2. School of Physics, University of Chinese Academy of Sciences, Beijing 100049, China
2. 中国科学院大学物理科学学院, 北京 100049
The development of rechargeable batteries, particularly rechargeable lithium-ion battery (LIB), greatly promotes the application of mobile electronic devices and environmentally friendly vehicles[1-5], which is subverting the human's life style and the way of energy application, initiating a new chapter of the new energy area. In order to further improve the performance of LIB, it is urgent to develop advanced electrode materials that provide rapid charging/ discharging rate, cyclic stability, satisfactory capacity, and safety[6-12].
In the past several decades, graphite has been used as traditional anode material, but its energy capacity (372mAh·g-1) and rate of Li are still far away from what are expected to meet the requirements of many applications[13-14]. Recent years, more and more attentions were focused on two-dimensional (2D) layered materials with the characteristics of high specific surface area, large interlayer spacing, and other peculiar properties[15-17]. For example, the well-studied graphene exhibits the unique capacity (774mAh·g-1) because of its high charge carrier mobility, large surface area, and a broad electrochemical window[18-22]. Up to date, graphene has been utilized as both cathode and anode materials with great success[23-27]. MoS2 has been explored to exhibit a high reversible lithium storage capacity and superior rate capability[28-33]. Black phosphorus was shown to have a series of good characteristics as Li-battery electrode[34-36]. With the decreasing of dimensionality, the 2D phosphorene has been validated as ideal anode material with a low energy barrier (0.08eV) of Li and higher average charging voltage (2.9V) than other 2D materials[37-39]. In addition, the group Ⅳ-Ⅵ compounds MX (M=Ge, Sn; X=S or Se), which display puckered structures similar to black phosphorus and form buckled honeycomb lattices, has received much attention as electrode materials of LIB[40-44]. Based on the interlayer spacing from 3.1 Å to 3.26 Å, their structural anisotropy directly leads to anisotropy of the migration direction of lithium ion. Sn-based materials, like SnS2 and SnO2 composites, provide ideal space for Li atom intercalation and exhibit high reversible capability and good cycling performances when they are used as anode materials[45-48]. However, as far as we know, the properties and performance of SnSe2 as electrode material have not been studied, while SnSe2 presents prominent characteristic of large interlayer spacing up to 4.175 and hence provides more possibility for the adsorption and diffusion of the Li ions. In this work, the Li-ion adsorption properties and migration mechanism in monolayer/bilayer/bulk SnSe2 materials have been systematically investigated.
Our study shows that the binding energy is significantly higher than that on graphene, phosphorene, MoS2, and some other two-dimensional (2D) layered materials, indicating a strong interaction between the Li atom and SnSe2 substrate. Bader charge analysis reveals that Li exists in cationic state with its 2s electron being completely transferred to SnSe2. The extremely low energy barrier (0.197eV) on the monolayer SnSe2 guarantees rapid diffusion of the Li atom. Due to the unique anionic framework structure of bulk SnSe2 and formation of the Oh-Th-Oh migration channel for the Li ion, the energy barrier arises to 0.483eV. Moreover, a remarkably large average voltage of 3.05V is predicted, which is even lerger than on phosphorene (2.9V). The intercalation of Li leads to a transition from semiconductor to metallic state, which gives rise to a good electrical conductivity. These findings provide insights into the Li-ion adsorption properties and migration mechanism in layered transition-metal dichalcogenides (TMDS).
