Protein adsorption on material surfaces has been widely studied in different fields such as biochemistry, biological materials, biophysics, and biomedical materials[1-3]. Proteins and peptides are easily adsorbed on various materials and interfaces such as hydrophobic graphite,hydrophilic titanium dioxide, kinds of SAMs, etc.[4-7]. However, adsorption capacities of these materials are dependent on the properties of solute such as ionic strength, chemical composition, temperature, pH, etc.[8] In addition, the final structure and catalytic activity of the protein adsorbed onto the surfaces are influenced by the specific properties of surfaces such as charge, topography, composition, hydrophobicity, and heterogeneity. The vital role of electrostatic force during protein adsorption process has been well documented[9-11]. The biological response of protein is activated when it is adsorbed on a surface, which may result in targeted features such as those in regenerative medicines and bimolecular engineering[12]. Several studies have been conducted on the influence of surface charge. However, there is no common consensus for defining the prevailing mechanism. In some reported cases, surfaces attract or repel oppositely or alike charged proteins. For example, surfaces grafted with charged polymer attract oppositely charged proteins and repel alike charged or uncharged proteins[13]. In some cases, attraction between protein and similar charge was observed. Lesins et al. reported that lysozyme, α chymotrypsinogen A, cytochrome c, and ribonuclease, which have net positive charges, can be adsorbed on positively charged surface. They attributed this to the uneven internal distribution of charge which may result in patches of opposite charge to the net charge[14-15]. In some situations, charge of substrate appears to be the most important parameter. In determining the optimal reaction condition for enzymatic surface catalysis, the adsorption increases with the surface charge density. However, the catalysis can be suppressed by presence of a strong charge, due to very low enzyme mobility[16-17]. Nonetheless, electrostatic interaction has been shown to be insignificant[18-20]. A recent research revealed that the dominant interaction between Au surface and BSA protein is the vdW interaction. The adsorption behavior is nearly identical for Au surfaces with C=0|e|, +0.0025|e|, +0.0125|e|, and +0.0200|e|. Moreover, too strong repulsive electrostatic interaction will cause instability[19]. This present work is geared towards unraveling some of the basic rules regarding the relationship between adsorption behavior and surface charge. Bone morphogenetic protein-2(BMP-2) is a dimeric cysteine knot protein, which plays a vital role in inducing bone formation. It plays a stabilizing role for implant surface adsorption and interacts with receptors corresponding to its type Ⅰ and type Ⅱ receptors. The binding regions of BMP-2 have been discovered by Nickel et al.[21], and the binding sites of type ⅠA and type Ⅱ receptors were called wrist and knuckle epitopes, respectively. The wrist epitope contains the prehelix loop and helix α1 of one monomer and β8 strand with inner loop 1 of the other monomer. The knuckle epitope includes the ALA34 of β3 strand,the HIS39 of β4 strand,the SER88 and LEU 90 of β7, and LEU100 of β8 in each monomer[22-23].
Molecular dynamics (MD) simulation are designed to study the process of protein adsorption and its structure change on different surfaces. The information it offers at molecular level is supplementary to experimental results. Despite a volume of works available, in-depth understanding of the mechanism of protein adsorption is yet to be unraveled. In this study, we focus on the important role of electrostatic interaction between protein and SAMs. There have been many theoretical and experimental studies on the interaction between BMP-2 and artificial surfaces such as graphite, titanium dioxide, and hydroxyapatite[10, 24]. These studies explain some interaction mechanisms about the interaction between the protein and the surfaces. For example, it was found that the dominating factor between hydroxyapatite and BMP-2 was the electrostatic interaction. In addition, the two-layer water structure formed between BMP-2 and titanium dioxide prevented their direct interaction. However, the atomic-level details on how BMP-2 interacts with charged SAMs are still rarely reported. SAM is an ideal model surface because of its well defined surface chemistry and atomic topology at a nanoscale[25-26]. It is very suitable for studying protein-surface adsorption behaviors. Further, we performed MD simulations to understand the influence of differently charged SAMs on BMP-2, its behavior, and structural changes observed during the adsorption process. We applied two different kinds of SAMs, negatively charged CH3(CH2)9SO3- and positively charged CH3 (CH2)9N (CH3)3+, to explore the effects of their dissimilar surface charges.
