肥胖是体内脂肪,尤其是甘油三酯(TG)积累过多导致的一种状态.按其病因不同,肥胖分为原发性肥胖和继发性肥胖[7].肥胖患者的血液中TG、游离脂肪酸(FFA)、总胆固醇(TC)、低密度脂蛋白胆固醇(LDL-c)、载脂蛋白B100(ApoB-100)和极低密度脂蛋白胆固醇(VLDL-c)水平升高,以及高密度脂蛋白胆固醇(HDL-c)含量降低等代谢异常[8-9].尤其是肝脏脂质的代谢紊乱引起IR,影响肝细胞脂类的氧化、储存和运输,并抑制自噬的发生[10].并且,IR可加重脂肪组织和肌肉组织的脂质分解异常,引起肝细胞内的脂质过度积累和活性氧物质过量生成,导致线粒体功能紊乱和脂毒性等副作用[3-5].
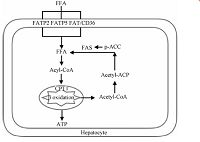
2 肝脏脂质代谢 2.1 肝脏脂质生成和氧化肝脏脂质代谢包括脂肪酸的摄入、生成和氧化(图 1).肝脏脂肪酸主要来源于血液中的FFA和肝细胞自身合成的内源性脂肪酸.血液FFA经脂肪酸转运蛋白2(FATP2)、脂肪酸转运蛋白5(FATP5)和脂肪酸移位酶(FAT/CD36)的主动运输进入肝脏[11-13].此外,乙酰辅酶A羧化酶(ACC)和脂肪酸合酶(FAS)参与内源性脂肪酸的生成[14].肝脏内的短或中长链的脂肪酸可以直接经线粒体内膜进入线粒体,而超长链脂肪酸则由肉毒碱棕榈酰基转移酶1(CPT1)转运至线粒体内进行β氧化,最终生成ATP[15].
|
Download:
|
| 图 1 肝细胞脂质代谢 Fig. 1 Lipid metabolism in hepatocyte | |
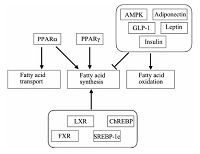
激素、核受体和细胞内相关蛋白质协同调节肝脏的脂代谢(图 2).脂肪组织分泌的脂联素既能上调CPT1的表达,增加肝脏脂质氧化;并抑制FAS和ACC的表达和肝脏脂质生成[16].瘦素则特异性抑制与肝脏脂肪合成相关的硬脂酰脱氢酶-1(SCD-1)mRNA表达水平,降低肝脏中TG和VLDL-c含量[17].胰岛素影响溶酶体DNA酶(DNL)活性,抑制固醇调节元件结合蛋白-1c(SREBP-1c)和激活Akt信号通路,降低VLDLs的水平[18],抑制线粒体β-羟-β-甲戊二酸单酰辅酶A(HMG CoA)转录水平,促进脂肪酸的氧化[19].高血糖素样肽1(GLP-1)增加cAMP抑制肝脏中脂质合成和促进脂质氧化[20].过氧化物酶体增殖物激活受体α(PPARα)上调肝脏FAT/CD36的表达,促进脂质转入肝细胞内[21].此外,肝脏内源性脂肪酸的合成受到PPARα和过氧化物酶体增殖物激活受体γ(PPARγ)调节[22-24].肝脏的核受体,如肝脏X受体(LXR)、FXR和孕烷受体(PXR),维持肝脏内脂质稳态[25].当肝脏内SREBP-1c和碳水化合物应答元件结合蛋白(ChREBP)表达量升高时,肝脏的脂质合成增加26-27],并参与FAS和ACC表达[28].另有研究表明,磷酸化AMP依赖蛋白激酶(p-AMPK)通过调节ACC磷酸化水平,促进肝细胞内脂质氧化,降低脂质含量[29].
|
Download:
|
| 图 2 肝脏脂质代谢的机制 Fig. 2 Mechanism of lipid metabolism in liver | |
自噬是真核生物细胞内普遍存在的一种自稳机制[30].在营养缺乏或者应激状态下,自噬通过消除/降解和再利用细胞内衰老或者失能细胞元件、细胞器和蛋白质,维持细胞内的能量平衡[31].研究发现,在饥饿时,自噬通过降解肝细胞内脂滴,将脂肪酸释放到细胞内线粒体进行氧化,调节肝细胞内的脂质储藏和能量平衡[32-33].若肝细胞的自噬受到抑制,易发生脂毒性和内质网应激[34].
