2. National Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei 230029, China
2. 中国科学技术大学国家同步辐射实验室, 合肥 230029
Methyl crotonate (CH3CH=CHCOOCH3, MC), containing a double bond and functional carboxyl group, is an α-β unsaturated ester widely used as monomers or comonomers in organic synthesis and preparation of spices[1]. They can be used for the preparation of paints and dispersants for paints, adhesives and inks, the manufacture of cleaning products, antioxidants and amphiphilic surfactants, water-based resins and dispersants for textiles and paper as well as be used as precursors of aromatic bases in cosmetics, shampoos, soaps, detergents, and cleaning agents[2-7].
Due to its high-vapor pressure ((1.986±0.027) kPa at 25 ℃), MC would be easily released into environment during industrial processes. In the atmosphere, MC can be oxidated or degraded by OH[4] and NO3[8] radicals and other oxidants like the O3[9-11] molecule or Cl[4, 12] atom, and then the products can be transformed into low-vapor pressure organic compounds, which may in turn contribute to the formation of secondary organic aerosol(SOA). Therefore, it is desirable to have a clear understanding of the unimolecular chemistry of MC so as to study its reaction with tropospheric oxidants and assess their impact on the air quality.
Up to now, many studies were performed for the reactions of MC with some atmospheric oxidatnts or radicals[4-12]. For example, Blanco et al.[12] studied the rate coefficients for the gas-phase reactions of MC with the Cl atom. Teruel et al.[4] also did some kinetic study for the reactions of MC with the OH radical and Cl atom. Only one photoionization study of MC was carried out by Wang et al.[13], and the IE of MC was determined to be 9.78 eV and only two fragments C4H5O2+ and C4H5O+ were identified. The detailed dissociative photoionization of MC is still less understood by the experimental and theoretical researchers.
In this work, in order to obtain accurate IE of MC and the appearance energies (AEs) of the fragment ions and to explore the mechanism of the complicated photodissociation process, tunable synchrotron vacuum ultraviolet (VUV) coupled with photoionization mass spectrometry (PIMS) was utilized to perform a study of dissociative photoionization of MC in the energy region 9.0-14.5 eV. The IE of MC and the AEs of major fragment ions were acquired by measurements of the photoionization efficiency curve (PIE) spectra. The major dissociative photoionization channels were proposed and discussed in detail on the basis of experimental measurements and theoretical calculations.
1 Experimental methods and computational methods 1.1 Experimental methodsThe whole experiments were carried out at the Atomic and Molecular Physics Beamline (BL09U), National Synchrotron Radiation Laboratory, Hefei, China, which have been reported in literatures[14-17], and only a short description is given here. The VUV synchrotron radiation (SR) is provided from an undulator of the 800 MeV electron storage ring which is monochromatized with a 6-m length monochromator. By using three interchangeable spherical gratings, photon energies can be adjusted between the range 7.5-124 eV. In this study, mass spectra and PIE curves of MC were measured in the energy region 9.0-14.5 eV, so only the 370 lines/mm grating was used which covers the photon energy range 7.5-22.5 eV. The absolute photon energy of monochromator is precisely calibrated with the known IEs of inert gases. The energy resolution (E/ΔE) of photons at 15.9 eV is measured by threshold photoelectron-photoion coincidence (TPEPICO) to be 9 meV (full width at half maximum), with an average photon flux of 1012 photons/s[18]. The home-made TOF-MS used in this study consists of seven electrostatic lenses that focus and accelerate the ions from the photoionization region, which provides a mass resolution M/ΔM of ~800.