1 Methods and materialsThe initial structure of the protein was obtained from the protein data base (PDB code 3REW)[27]. However, we only extracted the coordinates of the two chains of the BMP-2 homodimer with each monomer consisting of 103 residues. Then the BMP-2 molecule charged with -8e was solvated in a 1223 Å3 cubic box of TIP3P water. The whole system was neutralized by adding 24 Na+ and 16 Cl- for the purpose of approximating physiological conditions. We pre-equilibrated the system with restraints on heavy protein atoms by a 2-ns MD simulation, and subsequently the protein releasing the restrains was equilibrated for 2 ns. The equilibrated BMP-2 configuration was reserved for the foregoing adsorption simulations.
Two types of SAMs, CH3(CH 2)9SO3- and CH3 (CH2)9N (CH3)3+, were simulated to study their interactions with BMP-2. Each SAM surface had a (
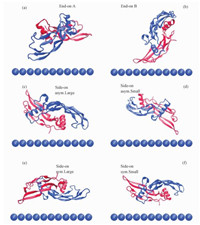
We extracted the coordinates of pre-equilibrated BMP-2 and two types of SAMs, and put them together with the initial separation distance of ~8 Å between BMP-2 and the surface. The initial separation distance represents the shortest distance between atoms of protein and atoms of SAM in the 3D box. In view of the computational demand and the size of the whole system, we put the BMP-2 with six starting orientations towards the surfaces, two end-on and four side-on orientations, show in Fig. 1. Four side-on orientations (symmetric-large, symmetric-small, asymmetric-large, asymmetric-small) were distinguished by both the orientation of each chain of the homodimer and the area of the BMP-2 around the surface[24]. We assumed “symmetric” if both chains of the BMP-2 are alike orientated to the surface, and “asymmetric” if the main part is directed to the surface. Moreover, whether it is large or small can be decided by the area of the protein in the vicinity of the surface and residues pertaining to the second monomer are marked by an asterisk (in red). All systems were simulated at 300 K using periodic boundary conditions in explicit TIP3P water with a cutoff distance of 12 Å for Lennard-Jones and real space electrostatic interactions. Long-range electrostatic interactions were computed using the particle mesh Ewald summation method (PME). The LINCS algorithm was used to restrain all bond lengths with a 2 fs time step. The production MD simulations were performed in an Isobaric-isochoric ensemble (NPT, T=300 K, P=1 atm). The BMP-2 and SAMs systems were pre-equilibrated for 2-ns MD with positional restraint on the protein. A full MD run of 70 ns was performed without any restraints on the BMP-2 subsequently. All MD simulations were performed using GROMACS (v4.6.1) simulation package with CHARMM27 force field.
|
Download:
|
|
Fig. 1 Initial orientations of BMP-2 homodimer on SAMs |
|
For each orientation, as shown in Table 1, we measure the adsorption stability of the BMP-2 to the surface by the numbers of contacts, defined as the number of protein atoms within 5 Å from the surface and by the total interaction energy (Utot) which represented the sum of vdW (UvdW) and electrostatic (Uele) energies[24]. At the end of simulation, in all six constructed systems, the BMP-2 shows a stable adsorption on the positively charged surfaces, but is adsorbed on the negatively charged surfaces only in end-on C configuration.
|
|
Table 1 Interaction energy between BMP-2 and SAM surface |
For the protein adsorbed on the positively charged surface, the end-on C configuration is obtained at the maximum level of interaction energy (536 kJ/mol) and the maximum number of contacts. We speculate that the final end-on C simulation configuration is likely to be the global-minimum-energy state orientation, and other orientations might be trapped in local-minimum-energy states[29]. Therefore, we choose the last simulation state of end-on C structure as the global-minimum-energy state in the subsequent analysis. The representative adsorption snapshots of the BMP-2 on two kinds of charged surfaces are shown in Fig. 2. Furthermore, BMP-2 is adsorbed onto two kinds of surfaces that are driven by both electrostatic and vdW interactions. In Table 1, we observed that the electrostatic interaction energy is much larger than the vdW interaction energy when the BMP-2 is adsorbed on both positively and negatively charged surfaces, respectively. Therefore,the dominant driving force for BMP-2 adsorption on charged surfaces is the electrostatic interaction. However, the vdW interaction still plays a role during the protein adsorption process. For the positively charged surface, the BMP-2 with each initial orientation can easily be adsorbed onto the surface. This is primarily because the protein carries a net charge of -8e. The protein interacts with the surface immediately due to the electrostatic attraction. Interestingly, the BMP-2 is adsorbed on the surface with “end-on” orientation instead of “side-on” orientation. The electrostatic map of BMP-2 is shown in Fig. 3. By combining Fig. 2, it is obvious that a partial negative patch of BMP-2 is adsorbed on the surface. This implies that positive patches on the other side of BMP-2 repel the protein away from the surface. This suggests that BMP-2 in this “end-on” state acquires a dynamic adsorption balance on the positively charged surface. It further implies that geometric feature of protein has vital influence on the protein adsorption process. In the case of negatively charged surface, BMP-2 was yet seen to be adsorbed onto the surface as shown in Fig. 2, despite the net charge on the protein (-8e). In Table 1, it is shown that the interaction energy between BMP-2 and negatively charged surface is larger than that between BMP-2 and positively charged surface. We can therefore conclude that the net charge is not the only key factor influencing protein adsorption but the distribution of charged amino acid residues on protein surface is of great importance. Liu et al.[30] have proved the important role of distribution of charge residues on the protein surface in determining the electrostatic interaction between the protein and the surface.The net charge is not the major standard for protein adsorption.

