2. School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing 100049, China ;
3. State Key Laboratory of Natural and Biomimetic Drugs, Peking University, Beijing 100191, China ;
4. State Key Laboratory of Systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China
2. 中国科学院大学化学与化工学院, 北京 100049 ;
3. 北京大学天然药物及仿生药物国家重点实验室, 北京 100191 ;
4. 中国科学院植物研究所系统与进化植物学国家重点实验室, 北京 100093
Origin of life has aroused widespread attention. Since the origin of life, biological circadian rhythms have been ubiquitous on all kinds of organisms in nature. However, the origin of biological rhythms is still a scientific challenge.
In previous related studies, people have realized that the environment factors mignt result in their own biological effects in the life system. These environment factors including the nano-particles[1], light [2], magnetic field[3], force field [4-6], and so on. It is worth noting that the circadian rhythm of geophysical environments, which was caused by the earth motion in space, might play an important role in the origin of circadian rhythms in biological systems [7-8]. Based on the rhythms of geophysical environments, a variety of hypotheses on origin of circadian rhythms were put forward[9-10]. Among all kinds of the ubiquitous environmental cycles, light-dark cycle was considered as an important factor to result in biological circadian rhythm [11-13]. Remarkably, the effects of the tidal rhythm on the natural biological circadian rhythms in coastal organisms and marine organisms have been reported [14-18]. These studies led us to consider the hypothesis that the circadian rhythm of natural gravity may play an important role in the origin of biological circadian rhythms.
Many researchers studied the effect of magnitude change of force on enzymatic activity. For example, the expression of the gene could be affected by microgravity and hypergravity, respectively [5, 19-20]. The hypergravity can improve the activity of DNA polymerase α, and this may result from the fact that the hypergravity increases the affinity of enzyme for template DNA[4]. In previous studies, researchers studied only the effect of forces with the different magnitudes on enzymatic activity. However, the effect of forces with the different directions on enzymatic activity was ignored[21].
In this work, both the magnitude and direction effects of the asymmetric gravity field on the activity of α-amylase are studied. Our results show that the activity of α-amylase is dependent on both the magnitude and direction of hypergravity. Considering the fact of the circadian rhythm of natural gravity on earth, we suggest that the circadian rhythm of natural gravity may play an important role in the origin of biological circadian rhythms. To the test of our knowledge, this is the first study on the effect of asymmetric hypergravity with different magnitudes and directions on enzymatic activity.
1 Experiment 1. 1 Materials and instrumentSoluble starch and α-amylase from Bacillus licheniformis were purchased from Beijing Solarbio Science & Technology Co. , Ltd. (Beijing, China). Other chemicals were obtained from Beijing Chemical Company (Beijing, China) and used as received. All chemicals used are of analytical grade and prepared using high pure water with a resistance of 18. 25MΩ·cm.
The asymmetric hypergravity fields (right-handed, left-handed) were obtained by two high-speed tabletop refrigerated centrifuges with clockwise-rotation and anticlockwise-rotation rotors, respectively (Xiang instrument, China). The pH of the solution was measured with PB-10 pH meter (Sartorius, Germany). UV-vis absorption spectra were acquired on a UV-2550 spectrophotometer (Shimadzu, Japan), using 1 cm path length quartz cuvettes for measurements.
1. 2 Influence of α-amylase concentration and temperature on enzymatic degradation0. 5 mL 0. 4g/L phosphate buffer was added into 0. 5mL 1×10-3 mol/L iodine solution, and it was diluted to 4 mL with water. 3, 4,and 5 g/L α-amylase solutions were prepared, respectively. The 0. 1mL three α-amylase solutions were added into starch-iodine solution and completely mixed, respectively. Their absorbencies at 660 nm were immediately determined along with time.
2. 5 mL 0. 4g/L phosphate buffer was added into 2. 5mL 1×10-3 mol/L iodine solution and the soltution was diluted to 20mL with water. 0. 5mL 4g/L α-amylase solution was added into the above starch-iodine solution. The sample absorbance at 660nm was determined at 20, 25, 30, 35, 40℃, respectively, and it was repeated for at least three times.
