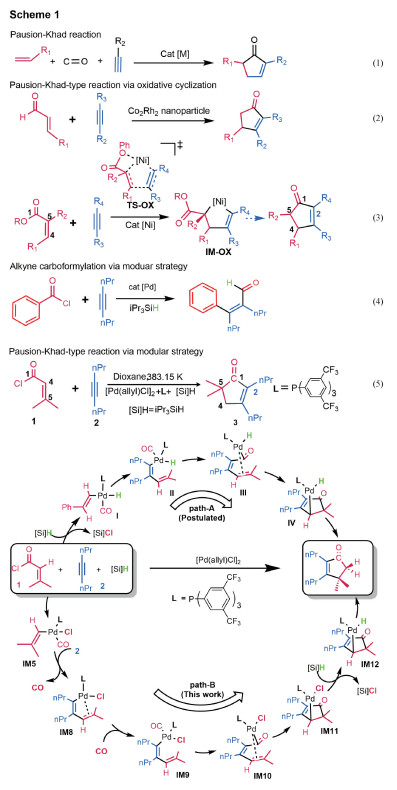
Cyclopentenones are valuable building blocks and prevalent motifs in bioactive molecules and natural products[1-9]. Development of effective and operationally simple methods to synthesize cyclopentenones is of significance in organic synthesis. The [2+2+1] cycloaddition of alkene, alkyne and carbon monoxide (CO), named as Pauson-Khand reaction (PKR), is a classical method to access cyclopentenones (Eq.(1) in Fig. 1). Since the first discovery of PKR[10], considerable efforts have been devoted to improve PKR[11-21]. A disadvantage of PKR is the use of toxic and flammable pressured CO gas, which is operationally inconvenient. To circumvent the problem, Chung et al.[22] in 2004 utilized α, β-unsaturated aldehydes to replace CO and alkene for the synthesis of cyclopentenones (Eq.(2)). In 2011, Montgomery group[23-24] and Ogoshi group[25] independently developed the nickel(0)-catalyzed synthesis of cyclopentenones from α, β-unsaturated esters and alkynes (Eq.(3)). Generally, these reactions undergo oxidative cyclization mechanism to form a five-membered metalacycle as a key intermediate (see TS-OX and IM-OX in Eq.(3))[22-26]. As such, these reactions maintain the integrity of the CO surrogates and add C1 and C4 to the ends of alkynes to afford cyclopentenones. Recently, Morandi and co-workers reported a mechanistically different method, called modular strategy, to synthesize cyclopentenones from α, β-unsaturated acid chlorides and alkynes, as exemplified by Eq.(5)[27]. The same strategy was also applied to perform carboformylation of alkynes with acid chlorides (Eq.(4))[28]. Interestingly, Eq.(5) affords no alkyne carboformylation product but cyclopentenones. Moreover, by fragmentation and reorganization, the strategy affords cyclopentenones with a quaternary carbon at C5, whereas the oxidative cyclization method furnishes cyclopentenones with C5 limited to secondary or tertiary carbon. Continuing our interest in the cyclization involving alkyne[29-30], we here report a detailed DFT mechanistic study to understand how the modular strategy works. As compared in Fig. 1, the study indicates that the reaction could proceed via the path-B mechanism, rather than path-A postulated by experimentalists[27].
|
Download:
|
|
Fig. 1 Researches on the synthesis of cyclopentenone and mechanisms of the modular strategy |
|
All structures were optimized at B3LYP[31]-D3[32]/BSI level in the gas phase. BSI represents a basis set with SDD[33] for Pd and 6-31G(d, p) for other atoms. Harmonic frequency analysis calculations were subsequently performed to verify the optimized geometries to be minima (no imaginary frequency) or transition states (TSs, having unique one imaginary frequency). The energies were then improved by M06-2X[34]/BSII single-point calculations with solvent effects accounted by the SMD solvent model[35], using the experimental solvent (dioxane). BSⅡ denotes a basis set with SDD for Pd and 6-311++G(d, p) for other atoms. All standard DFT calculations were carried out using Gaussian 09 program[36].