|
Download:
|
|
Fig. 2 Final structures of BMP-2 with side-on asymmetric-large orientations adsorbed on charged SAMs |
|
Several positive patches of the protein can be easily seen in the vicinity of the surface. Although previous researchers studied BMP-2 adsorption onto some artificial surfaces[10, 24],they only discussed the effect of hydrophilic and hydrophobic interaction. Our work studies detailed information on how BMP-2 interacts with charged SAMs. In addition, BMP-2 adsorptions were observed to be more stable on positively charged surfaces than negatively charged ones.
2.2 Binding sitesTo investigate the interaction pattern of BMP-2 on charged surfaces and whether the active binding sites are screened, the adsorption process and the final structures of BMP-2 with labeled contact amino acid residues on the differently charged surfaces are analyzed. The distribution maps of the contact residues are shown in Fig. 3. The contact residues are determined by the distance between the atoms of the protein and the top layer of surface along the Z-direction. When the distance is shorter than 5 Å, the residue including the corresponding atom is regarded as being in touch with the surface. Red parts show the negatively charged patches and blue parts show the positively charged patches.

|
Download:
|
|
Fig. 3 Electrostatic maps of BMP-2 in two different views |
|
As BMP-2 approaches the positively charged surface, the flexible loop containing two negatively charged residues (GLU94* and GLU96*) readily advances against the surface at about 1 ns. Though it is rather unstable, the two residues serve as fulcrums and the protein yields a significant rotation. Nonetheless, the process needs a short time until another flexible loop containing negatively charged residues (ASP22, ASP25, and ASP30) induces the process parallel to the surface, which starts at about 2.5 ns with ASP30 initially adsorbed to the surface. Subsequently, ASP29 is adsorbed onto the surface at 7.5 ns. Due to the repulsion of some positive residues (SER12, LYS15, LYS73, LYS76, and LYS73*), the protein finally forms an “end-on” pattern adsorbed on the surface, which leads to retreat of GLU94 from the surface. The adsorption becomes rather stable at about 35 ns, but subsequently the residues (ASP22, SER24 ASP25, ASN29, ASP30, LEU92, and GLU96) are adsorbed on the surface. It is worth mentioning that GLU96 is adsorbed on the surface all the time since it is in contact with the surface.
However, as BMP-2 is adsorbed on the negatively charged surface, the adsorption process and contact residues appear different from that on the positively charged surface. At the initial nanosecond of the MD simulation, positively charged residue SER12* primarily contacts with the surface. Subsequently LYS15* and LYS73 intermittently establish contacts to the surface at about 1.5 ns. This causes a slight rotation of the protein. These three positively charged residues induce the protein closer to the surface and also pull the negatively charged residue GLU46* close to the surface. Due to GLU46* interacting with the surface, the adsorption becomes slightly unstable and then stabilizes at about 30 ns. At distances langer than about 30 ns, the protein is adsorbed onto the surface mainly through SER12*, LYS15*,HIS17*,PRO18*,HIS44*,GLU46*,ASN71, SER72, and LYS73. In Fig. 2, we observed on the positively charged surface that the knuckle epitope of one monomer and the wrist epitope of another monomer are towards the surface, which indicates that some of active sites of BMP-2 are screened. Hence, the adsorption causes the catalytic capacity of the BMP-2 to reduce intensely. However, for the negatively charged surface, only the wrist epitope of one monomer faces the surface and most of the active sites are accessible. So the adsorption influences the activity of BMP-2 slight. In their experimental studies, Kloss et al.[31] found that BMP-2 in active form interacted strongly with oxygen-terminated nanocrystalline diamond, which may verify our results.