1. 3 Effects of magnitude and direction of asymmetric gravity field on activity of α-amylaseAccording to the definition of asymmetric force[22], the asymmetric gravity field was simulated by the external circular rotations in clockwise (L-rotation) and anticlockwise (R-rotation) centrifuges (facing to viewer) to obtain two types of the helical chiral force (radius 3 cm, RCF 0~11400×g). Rotation speed (r/min) was used to control the intensity of the asymmetric gravity field. 1. 5 mL 0. 4g/L phosphate buffer was added into 1. 5mL 1×10-3 mol/L iodine solution, and the solution was diluted to 12mL. 0. 3mL 4g/L α-amylase solution was added into the above starch-iodine solution and completely mixed. The solution was immediately equally distributed into three samples. Then they were put in a clockwise rotation centrifuge (L-rotation), anticlockwise rotation centrifuge (R-rotation), and natural condition as a control at 30℃, respectively. In the process of rotation, the sample time scanning at 660nm was determined at an interval of about 6min until the experiment was over. Similar experiments were conducted with different rotation speeds at 1000, 2000, 3000, 4000, 5000, 7000, and 9000g, respectively.
2 Results and discussionFigure 1 shows the linear relation between starch-iodine solution concentration and absorbance at 660nm, and it shows a good linearity with the concentration of starch solution ranging from 0 to 0. 4g/L. The linear regression equation is y=0. 80347x + 0. 03721 (R2 =0. 99862). This experiment was repeated three times at least, and the relative standard deviation of the same concentration is 0. 0115, which indicated that the method had good repeatability.

|
Download:
|
| Fig. 1 (a) UV-VIS spectra of starch-iodine solution with various concentrations of starch solution ranging from 0g/Lto 0. 4g/L and (b) linear relation between starch-iodine solution concentration and absorbance at 660nm | |
The concentration of α-amylase is an important factor to affect the enzymatic degradation of starch-iodine complex. Figure 2 (a) shows that the reaction time to reach the end point increases from 640s, 1480s to 2850s with the decrease of the α-amylase concentration from 5g/L, 4g/L to 3g/L at room temperature (25℃). Considering that about 30 min of reaction time is relatively suitable to explore the effect of external force on the enzymatic activity, 4 g/L was selected as the optimal α-amylase solution concentration for further experiments in this work.

|
Download:
|
| Fig. 2 Influence of reaction conditions on the enzymatic degradation: (a) the effect of concentration of α-amylase on enzymatic degradation and (b) the effect of temperature on enzymatic degradation | |
Temperature obviously affects the enzymatic reaction rate via changing protein conformation. Figure 2 (b) shows that the reaction time increases from 450s (7. 5min) to 5700s (95min) with the decline of temperature from 40℃ to 20℃, which indicates that the enzyme activity of α-amylase decreases. This shows that the enzyme activity of α-amylase significantly depends on the temperature of reaction. As mentioned above, considering about 30 min of reaction time is relatively suitable to explore the effect of external force on the enzymatic activity, 30℃ was selected as the optimal temperature for further experiments in this work.
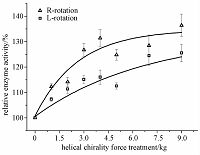
2.3 Dependence of α-amylase activity on both the magnitude and direction of gravityThe fresh starch-iodine solution with α-amylase was immediately treated under the different hypergravity field with L-rotation and R-rotation. Figure 3 shows that the activity of α-amylase increases with the hypergravity from 1g to 9000g. Obviously there were positive correlations between the activity of α-amylase and magnitude of hypergravity with either L-rotation or R-rotation. This is similar to the effects of hypergravity on other enzymes [4-5, 19-20, 23].
Interestingly, according to Fig. 3, L-rotation and R-rotation had different hypergravity effects when gravity was greater than 1g. The hypergravity field with R-rotation had a bigger effect on improving the activity of α-amylase than that with L-rotation. These novel results showed that the α-amylase activity is not only dependent on the magnitude of hypergravity but also on the direction of hypergravity.
|
Download:
|
| Fig. 3 Increases in activity of α-amylase with hypergravity from 1g to 9000g with both L-rotationand R-rotation | |
According to the concept of helix, chiral helix is produced by both axial vector and polar vector. In laboratory, axial vector can be delined by the circular rotation and the natural polar vector can be provided by the Earth’s moving along the Sun’s peculiar motion to Hercules[10, 21-22]. As a result, the clockwise and anticlockwise circular rotations on Northern Hemisphere will produce the artificial left- and right-handed helical motions and their corresponding helical force fields [10, 21-22].