In Gaussian program, the Gibbs free energies were obtained by using the ideal gas model under the conditions of 383.15 K and 1 atm. Considering that the reaction was carried out in the solvent, we corrected the gas phase Gibbs free energies to the values under the conditions of 383.15 K and 1 mol/L. The correction factor (ΔGcorr= 2.6 kcal/mol) for a species A can be estimated from Eq.(6), where Csol is equal to 1 mol/L and p is the standard atmosphere pressure (101 325 Pa).
| $ G_{[A(383.15 \mathrm{~K}, 1 \mathrm{~mol} / \mathrm{L})]}=G_{[A(383.15 \mathrm{~K}, 1 \mathrm{~atm})]}+\Delta G_{\mathrm{corr}^{*}} $ | (6) |
| $ \Delta G_{\text {cert }}=R T \times \ln \frac{C_{\text {sol }}}{C_{\mathrm{gs}}}=R T \times \ln \frac{C_{\text {sal }}}{p / R T}=26 \mathrm{kcal} / \mathrm{mol}. $ |
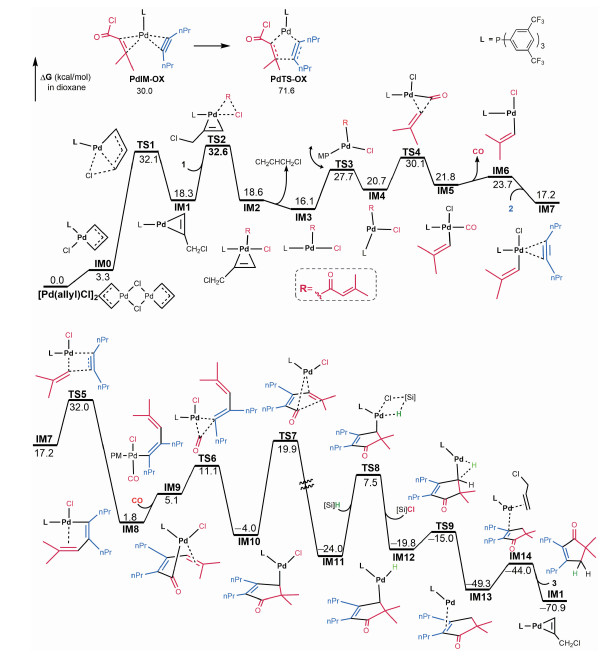
Figure 2 shows our computed energy profile for the reaction Eq.(5). To begin with, the palladium (Ⅱ) precursor [Pd(allyl)Cl]2 is activated to a palladium (0) complex IM1 via ligand L coordination, followed by reductive elimination with TS1. The C—Cl bond of acid chloride 1 then breaks through oxidative addition to IM1 with TS2. The resultant IM2 is coordinatively saturated, thus CH2CHCH2Cl is released via TS2, generating IM3 with a vacant active site. Ligand slippage via TS3 isomerizes trans IM3 to cis IM4, which allows the R group (i.e. -C(=O)-CH2=CMe2) to undergo reverse migratory insertion via TS4, eliminating the carbonyl group from 1. The processes from IM1 to IM5 fragmentize 1 into vinyl, CO, and Cl species. Subsequent to the fragmentation, the CO group in IM5 has to be liberated to continue the reaction (vide infra), allowing alkyne coordination to the resultant IM6, giving more stable IM7. By crossing a migration transition state TS5, the vinyl group and alkyne are bonded together, giving IM8. The C—C bond formation is favorable kinetically and thermodynamically, with a barrier of 14.8 kcal/mol and an exergonicity of 15.4 kcal/mol. IM8 possesses a vacant site available for CO recoordination to give IM9. The recoordination is uphill by 3.3 kcal/mol, but the CO group in IM9 is facile to undergo migratory insertion, giving IM10. Notably, the process from IM8 to IM10 is facile with a barrier of only 9.3 kcal/mol and exergonicity of 5.8 kcal/mol. The formation of IM10 assemblies the CO and vinyl group of 1 to the ends of alkyne. Proceeding forward, cyclization takes place via C=C bond insertion into the Pd-C(=O) bond, giving IM11. Subsequently, the transmetalation with [Si]H (iPr3SiH) via TS8 converts IM11 to a palladium (Ⅱ) hydride IM12. Finally, IM12 undergoes depalladation via reductive elimination, followed by ligand exchange, affording cyclopentenone 3 and regenerating the palladium (0) complex IM1.