Liquid MC (Aladdin, 98%) without further purification at room temperature was contained in a home-made sample reservoir. The MC sample was carried by Ar (purity 99.99%) and expanded into the ionization chamber through a nozzle with a diameter of 70 μm and one skimmer. The generated ions were drawn out of the photoionization region by a pulse extraction field triggered with a pulse generator (DG 535 SRS) and detected by a microchannel plate detector. During the experiments, higher harmonics of the undulator were effectively suppressed by using Ar as the filter gas with an operating pressure of 0.773 3 kPa. PIE spectra were obtained by scanning the monochromator with step width of 30 meV. Detailed information about the experimental apparatus has been reported elsewhere[15, 18].
1.2 Computational methodsAll the energies of the MC molecule and relevant ions involved in this work were calculated by quantum chemistry methods using Gaussian 09 suite of programs[19] on the supercomputing system in the Supercomputing Center of USTC, Hefei, China. The geometries of the parent molecule, fragment ions, intermediates, and transition states were optimized using density functional(DFT) method with the B3LYP/6-31g(d) hybrid functional. The description of the theoretical methods involved and the general calculations procedures were described in the literatures[20-21]. Harmonic vibrational frequencies were computed at the same level of theory, and they provide the zero-point vibrational energies(ZPTE) and can be used for making sure that the local minimum or transition state was located. In order to verify the reactants and products are connected by the computed transition states, the intrinsic reaction coordinate (IRC)[22] calculations at the same level were also performed. Accurate energies were obtained at the G3B3 level[23]. In order to better assess experimental data and to predict possible dissociation mechanisms, enthalpies of formation at 298 K (ΔHf, 298) for possible products were obtained by using the calculated enthalpy changes for their equations of formation at the B3LYP/6-31g(d) level and the experimental ΔHf, 298 for the isolated atoms of C, O, and H[24].
The adiabatic IE of C5H8O2+ is determined by using Eq.(1) and the adiabatic AE of M0+ is calculated by using Eq.(2):
| $ {\rm{IE}}\left( {{{\rm{C}}_5}{{\rm{H}}_8}{{\rm{O}}_2}} \right) = {E_0}\left( {{{\rm{C}}_5}{{\rm{H}}_8}{\rm{O}}_2^ + } \right) - {E_0}\left( {{{\rm{C}}_5}{{\rm{H}}_8}{{\rm{O}}_2}} \right) $ | (1) |
| $ {\rm{AE}}\left( {{\rm{M}}_0^ + } \right) = {E_{\max }} - {E_0}\left( {{{\rm{C}}_{\rm{5}}}{{\rm{H}}_{\rm{8}}}{{\rm{O}}_{\rm{2}}}} \right) $ | (2) |
where E0(C5H8O2+) and E0(C5H8O2) represent the absolute energies of ionic and neutral precursor, respectively, and Emax refers to the highest energy barrier involved along the formation pathway of corresponding ionic fragment M0+.
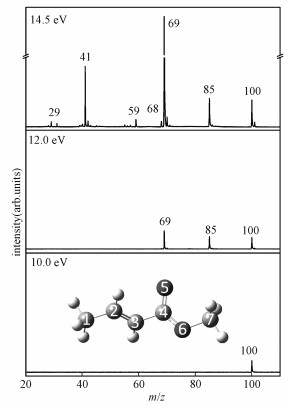
2 Results and discussion 2.1 VUV photoionization mass spectraThe photoionization mass spectra of MC at photon energies of 14.5, 12.0, and 10.0 eV are shown in Fig. 1. At low photon energy (10.0 eV), only the parent ion (m/z=100) is detected by mass spectra. Two fragments at m/z=85 (C4H5O2+) and 69 (C4H5O+) emerge as the photon energy increases to 12.0 eV. When photon energy increases to 15.5 eV, fragment ions at m/z=68, 59, 41, and 29 are obtained. The PIMS obtained from the TOF-MS is basically consistent with the 70 eV electron ionization mass spectra (EIMS)[25].