In addition, the chiral helical force field may result in an intrinsic energy difference between helical enantiomers, and the right-handed helical force stabilizes the right-handed conformations and destabilizes the left-handed conformations [22]. The α-amylases (EC 3. 2. 1. 1) distribute widely among various organisms, and it is reported that there are eight α-helices in its structure [24]. As α-helix is right-handed conformation, the right-handed helical hypergravity field may have a bigger effect on improving the activity of α-amylase than the left-handed helical hypergravity field. The experimental data shown in Fig. 3 demonstrated that the α-amylase activity is not only dependent on the magnitude of hypergravity but also dependent on the direction of hypergravity.
2.5 Possible relationship between circadian rhythm of natural gravity and circadian rhythmsIn this study, we found that there is a positive correlation between the activity of α-amylase and the magnitude of the external force (Fig. 3). This is similar to the effects of hypergravity on other enzymes[4-5, 19-20, 23]. It is suggested that the enzyme activity would have the corresponding circadian rhythm, when the external force is circadian rhythm, and then would result in the corresponding circadian rhythm in physiological change.
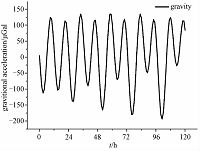
The circadian rhythmic change of natural gravity is mainly caused by the earth tide, which has been generally reported and accepted[17, 25-26]. Figure 4 shows the curve of theoretical value of earth tide fluctuation in Beijing (north latitude 39. 9° east longitude 116. 3°) within 120h since 1 April 2011, which was calculated by using the GOTIC2 program[26]. The change of gravitational acceleration is about 200 μGal [17, 25]. According to the dependence of enzyme activity on gravity, we believe that the circadian rhythmic change of natural gravity would be able to be transfer to the enzyme activity, then to result in the origin of circadian rhythm in biological systems. In other words, the circadian rhythm of natural gravity may play an important role in the origin of biological circadian rhythms.
|
Download:
|
| Fig. 4 Theoretical value of earth tide fluctuation inBeijing within 120h since 1 April 2011 | |
In the present work a degradation reaction of starch hydrolysis by α-amylase was used as model system to assess that the enzymatic activity depends on the magnitude and direction of hypergravity. Our data show that the activity of α-amylase is dependent on both the magnitude and chiral direction of hypergravity. Considering the circadian rhythm of natural gravity on earth, we suggest that the circadian rhythm of natural gravity may play an important role in the origin of biological circadian rhythms.
| [1] | Yuan L F, He Y J. Effect of surface charge of pdda-protected gold nanoparticles on the specificity and efficiency of DNA polymerase chain reaction[J]. Analyst , 2013, 138 (2) :539–545. DOI:10.1039/C2AN36145K |
| [2] | Jeon B W, Hwang J U, Hwang Y Y, et al. The Arabidopsis small G protein ROP2is activated by light in guard cells and inhibits light-induced stomatal opening[J]. Plant Cell , 2008, 20 (1) :75–87. DOI:10.1105/tpc.107.054544 |
| [3] | Xu C, Yin X, Lü Y, et al. A near-null magnetic field affects cryptochrome-related hypocotyl growth and flowering in Arabidopsis[J]. Advances in Space Research , 2012, 49 (5) :834–840. DOI:10.1016/j.asr.2011.12.004 |
| [4] | Takemura M, Yoshida S. Stimulation of DNA polymerase alpha by hypergravity generated by centrifugal acceleration[J]. Biochemical and Biophysical Research Communications , 2001, 289 (2) :345–349. DOI:10.1006/bbrc.2001.5986 |
| [5] | Kozeko L, Kordyum E. Effect of hypergravity on the level of heat shock proteins 70and 90in pea seedlings[J]. Microgravity Science and Technology , 2009, 21 (2) :175–178. |
| [6] | Allan D F. The effects of simulated microgravity on the seminiferous tubules of rats[J]. Advances in Space Research , 2012, 49 (4) :807–811. DOI:10.1016/j.asr.2011.11.033 |