2.3 RMSD, RMSF and superimposed structuresTo explore the conformation stability of BMP-2 adsorbed onto differently charged surfaces, root-mean-square deviation (RMSD), root-mean-square fluctuation (RMSF), and superimposed structures are analyzed[32]. The RMSD value is calculated by comparing the backbone structure between crystal structure and the adsorbed structure. RMSD values of BMP-2 adsorbed onto positively and negatively charged surfaces are not larger than 0.35 nm and 0.38 nm, respectively (see Fig. 4). In view of size of protein, it is indicated that the protein does not deform significantly and the original structure of the backbone of the BMP-2 is well reserved. RMSD is analyzed to illustrate the entire structural change of BMP-2 adsorbed onto the charged surfaces. Meanwhile, RMSF and superimposed structures are analyzed to reveal the local structural changes. The RMSF results are shown in Fig. 5. The RMSF values of the N-terminal and C-terminal of BMP-2 adsorbed onto differently charged surfaces are not larger than 0.2 nm. This indicates that they undergo only slight fluctuation. For the RMSF value of each monomer of BMP-2, there are mainly three parts displaying obvious changes, and they are the hydrophilic groups SER72 and LYS73, the segment of the wrist epitope including residues PHE49 to ASP 53, and the flexible loop comprising couple of residues (GLU94*, ASN95*, GLU96*, and LYS97*). These sections all contain charged residues with two parts directly facing the charged surfaces. For the negatively charged surface, the flexible loop (GLU94*, ASN95*, GLU96*, and LYS97*) reaches a larger RMSF (0.32 nm), which is mainly induced by the electrostatic repulsion and this part easily deviates from its original positions. However, on the positively charged surface, the hydrophilic groups SER72 and LYS73 acquire the largest value (0.33 nm). It further suggests that the electrostatic repulsion makes the region the region move away from the surface.

|
Download:
|
|
Fig. 4 RMSD of BMP-2 on charged SAMs |
|

|
Download:
|
|
Fig. 5 RMSF of two monomers of BMP-2 adsorbed on charged SAMs |
|
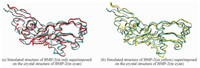
In addition, the stable conformations of BMP-2 adsorbed onto charged surfaces are superimposed with their crystal conformations (see Fig. 6). Although there are slight structural changes in the flexible α-helix, coils, and turns,the activity of BMP-2 is not obviously influenced.
|
Download:
|
|
Fig. 6 Simulated structure of BMP-2 adsorbed on charged SAMs superimposed on the crystal structure of BMP-2 |
|
In this work, we employ MD simulation to investigate the adsorption of BMP-2 on model anionic and cationic SAMs. The simulation results show that the BMP-2 is adsorbed onto both differently charged SAMs. Kloss et al. have determined that the contribution of electrostatic forces comprises around 75%-80% between BMP-2 and nanocrystalline diamond films using ab initio force-field-calculations and single molecule force spectroscopy measurements[33]. During the adsorption processes, the key inducer is the interaction between the SAMs and the oppositely charged residues. We also find that the geometric feature of the protein has vital influence. However, we find that the adsorption strength depends on both the net charge of BMP-2 and the distribution of charged amino acid residues on protein surface. In particular, the adsorption of BMP-2 on the positively charged surface is weaker than on the negatively charged surface, even though the net charge of BMP-2 is -8e. Moreover, the active site of BMP-2 is oriented toward the surface when it is adsorbed onto the positively charged surface, whereas the active site of BMP-2 is oriented toward the solution when it is adsorbed onto the negatively charged surface. Meanwhile, we observe that the main secondary structures are preserved on the two differently charged surfaces. This work provides detailed information about the adsorption of BMP-2 onto differently charged SAMs.