|
Download:
|
|
Fig. 1 Photoionization mass spectra of MC at different photon energies |
|
All the fragments are identified to be generated from dissociation of the parent ion C5H8O2+ since there is no signal at mass greater than that of C5H8O2+ (m/z=100) except the detected isotopomer of the molecular ion (m/z=101). Two major fragment peaks at m/z=69 and 41 are assigned to C4H5O+ and C3H5+, respectively. Two medium peaks at m/z=100 and 85 are proposed to be C5H8O2+ and C4H5O2+, respectively. The other three weak peaks appearing at m/z=68, 59, and 29 are assigned to C4H4O+, C2H3O2+, and C2H5+, respectively. It should be noted that in our data processing, no correction is made for possible kinetic shifts in determining the AEs. In addition, in view of the nozzle supersonic expansion condition mentioned above, the thermal energy distribution of the parent molecule is ignored[15, 26].
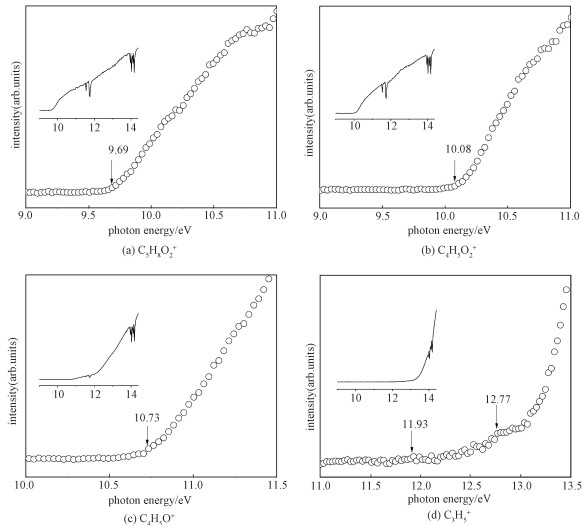
The PIE curves of the MC radical cation and the main fragment ions shown in Fig. 2 are obtained by integrating the area of each mass peak as a function of photon energy, followed by background subtraction and normalization to the photon flux.
|
Download:
|
|
Fig. 2 The PIE curves of the parent ion C5H8O2+ (a) and the fragmentions C4H5O2+ (b), C4H5O+ (c), and C3H5+ (d) |
|
For the parent ion, the IE determined from the PIE curve is (9.69±0.03) eV, which is in good agreement with the calculated value of 9.68 eV at G3B3 level in this study and the experimental value of 9.78 eV obtained by Wang et al.[13]. The experimental AEs of the fragments (C4H5O2+, C4H5O+, C4H4O+, C2H3O2+, C3H5+, C2H5+) are presented in Table 1, together with the calculated IE and AEs of the fragment ions. As shown in Fig. 1, the carbon atom numbering starts from the methyl group attached to the olefinic bond, then proceeds along the chain. The carbon and oxygen atoms of the carbonyl and -OCH3 group are numbered as C4, O5, O6, and C7, respectively.
|
|
Table 1 Theoretical and experimental values of the ionization energies and appearance energies for main fragment ions from dissociative photoionization of MC |
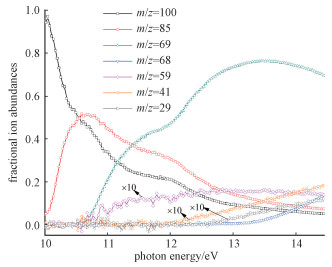
The relative branching ratios of the molecular ion of MC and its major fragment ions as functions of photon energy are derived from data of PIE curves and are plotted in Fig. 3. The sum of ion abundances is normalized to 1 at any photon energy. In the energy region 10-12 eV, the fractional abundance of the molecular ion decreases abruptly while those of the fragment ions at m/z 85 and 69 rise, implying that these fragments originate from the molecular ion. In the energy region 12-14 eV, the fractional abundances of the molecular ion and ion at m/z 85 decrease while those of the fragment ions at m/z 68, 59, 41, and 29 rise, implying that these fragments compete with the fragment ion at m/z 85 in the dissociation channels.