|
Download:
|
|
Fig. 2 Energy profile for the reaction Eq.(5) |
|
Examining the energy profile, the intermediates and transition states prior to IM8 have high relative energies up to 32.6 kcal/mol and the transmetalation from IM11 to TS8 has a high barrier of 31.5 kcal/mol. The somewhat unfavorable energetics explains why elevated temperature (383.15 K)was applied for the reaction. The energy profile suggests that the reaction could be improved from the following two aspects: (i) Because of the low barriers of the elementary reactions involved in the fragmentation of 1, using a catalytic system which could be initiated to easily generate a palladium (0) active species irreversibly to facilitate the fragmentation of 1. Note that Pd(PPh3)4 was used to catalyze reaction, but gave low yield, which we attribute to that Pd(PPh3)4 is not easy to give PdPPh3 active species via dissociation. (ii) Using alternative hydrogen source to low the transmetalation barrier.
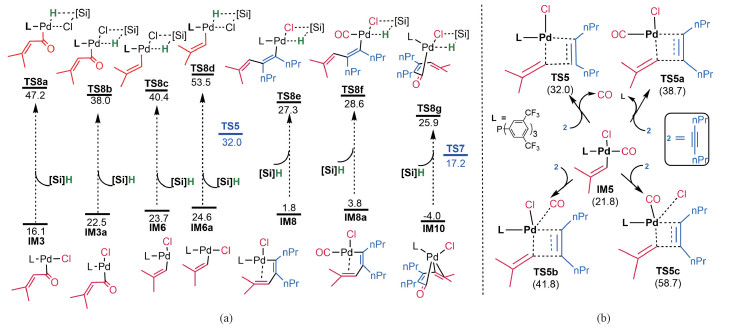
Morandi et al have considered four pathways for the reaction, among which path-A in Fig. 1 was considered to be most likely. Comparing path-A and our predicted path-B, the two pathways are different with two key differences. First, as the transmetalation in path-B takes place at the very late stage, the process in path-A occurs at the early stage. Second, the CO group in path-A coordinates to palladium throughout the reaction after fragmentation, whereas the CO group in path-B is released from IM5 and then re-coordinates after IM5 converts to IM8. The differences motived us to inspect our mechanism further. For transmetalation process, we considered various possible transmetalations, Fig. 3(a). For the intermediates prior to TS5, IM1, IM4 and IM5 which possess no vacant site cis to Cl are not viable for transmetalation. The transmetalation barriers of the coordinatively unsaturated IM3/IM3a and IM6/IM6a are much higher than TS5. The energetics indicates that fragmentation of the acid chlorides could not be interpreted by [Si]H and straightforwardly leads to IM5. Because the transmetalation barriers of IM8/IM8a and IM10 are much higher than TS7, these transmetalations can be ruled out, affirming that the transmetalation can only take place after ring-closure to form IM11.
|
Download:
|
|
Fig. 3 Alternative transmetalations (a) and comparing the alkyne-vinyl coupling with or without liberation of monoxide (b) |
|
To address the second difference, we considered alternative mechanisms for the reaction of IM5 with 2 without releasing CO, as shown in Fig. 3(b). The alkene migration described by TS5a with L released from IM5 is 6.7 kcal/mol less favorable than that (TS5) with CO released, due to the stronger coordination of phosphorus ligand L than CO. Note that the CO release from IM5 only cost 1.9 kcal/mol (Fig. 2). In TS5b and TS5c, the CO group or Cl atom maintain contact with Pd, however, these alkene migration transition states are significantly higher than TS5. These energetic results indicate that the CO ligand in IM5 must be liberated to drive the reaction forward. We considered two scenarios for CO liberation. The first scenario is that the CO release takes place in the solvent cage. When CO is released, it is confined by the solvent cage. After IM5 reacts with 2 to generate IM8 with accessible vacant site, the CO in the cage then coordinates to IM8 to continue the reaction. Alternatively, the liberated CO escapes the solvent cage and enters the gas phase of the reaction system. Given that the process from IM5 to IM8 is very facile and could be much faster than CO escape from the cage, it is more likely that the liberation and re-coordination of CO take place in the solvent cage. Experiments using isotopic acid chloride in the CO atmosphere or regular acid chloride in the isotopic CO atmosphere could verify our assumption.