1 Computational methodsAll the calculations were performed using Vienna ab initio simulation package (VASP) based on density functional theory[49]. The interactions between ion cores and valence electrons were described using the projector augmented wave (PAW) potentials[50]. The Perdew-Burke-Ernzerhof (PBE) generalized gradient approximation (GGA) was carried out for the electron exchange-correlation interactions[51-52]. The cutoff of plane-wave kinetic energy was set to be 550eV for all the calculations. Atomic relaxation was performed with the convergence of total energy less than 10-5eV and all the forces on each atom smaller than 0.0 1eV/ Å. The climbing image nudged elastic band method (CI-NEB) was used to search the minimum energy path (MEP) of Li migration[53]. For the Li adsorption and diffusion studies, we selected supercell containing 4×4 primitive cells for eliminating the interaction between Li atoms. For the monolayer/bilayer SnSe2, we set a vacuum space of 20 Å between adjacent layers to avoid mirror interaction. K-point meshes of 5×5×1 for monolayer/bilayer SnSe2 and 5×5×5 for bulk SnSe2 were employed in all calculations, and denser K-point meshes of 12×12×1 and 12×12×10 were adopted for monolayer and bulk band structures. Bader charge analysis[54] method was used to estimate the charge transfer between Li and substrate SnSe2. In addition, van der Waals correction was taken into account using the D2 method of Grimme[55] in the bilayer and bulk calculations.
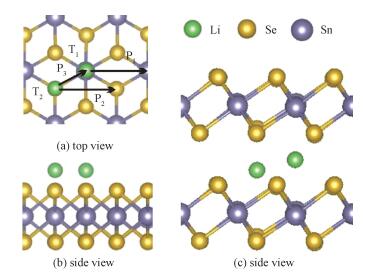
2 Results and discussion 2.1 Adsorption of Li on monolayer SnSe2 surface and in bilayer/bulk SnSe2Bulk SnSe2 possesses the typical layered TMDS structure, in which per SnSe2 layer has the sandwich-like structure with the Sn layer sandwiched between two Se layers, as shown in Fig. 1.
|
Download:
|
|
Fig. 1 Top (a) and side (b) views for Li adsorbed on monolayer, and Li in bulk (c) |
|
We investigated the adsorption of one Li atom on the surface of monolayer SnSe2 and in the bilayer/bulk SnSe2. In Fig. 1(a) and 1(b) shown are the two possible adsorption sites T1 and T2 on SnSe2 monolayer. The T1 site is directly above the Sn atom that is located in the second atomic layer and the T2 site is directly above the Se atom that is located in the third atomic layer. As for bilayer/bulk SnSe2, there are also two possible adsorption sites: the tetrahedral (Th) site formed by three Se atoms from lower triple layers and one Se atom from upper layer and the octahedral (Oh) site at which Li binds three Se atoms from the triple layers as shown in Fig. 1(c). The binding energy (Eb) was defined
| $ {E_{\rm{b}}} = {E_{{\rm{Li - SnSe}}}}_{_2} - {E_{{\rm{SnSe}}}}_{_2} - {E_{{\rm{Li}}}}, $ | (1) |
where ELi-SnSe2, ESnSe2, and ELi are the total energies of Li-adsorbed SnSe2, SnSe2, and a Li atom, respectively. A negative value of Eb suggests an exothermic reaction, and a more absolute value of Eb indicates a stronger interaction between Li and SnSe2. The values of Eb for Li are -3.01eV (T1) and -2.98eV (T2) on monolayer SnSe2, -3.52eV (Th) and -3.56eV (Oh) in bilayer SnSe2, and -3.70eV (Th) and -3.91eV (Oh) in bulk SnSe2, which are significantly higher than those on graphene, phosphorene, MoS2, and some other two-dimensional (2D) layered materials.