|
Download:
|
|
Fig. 3 Relative branching ratios of the molecular ion of MC and its major fragment ions as functions of photon energy |
|
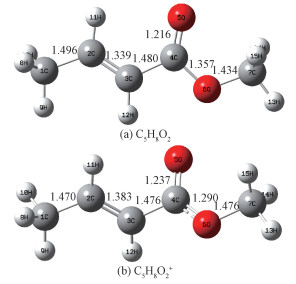
In this study, the parent ion C5H8O2+ is considered to be formed directly by VUV SR single-photon ionization. As the photon energy increases, the parent ion will undergo two types of dissociation reactions to form fragments: direct simple bond cleavage and indirect bond cleavage via transition states and intermediates. The proposed fragmentation channels for the main product ions are illustrated in Channel 1-6, where the geometries of transition states, intermediates, and dissociation products of MC cation are displayed. It is worth pointing out that the calculated energies shown in Channel 1-6 are relative to the ground state energy of the neutral MC which is defined as zero in this research. The optimized geometries of C5H8O2 and C5H8O2+ are given in Fig. 4. The unit of the bond lengths is Å(1 Å=0.1 nm). The calculated enthalpies of MC dissociation products (H298) and the derived standard enthalpies of formation at 298 K (ΔHf, 298) are listed in Table 2.
|
Download:
|
|
Fig. 4 Geometries of C5H8O2 (a) and C5H8O2+ (b) optimized at the B3LYP/6-31g(d) level of theory |
|
|
|
Table 2 Enthalpies of formation at 298 K (ΔHf, 298) for possible products of MC photoionization |
Channel 1 C4H5O++CH3O
The experimental AE of C4H5O+, as the dominant dissociation product of C5H8O2+ throughout the entire energy region examined in this study, is 10.73 eV, which is in good agreement with the experimental value of 10.78 eV by Wang et al.[13]. This is a direct bond dissociation channel, and theoretical AE is obtained from the potential curve for elimination of CH3O from C5H8O2+. In the barrier path, the C-O bond directly ruptures, leading to the products C4H5O+ and CH3O. As shown in Fig. 4, the C4-O6 bond length in the parent molecule is 1.357 Å, while in the molecular ion the bond length becomes 1.29 Å. The reaction path for the bond dissociation is computed at the B3LYP/6-31g(d) level. No distinct transition states are found along the pathway. We can further prove that this is a direct bond rupture process.
2.4 Indirect dissociation channelsChannel 2 C3H5++C2H3O2(CH3O+CO)
Obviously, there are two inflection points of the PIE of the fragment ion C3H5+, whose structure and stability has been theoretically investigated previously[27]. In this study, there are two possible structures of C3H5+: CH3-C=CH2+ and CH2=CH-CH2+. Besides, there are two possibilities of the neutral molecule: C2H3O2 or CH3O plus CO, i.e. C5H8O2+→C3H5++C2H3O2 or C5H8O2+→C3H5++CH3O+CO, which means that four possible channels may occur with the generation of m/z=41 fragment ion. As shown in Fig. 5, for the formation of C3H5+, no matter from which channel, one H atom from C2 migrates to C3 at first via transition state ts1 with an energy barrier of 1.57 eV, leading to the formation of intermediate int1.