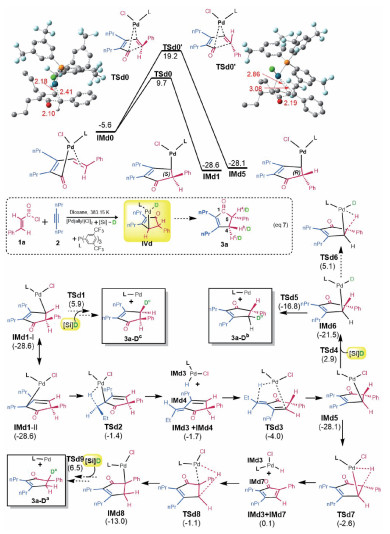
As our path-B is supported by computed energetics, path-A was proposed on the basis of deuterium-labeling experimental results, raising a question if our path-B contradicts to the experimental results. As shown in Eq.(7) in Fig. 4, H/D scrambling was observed at the hydrogen atoms bonded to both C4 and C5. Experimentalists attributed their observations to that the palladium hydride IVd in Eq.(7) could undergo competitive β-H elimination and reductive elimination. Because our path-B also involves a similar intermediate (i.e. IM12), our mechanism would not contradict to the experimental results if their elucidations were correct. However, according to our mechanism, we reasoned that the deuteration should not take place at the palladium hydride, because the barrier for subsequent reductive elimination is too low, being 4.8 kcal/mol from IM12 to TS9 (Fig. 2). Indeed as shown in Fig. 4, TSd5 for reductive elimination is 21.9 kcal/mol more favorable than TSd6 for β-H elimination. Thus, when IMd6 is formed, it would give 3a-Db directly, leaving no chance for β-H elimination to give 3a-Da. According to our computed pathway in Fig. 2, we reasoned that the structural isomerizations for deuteration should occur at the deeper valley corresponding to IM11. Unlike the achiral IM11, the counterparts of 1a are stereoisomers, namely IMd1 and IMd5 in Fig. 4. As such, we first examined which enantiomer is kinetically preferred. As compared, IMd1 is more kinetically favorable than IMd5 by 9.7 kcal/mol, which can be attributed to the stronger coordination interaction in TSd0 than in TSd0'. Supportively, the two marked Pd-C distances in the former are much shorter than those in the latter. Although IMd1 is not able to undergo β-H elimination, it is kinetically more favorable. As such, we should start with IMd1 to study the deuteration process. Considering IMd1, it can be represented by two resonance structures, namely IMd1-I and IMd1-Ⅱ. IMd1-I can lead to 3a-Dc after transmetalation and reductive elimination. IMd1-Ⅱ can convert to IMd5 via β-H elimination with nPr group (TSd2), IMd4 flip, and reverse β-H elimination (TSd3). Subsequently, IMd5 proceeds via two pathways. On the one hand, it can afford 3a-Db after transmetalation to give IMd6, followed by reductive elimination vis TSd5. Alternatively, it can convert to IMd8 via β-H elimination (TSd7), structural adjustment, and reverse β-H elimination (TSd8). Finally IMd8 affords 3a-Da via subsequent transmetalation and reductive elimination. The transmetalations of IMd1-I, IMd6, and IMd8 are the rate-determining step to give 3a-Dc, 3a-Db, and 3a-Da, respectively. Relative to IMd1, the transmetalation barriers are 35.1 (TSd9), 31.5 (TSd4), and 34.5 kcal/mol (TSd1). The energetic results reasonably explain the experimental observations.