3.1 自噬的形成自噬主要为大自噬、小自噬和分子伴侣介导的自噬[35-29].小自噬和分子伴侣介导的自噬分别指不同的特殊细胞自噬过程,而大自噬则指传统的细胞自噬.自噬体是由双层膜包裹的细胞质组成,自噬体与溶酶体融合后,溶酶体中酶降解自噬小体中的细胞组分[30].在生理条件下,自噬受到外界营养物质、以及脂质代谢相关蛋白和胰岛素等因素的调节[6].
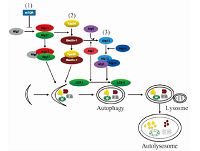
细胞内自噬小体的形成机制较为复杂,由30多个自噬相关基因共同协调完成[37].如图 3所示,调节细胞内大自噬生成的主要途径有:1)哺乳动物雷帕霉受体蛋白(mTOR)阻止Atg1与Atg13-Atg17结合抑制自噬,当mTOR抑制时,自噬水平升高[38];2)活化的Atg1-Atg13-Atg17复合体促进脂质细胞膜上beclin-1-Vps34复合体的形成[39-40];3)Atg7介导Atg12与Atg5的结合,与Atg16发生作用,在自噬体膜上形成Atg12-Atg5-16复合体后与自噬小体分离[41-42].在自噬信号的作用下,Atg7诱导LC3-Ⅰ与自噬小体膜上的磷脂酰乙醇胺形成LC3-Ⅱ,引起自噬小体膜的延伸和闭合[43].
|
Download:
|
| 图 3 自噬形成的分子机制 Fig. 3 Mechanism of autophagy | |
当肝脏中的脂质积累升高时,引起自噬水平降低,自噬小体的膜结构发生改变,抑制自噬小体与溶酶体结合,影响自噬降解脂质的作用[44].研究表明,用自噬的抑制剂3-MA处理肝细胞后,细胞TG和胆固醇的水平明显升高;Atg5或者Atg7敲除小鼠的肝脏发生TG积累[45].另有的研究表明,当肝脏内过度脂质积累引起自噬水平降低时,发生单纯炎症向NAFLD及其并发症演变.如果降低肝脏的脂质积累,则可以恢复肝脏自噬水平,预防肝损伤或者NAFLD及其并发症的发生[46].
细胞内TG和胆固醇酯(CE)主要以脂滴(LD)的形式存在,脂滴广泛地存在于多种组织细胞中,如脂肪细胞、肝细胞和肌肉细胞等.脂滴含有TG构成的脂质核心,脂核表面覆盖有单层磷脂,在单层磷脂内镶嵌着在结构上具有相关性的PAT家族蛋白,包括脂滴包被蛋白(perilipin)、脂肪生成相关蛋白(ADRP)等,脂滴在脂类代谢与存储、膜转运、蛋白降解中起着重要的作用[47].研究发现,自噬可以分解脂肪和降解脂滴[45, 48].当包裹脂滴的自噬小体与溶酶体融合后,脂滴被降解成FFA,FFA在线粒体内氧化分解生成ATP[45, 49].在饥饿状态下,LC3出现在脂滴表面,其中,LC3-Ⅱ与脂滴包被蛋白相互作用,通过自噬降解脂质,该过程是由自噬小体识别脂滴表面的脂滴包被蛋白介导的[50].最新研究发现,分子伴侣介导的自噬降解 perilipin 2和perilipin 3参与肝细胞的脂质代谢[51].