|
Download:
|
|
Fig. 5 Formation pathways of two possible structures of C3H5+ |
|
At first sight, one may think C3H5+ would be formed from C-C bond rupture in int1, but the reaction path for the bond dissociation computed at the B3LYP/6-31g(d) level shows that there is a transition state for the C-C bond cleavage. Two carbon atoms C2 and C4 in int1 come close to each other, leading to the formation of a three-membered ring transition state ts2, and ultimately link together to form a C-C single bond. A stabilized intermediate int2 is formed. Then, there are two alternative pathways that may lead to the formation of CH3-C=CH2+ as described in Fig. 5(a). Int2 has its C2-C4 bond cleaved to produce CH3-C=CH2+ and C2H3O2, or its C4-O6 bond cleaved via ts21 to form C4H5O++CH3O, which then dissociates to three parts, CH3-C=CH2+, CH3O, and CO, with no transition states, which is in agreement with the computed results of C3H5+ fragment ion formed from the photodissociation of MMA[18], an isomer of MC. The AEs of C3H5+ ion produced through these two pathways are 12.07 and 12.74 eV respectively. As shown in Table 2, the calculated value ΔHf, 298(CH3-C=CH2+) is 231.89 kcal/mol, which is basically consistent with the value of 237 kcal/mol reported by Bowen et al.[27].
Besides the pathway discussed above, CH2=CH-CH2+ is also a possible product, a H atom from C1 migrates to C2 via ts3 to yield int3, then the C3-C4 bond cleaved to produce CH2=CH-CH2+ and C2H3O2, or dissociates to C4H5O++CH3O and ultimately form three parts, CH2=CH-CH2+, CH3O, and CO, with no transition states. The theoretical AEs of these two C3H5+ ions are 11.76 and 12.42 eV, showing reasonable agreement with our experiment result. In addition, as shown in Table 2, the calculated value ΔHf, 298(CH2=CH-CH2+) is 223.812 kcal/mol, which is basically consistent with the 226.1 kcal/mol reported by Maccoll[28]. Compared with the experimental values of 11.93 and 12.77 eV, our calculations indicate that C3H5+ ions can proceed via several different pathways outlined in Fig. 5.
Channel 3 C4H5O2++CH3
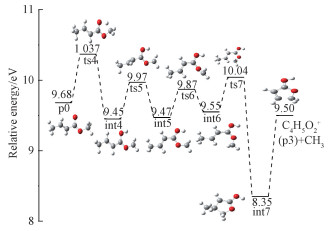
For the formation of C4H5O2+, obviously there are two nonequivalent methyl groups in the parent ion. Thus direct elimination of one methyl group is firstly considered. The theoretical AEs of products generated by C1 methyl elimination and C7 methyl elimination are calculated to be 12.87 and 11.67 eV, respectively, which are both much higher than the experimental value of (10.11±0.03) eV. Thus, formation of C4H5O2+ with the elimination of CH3 is not a one-step bond cleavage process. We then propose an energetically feasible pathway involving five-membered ring formation and hydrogen migrations, which is shown in detail in Fig. 6.
|
Download:
|
|
Fig. 6 Formation pathway of C4H5O2+ |
|
From C5H8O2+, firstly, one H atom from C7 migrates to O5 via transition state ts4 with an energy barrier of 0.69 eV, forming a relatively stable intermediate int4 which is 0.23 eV lower in energy than the molecular ion. Then, positional interchange of the H group and the CH3CH group occurs by rotation about the C2-C3 bond, giving rise to int5 via transition state ts5. Afterward, rotation around the C7-O6 bond transforms int5 into intermediate int6. Subsequently, two carbon atoms C2 and C7 in int6 come close to each other and ultimately link together to form a C-C single bond, leading to the formation of a more stable five-membered ring intermediate int7. Subsequently, the loss of the C1 methyl occurs to generate p3 through cleavage of the C1-C2 bond. According to theoretical calculations, ts4 has the highest energy along the entire pathway, and the corresponding appearance energy is 10.37 eV, showing good agreement with the experimental AE value of 10.11 eV.