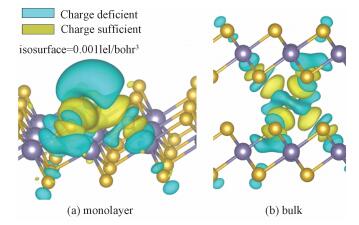
The spatial distributions of the charge difference between Li and SnSe2 for monolayer/bulk SnSe2 are illustrated in Fig. 2. The large charge deciency at Li and the charge excess around nearby the Se atom indicate strong electron transfer from Li to SnSe2. Bader charge analysis reveals that Li transferred almost the whole charge of 2s electron to SnSe2 (see Table 1) and thus exists in the cationic state, which gives an explanation for the strong interaction between Li and SnSe2.
|
Download:
|
|
Fig. 2 Charge density difference between Li and substrate SnSe2 |
|
|
|
Table 1 Transferred charges of Li at T1 (q1) and T2 (q2) sites on monolayer and at Th (q1) and Oh (q2) sites in bulk SnSe2 |
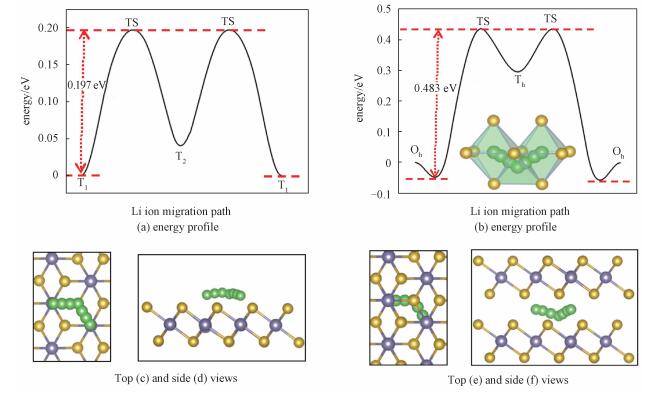
The performances on charging/discharging and circuit rate in Li ion battery are highly dependent on the diffusivity of Li atom in the electrode material. As shown in Fig. 1(a), three possible diffusion pathways (P1: Sn-Sn; P2: Se-Se; P3: Se-Sn) are considered to explore the optimistic migration path on monolayer SnSe2 surface. As shown in Fig. 3(a), the Li ion migrates with a remarkably low barrier of only 0.197eV along path P1 from one T1 site to another T1 site, passing through a T2 site (Fig. 3(c) and (d)). Compared with the other 2D materials, such as MoS2, VS2, and graphene, the Li diffusion is essentially isotropic in the plane, and the migration barriers are 0.25eV on MoS2[28], 0.22eV on VS2[56], and 0.327eV on graphene[23], showing that the monolayer SnSe2 is more favorite for Li ion migration. For bulk SnSe2, it is found that Li ions prefer the Oh-Th-Oh migration path[57], which connects two neighboring Oh sites by passing through a face-sharing Th site, as shown in Fig. 3(b), 3(e), and 3(f). The corresponding energy barrier arises to 0.483eV, implying that the diffusion of the Li ions on surface of SnSe2 are much faster than in bulk SnSe2.
|
Download:
|
|
Fig. 3 Energy profiles and schematic representations for Li diffusion in monolayer (a, c, d) and bulk SnSe (b, e, f) |
|
The open-circuit-voltage (OCV) is widely used to characterize the charging/discharging performance of Li ion battery. Thus, to further understand the performance of SnSe2-based Li ion battery, the open-circuit-voltage has been derived. In theory, the open circuit voltage curve can be obtained by calculating the average voltages over different Li concentrations. The charging/discharging process of SnSe2-based anode can be assumed as
| $ {\rm{L}}{{\rm{i}}_{x1}}A + ({x_2} - {x_1}){\rm{L}}{{\rm{i}}^ + } + ({x_2} - {x_1}){{\rm{e}}^ - } \leftrightarrow {\rm{L}}{{\rm{i}}_{x2}}A. $ | (2) |
Therefore, the average voltage of LixSnSe2 in the x1≤ x ≤ x2 range can be evaluated using equation
| $ V \approx \frac{{{E_{{\rm{Li}}}}{{_{_{x1}}}_{{\rm{SnSe}}}}_{_2} - {E_{{\rm{Li}}}}{{_{_{x2}}}_{{\rm{SnSe}}}}_{_2} + ({x_2} - {x_1}){E_{{\rm{Li}}}}}}{{({x_2} - {x_1}){\rm{e}}}}, $ | (3) |
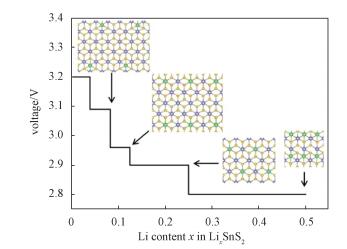