4 自噬与调节脂质代谢相关蛋白自噬小体的形成受到Atg家族相关蛋白和肝脏脂质代谢相关蛋白的调节.而且,肝脏脂质相关蛋白也影响肝细胞的自噬水平.
4.1 自噬对脂质代谢相关蛋白表达调节研究发现,下丘脑Atg7缺失小鼠的摄食量增加,能量消耗降低,体重升高,表明下丘脑自噬受到抑制后,降低瘦素水平[52].在营养充足时,细胞自噬水平受到抑制,mTORC1磷酸化抑制瘦素入核,上调FAS表达水平[53].细胞实验发现,3-MA抑制肝细胞自噬,其胞内的PXR水平增加[54].
4.2 脂质代谢相关蛋白调节自噬形成研究表明,AMPK磷酸化升高肝细胞自噬水平,促进脂质氧化[55].脂联素、SREBP-2和PPARγ上调LC3-Ⅱ蛋白的表达水平,升高细胞内自噬水平[56-58],而PPARα和FXR抑制肝脏的自噬水平[59].此外,高胰岛素血症和胰岛素抵抗与自噬水平的变化有关[56].高胰岛素血症和高脂饮食小鼠的肝脏的LC3-Ⅱ蛋白表达水平降低,自噬受到抑制;同时还发现,当肝脏中p62蛋白表达增加时,其Atg5和Atg7的表达下降[56].尽管胰岛素通过mTOR信号通路调节自噬形成,但不影响Atg相关基因的表达;胰岛素抑制FOXO1转录,降低细胞内自噬水平[56].动物实验也发现,核受体PXR缺失引起小鼠肝细胞的LC3-Ⅱ和Beclin-1蛋白水平下降[60].
5 小结近年来,肥胖导致的肝脏脂代谢紊乱对健康的影响倍受关注.上述研究表明,自噬在肝脏脂代谢中起到的关键作用,主要通过自噬溶酶体降解细胞内脂滴,降低肝脏脂质堆积,从而改善脂代谢紊乱.同时,参与脂质代谢相关的激素、核受体和蛋白也调节肝细胞内自噬水平.自噬在肝脏脂质代谢的分子机制为明确肥胖导致肝脏脂代谢紊乱的发病机制及其药物治疗提供了一定的理论依据.
| [1] | Musso G, Gambino R, Cassader M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD)[J]. Progress in Lipid Research , 2009, 48 (1) :1–26. DOI:10.1016/j.plipres.2008.08.001 |
| [2] | Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome[J]. Hepatology , 2003, 37 (4) :917–923. DOI:10.1053/jhep.2003.50161 |
| [3] | Sanyal A J, Campbell-Sargent C, Mirshahi F, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities[J]. Gastroenterology , 2001, 120 (5) :1183–1192. DOI:10.1053/gast.2001.23256 |
| [4] | Neuschwander-Tetri B A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites[J]. Hepatology , 2010, 52 (2) :774–788. DOI:10.1002/hep.23719 |
| [5] | Fu S, Yang L, Li P, et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity[J]. Nature , 2011, 473 (7348) :528–531. DOI:10.1038/nature09968 |
| [6] | Yin X M, Ding W X, Gao W T. Autophagy in the liver[J]. Hepatology , 2008, 47 (5) :1773–1785. DOI:10.1002/hep.22146 |
| [7] | Sweeting H N. Measurement and definitions of obesity in childhood and adolescence: a field guide for the uninitiated[J]. Nutrition Journal , 2007, 6 (1) :125–135. |
| [8] | Zierath J R, Livingston J N, Thorne A, et al. Regional difference in insulin inhibition of non-esterified fatty acid release from human adipocytes: relation to insulin receptor phosphorylation and intracellular signalling through the insulin receptor substrate-1 pathway[J]. Diabetologia , 1998, 41 (11) :1343–1354. DOI:10.1007/s001250051075 |
| [9] | Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease:a feature of the metabolic syndrome[J]. Diabetes , 2001, 50 (8) :1844–1850. DOI:10.2337/diabetes.50.8.1844 |
| [10] | Czaja M J, Ding W X, Donohue T M, et al. Functions of autophagy in normal and diseased liver[J]. Autophagy , 2013, 9 (8) :1131–1158. DOI:10.4161/auto.25063 |
| [11] | Berk P D. Regulatable fatty acid transport mechanisms are central to the pathophysiology of obesity, fatty liver, and metabolic syndrome[J]. Hepatology , 2008, 48 (5) :1362–1376. DOI:10.1002/hep.22632 |
| [12] | Ge F, Zhou S, Hu C, et al. Insulin-and leptin-regulated fatty acid uptake plays a key causal role in hepatic steatosis in mice with intact leptin signaling but not in ob/ob or db/db mice[J]. American Journal of physiology Gastrointestinal and Liver Physiology , 2010, 299 (4) :G855–G856. DOI:10.1152/ajpgi.00434.2009 |