|
Download:
|
|
Fig. 4 Rationalizing the deuterium-labeling experimental results |
|
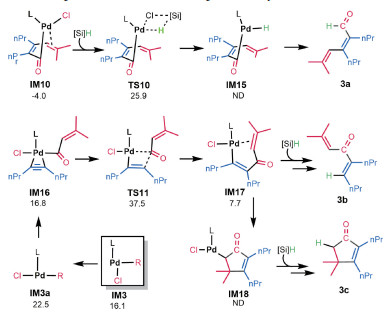
According to the experimental studies, [9-10] in addition to 3, the reaction could also afford 3a-3c (Fig. 5). To further corroborate our mechanism and to understand the selectivity of the reaction, we examined if our mechanism can rationalize the selectivity of the reaction to give 3. 3a is the alkyne carboformylation product in the Pd-catalyzed reaction of benzoyl chloride with alkyne (Eq.(4)). According to the mechanism in Fig. 2, 3a could be produced via transmetalation of IM10, followed by reductive elimination. The transition state (TS10) for transmetalation is 8.4 kcal/mol higher than TS7 for alkene insertion, excluding the production of 3a. It should be noted that, since the transmetalation barrier (29.9 kcal/mol from IM10 to TS10) is accessible, if there is no vinyl group in acid chloride, the reaction could afford carboformylation product, as shown in Eq.(4). The formations of 3b and 3c both start from IM3. First, cis IM3 converts to trans IM3a. Coordination of alkyne 2 to IM3a results in IM15. Alkyne insertion to Pd-C(O) via TS11 gives IM17. On the one hand, IM17 undergoes transmetalation, followed by reductive elimination, affording 3b. On the other hand, IM17 undergoes alkene insertion to give IM18, which then undergoes transmetalation and reductive elimination, affording 3c. Because the transition state TS11 (ΔG≠=37.5 kcal/mol) is much higher than TS4 (ΔG≠=30.1 kcal/mol) and TS5 (ΔG≠=32.0 kcal/mol), the reaction channels should not open to the formations of 3b and 3c. 3c could also be generated via oxidative cyclization, however, the highly unfavorable energies (i.e. see the process from PdIM-OX to PdTS-OX in Fig. 1) for the process exclude the possibility.
|
Download:
|
|
Fig. 5 Explaining the selectivity of 3 over 3a-3c |
|
In summary, DFT calculations have been applied to understand how the palladium catalyst assembles α, β-unsaturated acid chlorides and alkynes to cyclopentenones in the presence of hydrosilane. Instead of undergoing oxidative cyclizatoin to form a five-membered metalacycle, the transformation proceeds via the sequence: the disassembly of α, β-unsaturated acid chloride into vinyl, carbonyl, and Cl units, carbon monoxide release, coupling of alkyne with vinyl, re-coordination of carbon monoxide and migratory insertion to form another C—C bond, ring-closure via C=C bond insertion, transmetalation with hydrosilane, C, H-reductive elimination to release the product. Different from the mechanism proposed by experimentalists, the CO group is involved in the reaction via separate liberation and re-coordination in the solvent cage, rather than persistent coordination with palladium. The transmetalation takes place at the late stage of the reaction and is a bottleneck of the transformation, instead of at the early disassembly stage. Expectedly, the deep insights could help advance the modular strategy for new transformations.
| [1] |
Gibson S E, Lewis S E, Mainolfi N. Transition metal-mediated routes to cyclopentenones[J]. Journal of Organometallic Chemistry, 2004, 689(24): 3873-3890. DOI:10.1016/j.jorganchem.2004.04.045 |
| [2] |
Lindquist N, Fenical W, Sesin D F, et al. Isolation and structure determination of the didemnenones, novel cytotoxic metabolites from tunicates[J]. Journal of the American Chemical Society, 1988, 110(4): 1308-1309. DOI:10.1021/ja00212a059 |
| [3] |
Pagani A, Navarrete C, Fiebich B L, et al. Synthesis and biological evaluation of 12-aminoacylphorboids[J]. Journal of Natural Products, 2010, 73(3): 447-451. DOI:10.1021/np9006553 |
| [4] |
Trzoss L, Xu J, Lacoske M H, et al. Enantioselective synthesis of (-)-jiadifenin, a potent neurotrophic modulator[J]. Organic Letters, 2011, 13(17): 4554-4557. DOI:10.1021/ol201742j |