whereELix1SnSe2, ELix2SnSe2, and ELi are the energies of Lix1SnSe2, Lix2SnSe2, and metallic Li, respectively. In this work, a series of LixSnSe2 (x=0.04, 0.083, 0.125, 0.25, 0.5) are considered by adopting the 1×1, 2×2, 2×4, 3×4, and 5×5 supercells with one Li atom absorbed on monolayer SnSe2 surface. The voltage profile and geometrical configuration are shown in Fig. 4, and there is a slight drop from 3.2V to 2.84V. The calculated average voltage by averaging numerically the voltage profile is 3.05V in the 0≤x≤ 0.25 range, which is obviously higher than the values of 2.9V for phosphorene[38], 1.5V for graphite and TiO2[25], and 0.93V for VS2[56], indicating that the monolayer SnSe2 provides a higher charging voltage. Furthermore, for the intercalation of Li in bulk-SnSe2, a series of Li concentrations were considered by using 1×1, 2×2, 2×4, 3×4, and 5×5 supercells with one Li atom intercalated in bulk-SnSe2. The obtained average OCV is about 3.7V, which is even higher than the value for monolayer SnSe2. Besides, the OCV does not drop a lot when the Li concentration increases from 0.04 to 0.25.
|
Download:
|
|
Fig. 4 Calculated voltage profile with respect to Li content from 0 to 0.5 |
|
As we know, the Li ion battery has been one of promising battery systems, used to power a great deal of devices in many applications, such as low-current cells applied in portable electronics and memory backup and high-current cells used in military applications. Thus, it is essential for us to investigate the electronic properties of electrode materials.
As revealed by Bader charge analysis, the Li atoms transfer almost the whole charge of 2s electron to substrate SnSe2, which affects the electronic structure of the whole system distinctly, such as shift of Fermi energy level compared to the original band structures. The electronic band structures of SnSe2 with different Li concentrations have been calculated, and the results are shown in Fig. 5. Obviously, Fermi energy levels shift up with increasing of Li concentration, and the whole system transits from original semiconducting state to metallic state during the process of Li intercalation, giving rise to a good electrical conductivity.
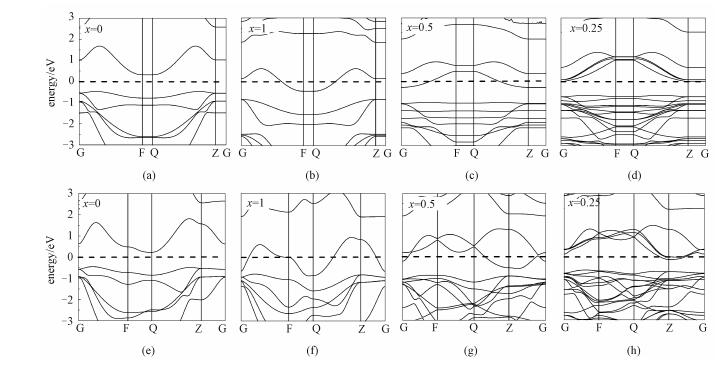
|
Download:
|
|
Dashed lines denote the Fermi level. Fig. 5 Electronic band structures of the LixSnSe2 monolayer (a, b, c, d) and bulk (e, f, g, h) |
|
In conclusion, based on first-principle calculations, we performed a systematically study on the adsorption properties and migration mechanism of the Li atoms in monolayer/bilayer/bulk SnSe2 systems. Our study shows that the Li atom is able to form stable adsorption with SnSe2 with a higher binding energy than with some common 2D layered materials. Upon adsorption, the Li atom transfers its 2s electron to substrate SnSe2 and exists in the cationic state. The adsorption complex becomes metallic with Li interaction, giving rise to a good electrical conductivity, which is essential for an electrode. The energy barrier is only 0.197eV for Li in monolayer SnSe2, significantly lower than those on other 2D materials, such as MoS2, graphene, SnS2, VS2, etc. Due to the unique anionic framework of bulk SnSe2, the Oh-Th-Oh migration channel turns out to be preferred. Furthermore, the average voltage for monolayer SnSe2 has been estimated to be 3.05V, which is obviously suitable for high charging voltage applications. On the basis of our present findings, monolayer SnSe2 is expected to be one of candidates for electrode materials. These findings provide insights into the Li-ion adsorption properties and migration mechanism in layered TMDS.