| [1] | Gray J J. The interaction of proteins with solid surfaces[J]. Current Opinion in Structural Biology , 2004, 14 (1) :110–115. DOI:10.1016/j.sbi.2003.12.001 |
| [2] | Dong X L, Wang Q, Wu T, et al. Understanding adsorption-desorption dynamics of BMP-2 on hydroxyapatite (001) surface[J]. Biophysical Journal , 2007, 93 (3) :750–759. DOI:10.1529/biophysj.106.103168 |
| [3] | Kandori K, Masunari A, Ishikawa T. Study on adsorption mechanism of proteins onto synthetic calcium hydroxyapatites through ionic concentration measurements[J]. Calcified Tissue International , 2005, 76 (3) :194–206. DOI:10.1007/s00223-004-0102-4 |
| [4] | K im, S R, Lee J H, Kim Y T, et al. Synthesis of Si, Mg substituted hydroxyapatites and their sintering behaviors[J]. Biomaterials , 2003, 24 (8) :1389–1398. DOI:10.1016/S0142-9612(02)00523-9 |
| [5] | Latour R A, Hench L L. A theoretical analysis of the thermodynamic contributions for the adsorption of individual protein residues on functionalized surfaces[J]. Biomaterials , 2002, 23 (23) :4633–4648. DOI:10.1016/S0142-9612(02)00213-2 |
| [6] | Latour R A, Rini C J. Theoretical analysis of adsorption thermodynamics for hydrophobic peptide residues on SAM surfaces of varying functionality[J]. Journal of Biomedical Materials Research , 2002, 60 (4) :564–577. DOI:10.1002/(ISSN)1097-4636 |
| [7] | Wilson K, Stuart S J, Garcia A, et al. A molecular modeling study of the effect of surface chemistry on the adsorption of a fibronectin fragment spanning the 7-10th type-Ⅲ repeats[J]. Journal of Biomedical Materials Research Part A , 2004, 69A (4) :686–698. DOI:10.1002/(ISSN)1097-4636 |
| [8] | Zhang Z, Dalgleish D G, Goff H D. Effect of pH and ionic strength on competitive protein adsorption to air/water interfaces in aqueous foams made with mixed milk proteins[J]. Colloids and Surfaces B-Biointerfaces , 2004, 34 (2) :113–121. DOI:10.1016/j.colsurfb.2003.11.009 |
| [9] | Feng B, Chen J Y, Zhang X D. Interaction of calcium and phosphate in apatite coating on titanium with serum albumin[J]. Biomaterials , 2002, 23 (12) :2499–2507. DOI:10.1016/S0142-9612(01)00384-2 |
| [10] | Shen J W, Wu T, Wang Q, et al. Molecular simulation of protein adsorption and desorption on hydroxyapatite surfaces[J]. Biomaterials , 2008, 29 (5) :513–532. DOI:10.1016/j.biomaterials.2007.10.016 |
| [11] | Zhou H, Wu T, Dong X, et al. Adsorption mechanism of BMP-7 on hydroxyapatite (001) surfaces[J]. Biochemical and Biophysical Research Communications , 2007, 361 (1) :91–96. DOI:10.1016/j.bbrc.2007.06.169 |
| [12] | DeConde A S, Sidell D, Lee M, et al. Bone morphogenetic protein-2-impregnated biomimetic scaffolds successfully induce bone healing in a marginal mandibular defect[J]. Laryngoscope , 2013, 123 (5) :1149–1155. DOI:10.1002/lary.v123.5 |
| [13] | Kato K, Sano S, Ikada Y. Protein adsorption onto ionic surfaces[J]. Colloids and Surfaces B-Biointerfaces , 1995, 4 (4) :221–230. DOI:10.1016/0927-7765(94)01172-2 |
| [14] | Lesins V, Ruckenstein E. Patch controlled attractive electrostatic interactions between similarly charged proteins and adsorbents[J]. Colloid and Polymer Science , 1988, 266 (12) :1187–1190. DOI:10.1007/BF01414409 |
| [15] | Ravichandran S, Madura J D, Talbot J. A brownian dynamics study of the initial stages of hen egg-white lysozyme adsorption at a solid interface[J]. Journal of Physical Chemistry B , 2001, 105 (17) :3610–3613. DOI:10.1021/jp010223r |
| [16] | Feller B E, Kellis J T, Cascao-Pereira L G, et al. Interfacial biocatalysis on charged and immobilized substrates:the roles of enzyme and substrate surface charge[J]. Langmuir , 2011, 27 (1) :250–263. DOI:10.1021/la103079t |