| [7] | Brown F A. Response to pervasive geophysical factors and the biological clock problem[J]. Cold Spring Harbor Symposia on Quantitative Biology , 1960, 25 :57–71. DOI:10.1101/SQB.1960.025.01.007 |
| [8] | Foster R G, Roenneberg T. Human responses to the geophysical daily, annual and lunar cycles[J]. Current Biology , 2008, 18 (17) :784–794. DOI:10.1016/j.cub.2008.07.003 |
| [9] | Brown F A. The biological clock phenomenon: exogenous timing hypothesis[J]. Journal of Interdisciplinary Cycle Research , 1983, 14 (2) :137–162. DOI:10.1080/09291018309359807 |
| [10] | He Y J, Qi F, Qi S C. Periodicity of Earth's orbital chirality and possible mechanism of biological rhythms[J]. Medical Hypotheses , 2000, 55 (3) :253–256. DOI:10.1054/mehy.1999.1186 |
| [11] | Crosthwaite S K, Dunlap J C, Loros J J. Neurospora wc-1and wc-2: Transcription, photoresponses, and the origins of circadian rhythmicity[J]. Science , 1997, 276 (5313) :763–769. DOI:10.1126/science.276.5313.763 |
| [12] | Panda S, Hogenesch J B, Kay S A. Circadian rhythms from flies to human[J]. Nature , 2002, 417 (6886) :329–335. DOI:10.1038/417329a |
| [13] | Abe T, Ishikawa T, Masuda T, et al. Molecular analysis of Dec1 and Dec2 in the peripheral circadian clock of zebrafish photosensitive cells[J]. Biochemical and Biophysical Research Communications , 2006, 351 (4) :1072–1077. DOI:10.1016/j.bbrc.2006.10.172 |
| [14] | Chandrashekaran M K, Sharma V K. Tidal rhythms[M]. The Netherlands: Springer Press, 2008 : 1 . |
| [15] | Holley D C, DeRoshia C W, Moran M M, et al. Chronic centrifugation (hypergravity) disrupts the circadian system of the rat[J]. Journal of Applied Physiology , 2003, 95 (3) :1266–1278. DOI:10.1152/japplphysiol.00707.2002 |
| [16] | Hoban-Higgins T M, Alpatov A M, Wassmer G T, et al. Gravity and light effects on the circadian clock of a desert beetle, Trigonoscelis gigas[J]. Journal of Insect Physiology , 2003, 49 (7) :671–675. DOI:10.1016/S0022-1910(03)00068-4 |
| [17] | Peters A, Chung K Y, Chu S. Measurement of gravitational acceleration by dropping atoms[J]. Nature , 1999, 400 (6747) :849–852. DOI:10.1038/23655 |
| [18] | Fuller C A. The effects of gravity on the circadian timing system[J]. Journal of gravitational physiology , 1994, 1 (1) :1–4. |
| [19] | Vukanti R, Mintz E, Leff L. . coli under conditions of modeled reduced gravity[J]. Microgravity Science and Technology , 2008, 20 (1) :41–57. DOI:10.1007/s12217-008-9012-9 |
| [20] | Li Y H, Huang Y, Dai Z Q, et al. Gravity, a regulation factor in the differentiation of rat bone marrow mesenchymal stem cells[J]. Journal of Biomedical Science , 2009, 16 :87. DOI:10.1186/1423-0127-16-87 |
| [21] | He Y J, Dai Z F, Zeng L X, et al. Earth's orbital chirality and its possible role in biomolecular evolution[J]. Neuroquantology , 2008, 6 (2) :119–125. |
| [22] | He Y J, Qi F, Qi S C. Effect of chiral helical force field on molecular helical enantiomers and possible origin of biomolecular homochirality[J]. Medical Hypotheses , 1998, 51 (2) :125–128. DOI:10.1016/S0306-9877(98)90105-0 |
| [23] | Shimoshige H, Kobayashi H, Usami R. Inhibition of gene expression in Escherichia coli under hypergravity[J]. Bioscience Biotechnology and Biochemistry , 2011, 75 (1) :175–177. DOI:10.1271/bbb.100671 |
| [24] | Prakash O, Jaiswal N. α-amylase: an ideal representative of thermostable enzymes[J]. Applied Biochemistry and Biotechnology , 2010, 160 (8) :2401–2414. DOI:10.1007/s12010-009-8735-4 |
| [25] | Sun W, Miura S, Sato T, et al. Gravity measurements in southeastern Alaska reveal negative gravity rate of change caused by glacial isostatic adjustment[J]. Journal of Geophysical Research-Solid Earth , 2010, 115 (B12) :B12406. DOI:10.1029/2009JB007194 |
| [26] | Matsumoto K, T, Sato T, Takanezawa. Gotic2: a program for computation of oceanic tidal loading effect[J]. Journal of the Geodetic Society of Japan , 2001, 47 (1) :243–248. |
 2016, Vol. 33
2016, Vol. 33 