Appendix

|
Download:
|
|
The dashed lines denote the Fermi levels. Fig. S1 Electronic band structures of the bulk LixSnSe2 without vdW corrections (a, b, c) and with vdW corrections (d, e, f) |
|
| [1] |
Goodenough J B, Park K S. The Li-ion rechargeable battery:a perspective[J]. Journal of the American Chemical Society, 2013, 135(4): 1167-1176. DOI:10.1021/ja3091438 |
| [2] |
Manthiram A, Fu Y, Yusheng S. In charge of the world:electrochemical energy storage[J]. Journal of Physical Chemistry Letters, 2013, 4(8): 1295-1297. DOI:10.1021/jz4006652 |
| [3] |
Song K, Seo D H, Jo M R, et al. Tailored oxygen framework of Li4Ti5O12 nanorods for high-power Li ion battery[J]. Journal of Physical Chemistry Letters, 2014, 5(8): 1368-1373. DOI:10.1021/jz5002924 |
| [4] |
Takamatsu D, Nakatsutsumi T, Mori S, et al. Nanoscale observation of the electronic and local structures of LiCoO2thin film electrode by depth-resolved X-ray absorption spectroscopy[J]. Journal of Physical Chemistry Letters, 2011, 2(20): 2511-2514. DOI:10.1021/jz2011226 |
| [5] |
Meini S, Elazari R, Rosenman A, et al. The use of Redox mediators for enhancing utilization of Li2S cathodes for advanced Li-S battery systems[J]. Journal of Physical Chemistry Letters, 2014, 5(5): 915-918. DOI:10.1021/jz500222f |
| [6] |
Idota Y, Kubota T, Matsufuji A, et al. Tin-based amorphous oxide:a high-capacity lithium-ion-storage material[J]. Science, 1997, 276(5317): 1395-1397. DOI:10.1126/science.276.5317.1395 |
| [7] |
Tarascon J M, Armand M. Issues and challenges facing rechargeable lithium batteries[J]. Nature, 2001, 414(6861): 359-367. DOI:10.1038/35104644 |
| [8] |
Oyama N, Tatsuma T, Sato T, et al. Dimercaptan-polyaniline composite electrodes for lithium batteries with high energy density[J]. Nature, 1995, 373(6515): 598-600. DOI:10.1038/373598a0 |
| [9] |
Armand M, Tarascon J M. Building better batteries[J]. Nature, 2008, 451(7179): 652-657. DOI:10.1038/451652a |
| [10] |
Dubal D P, Ayyad O, Ruiz V, et al. Hybrid energy storage:the merging of battery and supercapacitor chemistries[J]. Chemical Society Reviews, 2015, 44: 1777-1790. DOI:10.1039/C4CS00266K |
| [11] |
Simon P, Gogotsi Y, Dunn B. Where Do Batteries end and supercapacitors begin[J]. Science Magazine, 2014, 343(6176): 1210-1211. |
| [12] |
Arico A S, Bruce P, Scrosati B, et al. Nanostructured materials for advanced energy conversion and storage devices[J]. Nature Materials, 2005, 4: 366-377. DOI:10.1038/nmat1368 |
| [13] |
Dahn J R, Zheng T, Liu Y, et al. Mechanisms for lithium insertion in carbonaceous materials[J]. Science, 1995, 270(5236): 590-593. DOI:10.1126/science.270.5236.590 |
| [14] |
Winter M, Besenhard J O, Spahr M E, et al. Insertion electrode materials for rechargeable lithium batteries[J]. Advance Materials, 1998, 10(10): 725-763. DOI:10.1002/(ISSN)1521-4095 |
| [15] |