| [17] | Gong P, Szleifer I. Competitive adsorption of model charged proteins:the effect of total charge and charge distribution[J]. Journal of Colloid and Interface Science , 2004, 278 (1) :81–90. DOI:10.1016/j.jcis.2004.05.023 |
| [18] | Burns N L, Holmberg K, Brink C. Influence of surface charge on protein adsorption at an amphoteric surface:effects of varying acid to base ratio[J]. Journal of Colloid and Interface Science , 1996, 178 (1) :116–122. DOI:10.1006/jcis.1996.0099 |
| [19] | Hagiwara T, Sakiyama T, Watanabe H. Molecular simulation of bovine beta-lactoglobulin adsorbed onto a positively charged solid surface[J]. Langmuir , 2009, 25 (1) :226–234. DOI:10.1021/la8024149 |
| [20] | Kopaciewicz W, ROUNDS M A, FAUSNAUGH J, et al. Retention model for high-performance ion-exchange chromatography[J]. Journal of Chromatography , 1983, 266 (AUG) :3–21. |
| [21] | Nickel J, Dreyer M K, Kirsch T, et al. The crystal structure of the BMP-2:BMPR-IA complex and the generation of BMP-2 antagonists[J]. Journal of Bone and Joint Surgery-American Volume , 2001, 83A :S7–S14. |
| [22] | Mueller T D, Nickel J. Promiscuity and specificity in BMP receptor activation[J]. Febs Letters , 2012, 586 (14) :1846–1859. DOI:10.1016/j.febslet.2012.02.043 |
| [23] | Kirsch T, Nickel J, Sebald W. BMP-2 antagonists emerge from alterations in the low-affinity binding epitope for receptor BMPR-Ⅱ[J]. Embo Journal , 2000, 19 (13) :3314–3324. DOI:10.1093/emboj/19.13.3314 |
| [24] | Utesch T, Daminelli G, Mroginski M A. Molecular dynamics simulations of the adsorption of bone morphogenetic protein-2 on surfaces with medical relevance[J]. Langmuir , 2011, 27 (21) :13144–13153. DOI:10.1021/la202489w |
| [25] | Templeton A C, Wuelfing M P, Murray R W. Monolayer protected cluster molecules[J]. Accounts of Chemical Research , 2000, 33 (1) :27–36. DOI:10.1021/ar9602664 |
| [26] | Schreiber F. Structure and growth of self-assembling monolayers[J]. Progress in Surface Science , 2000, 65 (5-8) :151–256. DOI:10.1016/S0079-6816(00)00024-1 |
| [27] | Keller S, Nickel J, Zhang J L, et al. Molecular recognition of BMP-2 and BMP receptor IA[J]. Nature Structural & Molecular Biology , 2004, 11 (5) :481–488. |
| [28] | Soliman W, Bhattacharjee S, Kaur K. Adsorption of an antimicrobial peptide on self-assembled monolayers by molecular dynamics simulation[J]. Journal of Physical Chemistry B , 2010, 114 (34) :11292–11302. DOI:10.1021/jp104024d |
| [29] | Liu J, Yu G, Zhou J. Ribonuclease A adsorption onto charged self-assembled monolayers:A multiscale simulation study[J]. Chemical Engineering Science , 2015, 121 :331–339. DOI:10.1016/j.ces.2014.07.021 |
| [30] | Liu J, Liao C, Zhou J. Multiscale simulations of protein G B1 adsorbed on charged self-assembled monolayers[J]. Langmuir , 2013, 29 (36) :11366–11374. DOI:10.1021/la401171v |
| [31] | Kloss F R, Gassner R, Preiner J, et al. The role of oxygen termination of nanocrystalline diamond on immobilisation of BMP-2 and subsequent bone formation[J]. Biomaterials , 2008, 29 (16) :2433–2442. DOI:10.1016/j.biomaterials.2008.01.036 |
| [32] | Stocker U, Spiegel K, van Gunsteren W F. On the similarity of properties in solution or in the crystalline state:A molecular dynamics study of hen lysozyme[J]. Journal of Biomolecular Nmr , 2000, 18 (1) :1–12. DOI:10.1023/A:1008379605403 |
| [33] | Wildling L, Unterauer B, Zhu R, et al. Linking of sensor molecules with amino groups to amino-functionalized AFM tips[J]. Bioconjugate Chemistry , 2011, 22 (6) :1239–1248. DOI:10.1021/bc200099t |
 2017, Vol. 34
2017, Vol. 34 