| [13] | Liu F, Xie M X, Chen D L, et al. Effect of (VO)-O-IV(dipic-Cl)(H2O)(2) on lipid metabolism disorders in the liver of STZ-induced diabetic rats[J]. Journal of Diabetes Research , 2013 (1) :1–10. |
| [14] | Liang G S, Yang J, Horton J D, et al. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c[J]. The Journal of Biological Chemistry , 2002, 277 (11) :9520–9528. DOI:10.1074/jbc.M111421200 |
| [15] | Perez-Carreras M, Del Hoyo P, Martin M A, et al. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis[J]. Hepatology , 2003, 38 (4) :999–1007. DOI:10.1002/(ISSN)1527-3350 |
| [16] | Xu A, Wang Y, Keshaw H, et al. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice[J]. The Journal of Clinical Investigation , 2003, 112 (1) :91–100. DOI:10.1172/JCI200317797 |
| [17] | Cohen P, Miyazaki M, Socci N D, et al. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss[J]. Science , 2002, 297 (5579) :240–243. DOI:10.1126/science.1071527 |
| [18] | Stanhope K L, Schwarz J M, Keim N L, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans[J]. The Journal of Clinical Investigation , 2009, 119 (5) :1322–1334. DOI:10.1172/JCI37385 |
| [19] | Chakravarthy M V, Pan Z J, Zhu Y M, et al. “New” hepatic fat activates PPAR alpha to maintain glucose, lipid, and cholesterol homeostasis[J]. Cell Metabolism , 2005, 1 (5) :309–322. DOI:10.1016/j.cmet.2005.04.002 |
| [20] | Ding X K, Saxena N K, Lin S B, et al. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice[J]. Hepatology , 2006, 43 (1) :173–181. DOI:10.1002/(ISSN)1527-3350 |
| [21] | Wierzbicki M, Chabowski A, Zendzian-Piotrowska M, et al. Differential effects of in vivo PPAR alpha and gamma activation on fatty acid transport proteins expression and lipid content in rat liver[J]. Journal of Physiology and Pharmacology , 2009, 60 (1) :99–106. |
| [22] | Knight B L, Hebbachi A, Hauton D, et al. A role for PPARalpha in the control of SREBP activity and lipid synthesis in the liver[J]. Biochemical Journal , 2005, 389 (Pt 2) :413–421. |
| [23] | Schadinger S E, Bucher N L, Schreiber B M, et al. PPARgamma2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes[J]. American Journal of physiology Endocrinology and metabolism , 2005, 288 (6) :E1195–E1205. DOI:10.1152/ajpendo.00513.2004 |
| [24] | Samuel V T, Petersen K F, Shulman G I. Lipid-induced insulin resistance: unravelling the mechanism[J]. The Lancet , 2010, 375 (9733) :2267–2277. DOI:10.1016/S0140-6736(10)60408-4 |
| [25] | Edwards P A, Kast H R, Anisfeld A M. BAREing it all: the adoption of LXR and FXR and their roles in lipid homeostasis[J]. Journal of Lipid Research , 2002, 43 (1) :2–12. |
| [26] | Shimomura I, Matsuda M, Hammer R E, et al. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice[J]. Molecular Cell , 2000, 6 (1) :77–86. DOI:10.1016/S1097-2765(05)00010-9 |
| [27] | Uyeda KRepa J J. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis[J]. Cell Metabolism , 2006, 4 (2) :107–110. DOI:10.1016/j.cmet.2006.06.008 |
| [28] | Sul H S, Latasa M J, Moon Y, et al. Regulation of the fatty acid synthase promoter by insulin[J]. Journal of Nutrition , 2000, 130 (2S Suppl) :315S–320S. |