Fang W Z, Zhang L C, Yan Q B, et al. Effects of strain on mechanical and electronic properties of SnSe and SnS auxetic materials[J]. Journal of University of Chinese Academy of Sciences, 2017, 34(1): 8-14. |
| [16] |
Kang Y, Gong Y J, Hu Z J, et al. Plasmonic hot electron enhanced MoS2 photocatalysis in hydrogen evolution[J]. Nanoscale, 2015, 7: 4482-4488. DOI:10.1039/C4NR07303G |
| [17] |
Kang Y, Najmaei S, Liu Z, et al. Plasmonic hot electron induced structural phase transition in a MoS2 Monolayer[J]. Advanced Materials, 2014, 26(37): 6467-6471. DOI:10.1002/adma.201401802 |
| [18] |
Peigney A, Laurent Ch, Flahaut E, et al. Specific surface area of carbon nanotubes and bundles of carbon nanotubes[J]. Carbon, 2001, 39(4): 507-514. DOI:10.1016/S0008-6223(00)00155-X |
| [19] |
Novoselov K S, Geim A K, Jiang D, et al. Electric field effect in atomically thin carbon films[J]. Science, 2004, 306(5696): 666-669. DOI:10.1126/science.1102896 |
| [20] |
Trushin M, Schliemann J. Minimum Electrical and thermal conductivity of graphene:a quasiclassical approach[J]. Physical Review Letters, 2007, 99(21): 216602. DOI:10.1103/PhysRevLett.99.216602 |
| [21] |
Allen M J, Tung V C, Kaner R B. Honeycomb carbon:a review of graphene[J]. Chemical Reviews, 2010, 110(1): 132-145. DOI:10.1021/cr900070d |
| [22] |
Khantha M, Cordero N A, Molina L M, et al. Interaction of lithium with graphene:an ab initio study[J]. Physical Review B, 2004, 70(12): 125422. DOI:10.1103/PhysRevB.70.125422 |
| [23] |
Fan X F, Zheng W T, Kuo J L. Adsorption and diffusion of Li on pristine and defective graphene[J]. ACS Applied Materials & Interfaces, 2012, 4(5): 2432-2438. |
| [24] |
Liu Y, I V L, Liu M J, et al. Feasibility of lithium storage on graphene and its derivatives[J]. Journal of Physical Chemistry Letters, 2013, 4(10): 1737-1742. DOI:10.1021/jz400491b |
| [25] |
Jing Y, Zhou Z, Cabrera C R, et al. Graphene, inorganic graphene analogs and their composites for lithium ion batteries[J]. Journal of Materials Chemistry A, 2014, 2: 12104-12122. DOI:10.1039/C4TA01033G |
| [26] |
Wan W, W H D. First-Principles Investigation of adsorption and diffusion of ions on pristine, defective and B-doped Graphene[J]. Materials, 2015, 8(9): 6163-6178. DOI:10.3390/ma8095297 |
| [27] |
Lee S K, Rana K, Ahn J H. Graphene films for flexible organic and energy storage devices[J]. Journal of Physical Chemistry Letters, 2013, 4(5): 831-841. DOI:10.1021/jz400005k |
| [28] |
Li Y F, Wu D H, Zhou Z, et al. Enhanced Li adsorption and diffusion on MoS2 zigzag nanoribbons by edge effects:a computational study[J]. Journal of Physical Chemistry Letters, 2012, 3(16): 2221-2227. DOI:10.1021/jz300792n |
| [29] |
Ding S, Zhang D, Chen J S, et al. Facile synthesis of hierarchical Mo S2 microspheres composed of few-layered nanosheets and their lithium storage properties[J]. Nanoscale, 2012, 4: 95-98. DOI:10.1039/C1NR11552A |