| [5] |
Ding L J, Gu B B, Jiao W H, et al. New furan and cyclopentenone derivatives from the sponge-associated fungus hypocrea koningii PF04[J]. Marine Drugs, 2015, 13(9): 5579-5592. DOI:10.3390/md13095579 |
| [6] |
Saleh N, Blanchard F, Voituriez A. Synthesis of nitrogen-containing heterocycles and cyclopentenone derivatives via phosphine-catalyzed Michael addition/intramolecular Wittig reaction[J]. Advanced Synthesis & Catalysis, 2017, 359(13): 2304-2315. DOI:10.1002/adsc.201700313 |
| [7] |
Mikołajczyk M. Phosphonate reagents and building blocks in the synthesis of bioactive compounds, natural products and medicines[J]. Pure and Applied Chemistry, 2019, 91(5): 811-838. DOI:10.1515/pac-2018-1117 |
| [8] |
Burstein S H. The chemistry, biology and pharmacology of the cyclopentenone prostaglandins[J]. Prostaglandins & Other Lipid Mediators, 2020, 148: 106408. DOI:10.1016/j.prostaglandins.2020.106408 |
| [9] |
Yang Z. Navigating the Pauson-Khand reaction in total syntheses of complex natural products[J]. Accounts of Chemical Research, 2021, 54(3): 556-568. DOI:10.1021/acs.accounts.0c00709 |
| [10] |
Khand I U, Knox G R, Pauson P L, et al. A cobalt induced cleavage reaction and a new series of arenecobalt carbonyl complexes[J]. Journal of the Chemical Society D: Chemical Communications, 1971(1): 36a. DOI:10.1039/c2971000036a |
| [11] |
Billington D C, Kerr W J, Pauson P L, et al. The Khand reaction of some trisubstituted alkenes. Uses of the catalytic version of the reaction[J]. Journal of Organometallic Chemistry, 1988, 356(2): 213-219. DOI:10.1016/0022-328X(88)83092-4 |
| [12] |
Krafft M E, Juliano C A, Scott I L, et al. The directed Pauson-Khand reaction[J]. Journal of the American Chemical Society, 1991, 113(5): 1693-1703. DOI:10.1021/ja00005a038 |
| [13] |
Cassayre J, Gagosz F, Zard S Z. A short synthesis of (±)-13-deoxyserratine[J]. Angewandte Chemie International Edition, 2002, 41(10): 1783-1785. DOI:10.1002/1521-3773(20020517)41:10<1783:AID-ANIE1783>3.0.CD;2-1 |
| [14] |
Itami K, Mitsudo K, Fujita K, et al. Catalytic intermolecular Pauson-Khand-type reaction: strong directing effect of pyridylsilyl and pyrimidylsilyl groups and isolation of Ru complexes relevant to catalytic reaction[J]. Journal of the American Chemical Society, 2004, 126(35): 11058-11066. DOI:10.1021/ja047484+ |
| [15] |
Barluenga J, Álvarez-Fernández A, Suárez-Sobrino Á L, et al. Regio- and stereoselective synthesis of cyclopentenones: intermolecular pseudo-pauson-khand cyclization[J]. Angewandte Chemie International Edition, 2012, 51(1): 183-186. DOI:10.1002/anie.201105362 |
| [16] |
Grellepois F. Enantiopure trifluoromethylated β3, 3-amino acids: synthesis by asymmetric reformatsky reaction with stable analogues of trifluoromethyl N-tert-butanesulfinylketoimines and incorporation into α/β-peptides[J]. The Journal of Organic Chemistry, 2013, 78(3): 1127-1137. DOI:10.1021/jo302549v |
| [17] |
Asano K, Uesugi Y, Yoshida J. Pauson-Khand reactions in a photochemical flow microreactor[J]. Organic Letters, 2013, 15(10): 2398-2401. DOI:10.1021/ol4008519 |
| [18] |
Cochrane A R, Kerr W J, Paterson L C, et al. Advances in the cobalt-catalysed Pauson-Khand reaction: development of a sulfide-promoted, microwave-assisted protocol[J]. Tetrahedron, 2021, 78: 131805. DOI:10.1016/j.tet.2020.131805 |
| [19] |
Yamanaka M, Nakamura E. Density functional studies on the Pauson-Khand reaction[J]. Journal of the American Chemical Society, 2001, 123(8): 1703-1708. DOI:10.1021/ja005565+ |
| [20] |
Burrows L C, Jesikiewicz L T, Lu G, et al. Computationally guided catalyst design in the type I dynamic kinetic asymmetric Pauson-Khand reaction of allenyl acetates[J]. Journal of the American Chemical Society, 2017, 139(42): 15022-15032. DOI:10.1021/jacs.7b07121 |