| [29] | Hardie D G. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status[J]. Endocrinology , 2003, 144 (12) :5179–5183. DOI:10.1210/en.2003-0982 |
| [30] | Kobayashi S. Choose delicately and reuse adequately: the newly revealed process of autophagy[J]. Biological & Pharmaceutical Bulletin , 2015, 38 (8) :1098–1103. |
| [31] | Singh RCuervo A M. Autophagy in the cellular energetic balance[J]. Cell Metabolism , 2011, 13 (5) :495–504. DOI:10.1016/j.cmet.2011.04.004 |
| [32] | Singh R, Kaushik S, Wang Y J, et al. Autophagy regulates lipid metabolism[J]. Nature , 2009, 458 (7242) :1131–1135. DOI:10.1038/nature07976 |
| [33] | Rautou P E, Mansouri A, Lebrec D, et al. Autophagy in liver diseases[J]. Journal of Hepatology , 2010, 53 (6) :1123–1134. DOI:10.1016/j.jhep.2010.07.006 |
| [34] | Yang L, Li P, Fu S N, et al. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance[J]. Cell Metabolism , 2010, 11 (6) :467–478. DOI:10.1016/j.cmet.2010.04.005 |
| [35] | Mehrpour M, Esclatine A, Beau I, et al. Autophagy in health and disease. 1. Regulation and significance of autophagy: an overview[J]. American Journal of Physiology-Cell Physiology , 2010, 298 (4) :C776–C785. DOI:10.1152/ajpcell.00507.2009 |
| [36] | Orenstein S JCuervo A M. Chaperone-mediated autophagy: Molecular mechanisms and physiological relevance[J]. Seminars in Cell & Developmental Biology , 2010, 21 (7) :719–726. |
| [37] | Mizushima NLevine B. Autophagy in mammalian development and differentiation[J]. Nature Cell Biology , 2010, 12 (9) :823–830. DOI:10.1038/ncb0910-823 |
| [38] | Sarbassov D D, Ali S M, Sabatini D M. Growing roles for the mTOR pathway[J]. Current Opinion in Cell Biology , 2005, 17 (6) :596–603. DOI:10.1016/j.ceb.2005.09.009 |
| [39] | Liang X H, Jackson S, Seaman M, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1[J]. Nature , 1999, 402 (6762) :672–676. DOI:10.1038/45257 |
| [40] | Backer J M. The regulation and function of Class Ⅲ PI3Ks: novel roles for Vps34[J]. Biochemical Journal , 2008, 410 :1–17. |
| [41] | Mizushima N, Noda T, Yoshimori T, et al. A protein conjugation system essential for autophagy[J]. Nature , 1998, 395 (6700) :395–398. DOI:10.1038/26506 |
| [42] | Ohsumi Y, Mizushima N. Two ubiquitin-like conjugation systems essential for autophagy[J]. Seminars in Cell & Developmental Biology , 2004, 15 (2) :231–236. |
| [43] | Ichimura Y, Kirisako T, Takao T, et al. A ubiquitin-like system mediates protein lipidation[J]. Nature , 2000, 408 (6811) :488–492. DOI:10.1038/35044114 |
| [44] | Koga H, Kaushik S, Cuervo A M. Altered lipid content inhibits autophagic vesicular fusion[J]. The Journal of the Federation of American Societies for Experimental Biology , 2010, 24 (8) :3052–3065. DOI:10.1096/fj.09-144519 |
| [45] | Singh R, Kaushik S, Wang Y J, et al. Autophagy regulates lipid metabolism[J]. Nature , 2009, 458 (7242) :1131–1164. DOI:10.1038/nature07976 |
| [46] | Amir MCzaja M J. Autophagy in nonalcoholic steatohepatitis[J]. Expert Review of Gastroenterology & Hepatology , 2011, 5 (2) :159–166. |