| [30] |
Du G, Guo Z, Wang S, Zeng R, et al. Superior stability and high capacity of restacked molybdenum disulfide as anode material for lithium ion batteries[J]. Chemical Communication, 2010, 46: 1106-1108. DOI:10.1039/B920277C |
| [31] |
Hwang H, Kim H, Cho J. MoS2 Nanoplates consisting of disordered graphene-like layers for high rate lithium battery anode materials[J]. Nano Letters, 2011, 11(11): 4826-4830. DOI:10.1021/nl202675f |
| [32] |
Liu H, Su D, Zhou R, et al. Highly ordered mesoporous MoS2 with expanded spacing of the (002) crystal plane for ultrafast lithium ion storage[J]. Advanced Energy Materials, 2012, 2(8): 970-975. DOI:10.1002/aenm.v2.8 |
| [33] |
Julien C, Saikh S I, Nazri G A. Electrochemical studies of disordered MoS2 as cathode material in lithium batteries[J]. Material Science and Engineering B, 1992, 15(1): 73-77. DOI:10.1016/0921-5107(92)90034-7 |
| [34] |
Gen C G, Wei X L, Wang D, et al. Pristine and defect-containing phosphorene as promising anode materials for rechargeable Li batteries[J]. Journal of Materials Chemistry A, 2015, 3: 11246-11252. DOI:10.1039/C5TA01661D |
| [35] |
Yao Q S, Huang C X, Yuan Y B, et al. Theoretical prediction of phosphorene and nanoribbons as fast-charging Li ion battery anode materials[J]. Journal of Physical Chemistry C, 2015, 119(12): 6923-6928. DOI:10.1021/acs.jpcc.5b02130 |
| [36] |
Li W J, Chou S L, Wang J Z, et al. Simply mixed commercial red phosphorus and carbon nanotube composite with exceptionally reversible sodium-ion storage[J]. Nano Letters, 2013, 13(11): 5480-5484. DOI:10.1021/nl403053v |
| [37] |
Liu H, Neal A T, Zhu Z, et al. Phosphorene:an unexplored 2D semiconductor with a high hole mobility[J]. ACS Nano, 2014, 8(4): 4033-4041. DOI:10.1021/nn501226z |
| [38] |
Li W F, Yang Y M, Zhang G, et al. Ultrafast and directional diffusion of lithium in phosphorene for high-performance lithium-ion battery[J]. Nano Letters, 2015, 15(3): 1691-1697. DOI:10.1021/nl504336h |
| [39] |
Zhang R Q, Wu X J, Yang J L. Blockage of ultrafast and directional diffusion of Li atoms on phosphorene with intrinsic defects[J]. Nanoscale, 2016, 8: 4001-4006. DOI:10.1039/C5NR06856H |
| [40] |
Karmakar S, Chowdhury C, Datta A. Two-dimensional group Ⅳ monochalcogenides:anode materials for Li-ion batteries[J]. Journal of Physical Chemistry C, 2016, 120(27): 14522-14530. DOI:10.1021/acs.jpcc.6b04152 |
| [41] |
Im H S, Lim Y R, Cho Y J, et al. Germanium and Tin Sele nide nanocrystals for high-capacity lithium ion batteries:comparative phase conversion of germanium and tin[J]. Journal of Physical Chemistry C, 2014, 118(38): 21884-21888. DOI:10.1021/jp507337c |
| [42] |
Kim Y J, Kim Y L, Park Y, et al. SnSe alloy as a promising anode material for Na-ion batteries[J]. Chemical Communications, 2015, 51: 50-53. DOI:10.1039/C4CC06106C |
| [43] |
