |
Yang ML, Wang YL, Jiang F, et al. miR-71 and miR-263 jointly regulate target genes Chitin synthase and Chitinase to control locust molting[J]. PLoS Genet, 2016, 12(8): e1006257. DOI:10.1371/journal.pgen.1006257 |
 2023, Vol. 34
2023, Vol. 34