| [47] | Farese R V, Jr.Walther T C. Lipid droplets finally get a little R-E-S-P-E-C-T[J]. Cell , 2009, 139 (5) :855–860. DOI:10.1016/j.cell.2009.11.005 |
| [48] | Shibata M, Yoshimura K, Furuya N, et al. The MAP1-LC3 conjugation system is involved in lipid droplet formation[J]. Biochemical and Biophysical Research Communications , 2009, 382 (2) :419–423. DOI:10.1016/j.bbrc.2009.03.039 |
| [49] | Czaja M J. Autophagy in health and disease. 2. Regulation of lipid metabolism and storage by autophagy: pathophysiological implications[J]. American Journal of Physiology-Cell Physiology , 2010, 298 (5) :973–C978. DOI:10.1152/ajpcell.00527.2009 |
| [50] | Shibata M, Yoshimura K, Furuya N, et al. The MAP1-LC3 conjugation system is involved in lipid droplet formation[J]. Biochemical and Biophysical Research Communications , 2009, 382 (2) :419–423. DOI:10.1016/j.bbrc.2009.03.039 |
| [51] | Kaushik SCuervo A M. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis[J]. Nature Cell Biology , 2015, 17 (6) :759–770. DOI:10.1038/ncb3166 |
| [52] | Quan W, Kim H K, Moon E Y, et al. Role of hypothalamic proopiomelanocortin neuron autophagy in the control of appetite and leptin response[J]. Endocrinology , 2012, 153 (4) :1817–1826. DOI:10.1210/en.2011-1882 |
| [53] | Peterson T R, Sengupta S S, Harris T E, et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway[J]. Cell , 2011, 146 (3) :408–420. DOI:10.1016/j.cell.2011.06.034 |
| [54] | Sugatani J, Noguchi Y, Hattori Y, et al. Threonine-408 regulates the stability of human pregnane X receptor through Its phosphorylation and the CHIP/chaperone-autophagy pathway[J]. Drug Metabolism and Disposition , 2016, 44 (1) :137–150. |
| [55] | Egan D F, Shackelford D B, Mihaylova M M, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy[J]. Science , 2011, 331 (6016) :456–461. DOI:10.1126/science.1196371 |
| [56] | Liu H Y, Han J M, Cao S Y, et al. Hepatic autophagy is suppressed in the presence of insulin resistance and hyperinsulinemia inhibition of FoxO1-dependent expression of key autophagy genes by insulin[J]. The Journal of Biological Chemistry , 2009, 284 (45) :31484–31492. DOI:10.1074/jbc.M109.033936 |
| [57] | Seo Y K, Jeon T I, Chong H K, et al. Genome-wide localization of SREBP-2 in hepatic chromatin predicts a role in autophagy[J]. Cell Metabolism , 2011, 13 (4) :367–375. DOI:10.1016/j.cmet.2011.03.005 |
| [58] | Lin Z F, Wu F, Lin S Q, et al. Adiponectin protects against acetaminophen-induced mitochondrial dysfunction and acute liver injury by promoting autophagy in mice[J]. Journal of Hepatology , 2014, 61 (4) :825–831. DOI:10.1016/j.jhep.2014.05.033 |
| [59] | Lee J M, Wagner M, Xiao R, et al. Nutrient-sensing nuclear receptors coordinate autophagy[J]. Nature , 2014, 516 (7529) :112–115. |
| [60] | Wang K, Damjanov I, Wan Y J Y. The protective role of pregnane X receptor in lipopolysaccharide/D-galactosamine-induced acute liver injury[J]. Laboratory Investigation , 2010, 90 (2) :257–265. DOI:10.1038/labinvest.2009.129 |
 2016, Vol. 33
2016, Vol. 33 