| [21] |
Burrows L C, Jesikiewicz L T, Liu P, et al. Mechanism and origins of enantioselectivity in the Rh(I)-catalyzed Pauson-Khand reaction: comparison of bidentate and monodentate chiral ligands[J]. ACS Catalysis, 2021, 11(1): 323-336. DOI:10.1021/acscatal.0c03774 |
| [22] |
Park K H, Jung I G, Chung Y K. A Pauson-Khand-type reaction between alkynes and olefinic aldehydes catalyzed by rhodium/cobalt heterobimetallic nanoparticles: an olefinic aldehyde as an olefin and CO source[J]. Organic Letters, 2004, 6(7): 1183-1186. DOI:10.1021/ol049765s |
| [23] |
Jenkins A D, Herath A, Song M, et al. Synthesis of cyclopentenols and cyclopentenones via nickel-catalyzed reductive cycloaddition[J]. Journal of the American Chemical Society, 2011, 133(36): 14460-14466. DOI:10.1021/ja206722t |
| [24] |
Jenkins A D, Robo M T, Zimmerman P M, et al. Nickel-catalyzed three-component cycloadditions of enoates, alkynes, and aldehydes[J]. The Journal of Organic Chemistry, 2020, 85(5): 2956-2965. DOI:10.1021/acs.joc.9b02446 |
| [25] |
Ohashi M, Taniguchi T, Ogoshi S. Nickel-catalyzed formation of cyclopentenone derivatives via the unique cycloaddition of α, β-unsaturated phenyl esters with alkynes[J]. Journal of the American Chemical Society, 2011, 133(38): 14900-14903. DOI:10.1021/ja2059999 |
| [26] |
Ahlin J S E, Donets P A, Cramer N. Nickel(0)-catalyzed enantioselective annulations of alkynes and arylenoates enabled by a chiral NHC ligand: efficient access to cyclopentenones[J]. Angewandte Chemie International Edition, 2014, 53(48): 13229-13233. DOI:10.1002/anie.201408364 |
| [27] |
Lee Y H, Denton E H, Morandi B. Modular cyclopentenone synthesis through the catalytic molecular shuffling of unsaturated acid chlorides and alkynes[J]. Journal of the American Chemical Society, 2020, 142(50): 20948-20955. DOI:10.1021/jacs.0c10832 |
| [28] |
Lee Y H, Denton E H, Morandi B. Palladium-catalysed carboformylation of alkynes using acid chlorides as a dual carbon monoxide and carbon source[J]. Nature Chemistry, 2021, 13(2): 123-130. DOI:10.1038/s41557-020-00621-x |
| [29] |
Tao Y, Dang Y F., Wang Z X. Studies on Rh(Ⅲ)-catalyzed[5+2]/[3+2] cycloadditions of 2-hydroxystyrenes with alkynes[J]. Journal of University of Chinese Academy of Sciences, 2016, 33(1): 57-64. DOI:10.7523/j.issn.2095-6134.2016.01.009 |
| [30] |
Ren X J, Lu Y, Lu G, et al. Density functional theory mechanistic study of Ni-catalyzed reductive alkyne-alkyne cyclodimerization: oxidative cyclization versus outer-sphere proton transfer[J]. Organic Letters, 2020, 22(6): 2454-2459. DOI:10.1021/acs.orglett.0c00674 |
| [31] |
Becke A D. Density-functional thermochemistry. Ⅲ The role of exact exchange[J]. The Journal of Chemical Physics, 1993, 98(7): 5648-5652. DOI:10.1063/1.464913 |
| [32] |
Grimme S, Ehrlich S, Goerigk L. Effect of the damping function in dispersion corrected density functional theory[J]. Journal of Computational Chemistry, 2011, 32(7): 1456-1465. DOI:10.1002/jcc.21759 |
| [33] |
Roy L E, Hay P J, Martin R L. Revised basis sets for the LANL effective core potentials[J]. Journal of Chemical Theory and Computation, 2008, 4(7): 1029-1031. DOI:10.1021/ct8000409 |
| [34] |
Zhao Y, Truhlar D G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals[J]. Theoretical Chemistry Accounts, 2008, 120(1-3): 215-241. DOI:10.1007/s00214-007-0310-x |
| [35] |
Marenich A V, Cramer C J, Truhlar D G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions[J]. The Journal of Physical Chemistry B, 2009, 113(18): 6378-6396. DOI:10.1021/jp810292n |
| [36] |
Frisch M J, Trucks G W, Schlegel H B, et al. Gaussian 09, Revision A. 01[CP]. Gaussian Inc, Wallingford CT, 2009.
|
 2022, Vol. 39
2022, Vol. 39 