Zhu Z, Guan J, Liu D, et al. Designing isoelectronic counterparts to layered group V semiconductors[J]. ACS Nano, 2015, 9(8): 8284-8290. DOI:10.1021/acsnano.5b02742 |
| [44] |
Gomes L C, Carvalho A. Phosphorene analogues:isoelectronic two-dimensional group-Ⅳ monochalcogenides with orthorhombic structure[J]. Physical Review B, 2015, 92(8): 085406. DOI:10.1103/PhysRevB.92.085406 |
| [45] |
Zhuo L H, Wu Y Q, Wang L Y, et al. One-step hydrothermal synthesis of SnS2/graphene composites as anode material for highly efficient rechargeable lithium ion batteries[J]. RSC Advances, 2012, 2: 5084. DOI:10.1039/c2ra00002d |
| [46] |
Shi J, Shi W, Jin W, et al. Diffusion of lithium in α-Sn and β-Sn as anode materials for lithium ion batteries[J]. International Journal of Electrochemical Science, 2015, 10: 4793-4800. |
| [47] |
Gao P, Wang L P, Zhang Y, et al. High-resolution tracking asymmetric lithium insertion and extraction and local structure ordering in SnS2[J]. Nano Letters, 2016, 16(9): 5582-5588. DOI:10.1021/acs.nanolett.6b02136 |
| [48] |
Huang Y C, Ling H Y, Chen X, et al. SnS2 nanotubes:a promising candidate for theanode material for lithium ion batteries[J]. RSC Advances, 2015, 5: 32505-32510. DOI:10.1039/C5RA01211B |
| [49] |
Kresse G, Furthmuller J. Efficienct iterative schemes for initio total-energy calculations using a plane-wave basis set[J]. Physical Review B, 1996, 54(16): 11169. DOI:10.1103/PhysRevB.54.11169 |
| [50] |
Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method[J]. Physical Review B:Condensed Matter Mater. Phys, 1999, 59(3): 1758. DOI:10.1103/PhysRevB.59.1758 |
| [51] |
Vanderbilt D. Soft Self-consistent pseudopotentials in a generalized eigenvalue formalism[J]. Physical Review B:Condensed Matter Mater. Phy, 1990, 41(11): 7892. DOI:10.1103/PhysRevB.41.7892 |
| [52] |
Perdew J P, Burke K, Ernzerhof M. Generalized gradient approximation made simple[J]. Physical Review Letters, 1996, 77(18): 3865. DOI:10.1103/PhysRevLett.77.3865 |
| [53] |
Henkelman G, Jónsson H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points[J]. Journal of Chemistry Physical, 2000, 113(22): 9978-9985. DOI:10.1063/1.1323224 |
| [54] |
Sanville E, Kenny S D, Smith R, et al. Improved grid-based algorithm for bader charge allocation[J]. Journal of Computational Chemistry, 2007, 28(5): 899-908. DOI:10.1002/(ISSN)1096-987X |
| [55] |
Grimme S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction[J]. Computer& Chemistry, 2006, 27(15): 1787-1799. |
| [56] |
Jing Y, Zhou Z, Cabrera C R, et al. Metallic VS2 monolayer:a promising 2D anode material for lithium ion batteries[J]. Journal of Physical Chemistry C, 2013, 117(48): 25409-25413. DOI:10.1021/jp410969u |
| [57] |
Yan W, Richards W D, Ong S P, et al. Design principles for solid-state lithium superionic conductors[J]. Nature Materials, 2015, 14: 1026-1031. DOI:10.1038/nmat4369 |
 2018, Vol. 35
2018, Vol. 35 