Channel 4 C2H3O2++C3H5
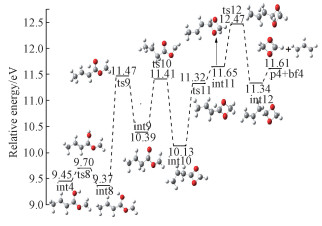
At first sight, the C2H3O2+ cation can be readily produced from a simple cleavage of the C3-C4 bond in the molecular ion. However, the expected structure of ionic fragment could not be located at the G3B3 level, indicating that formation of C2H3O2+ from MC should proceed through a more complicated mechanism. According to our theoretical calculations, an energetically feasible pathway is then proposed which is displayed in detail in Fig. 7.
|
Download:
|
|
Fig. 7 Formation pathway of C2H3O2+ |
|
Starting from int4, rotation of O5 happens to yield the intermediate int8 overcoming a barrier of 0.25 eV. Then, the H atom shifts to C3 to yield int9. Then, a four-membered ring intermediate int10 is produced via transition state ts10. Then, H atom shifts from C3 to C4 yielding int11, followed by H migration between C1 and C2. The final product p4 is yielded by breaking the C3-C4 bond in int12, accompanied with the loss of C3H5 radical. According to theoretical calculations, ts6 has the highest energy along the entire pathway, and the corresponding appearance energy is 12.47 eV, showing good agreement with the experimental AE value of 12.16 eV.
Channel 5 C4H4O++CH4O
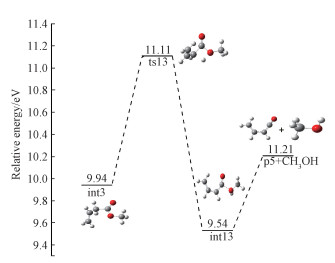
A proposed channel for this reaction to form C4H4O+ (p5) is shown in Fig. 8 which is actually branched from channel 2-b. Starting from int3, H atom shifts from C3 to O6 to yield int13 by overcoming a barrier of 1.17 eV. The final product p5 is yielded by breaking the C-O bond in int13, accompanied with the loss of CH3OH. The reaction path for the bond dissociation is computed at the B3LYP/6-31g(d) level, and no distinct transition states are found in the bond cleavage. The calculated AE for p5 is 11.26 eV, which is a little higher than the experiment value. In addition, as shown in Table 2, the calculated ΔHf, 298(C4H4O+) value is 198.71 kcal/mol, which is basically consistent with the value of 195.27 kcal/mol reported by Lias[24].
|
Download:
|
|
Fig. 8 Formation pathway of C4H4O+ |
|
Channel 6 C2H5++C3H3O2
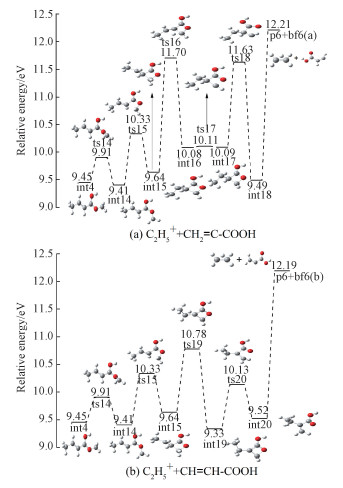
The species at m/z=29 is assigned to C2H5+. The results of our computations suggest a plausible formation pathway which is depicted in Fig. 9. Starting from int4, rotation around the O6-C7 bond via transition state ts14 generates int14. Secondly, a ring-formation step takes place, which leads to the formation of int15. Then, there are two different transformation pathways for int15. As shown in Fig. 9(a), an H atom of C7 migrates to C2 via ts16 with an energy barrier of 2.06 eV, followed by rotation of the H atom around C7-C3 to form int17. If the C2-C3 bond ruptures directly, the calculated energy for the pathway does not agree with the experiment value. Then the four-membered ring opening in int17 coupled with an insert step, insertion of C7 between C2 and C3, via transition state ts18, which leads to the formation of int18. It is further confirmed by IRC computations that ts18 really connects int17 and int18 directly, without any intermediates in between. Finally, the C2H5+ (p6) and a neutral part C3H3O2 (bf6a) are produced by the fission of the C2-C7 bond without any apparent transition state. There is another possible pathway of the formation of C2H5+ which is shown in Fig. 9(b). Starting from int15, an H atom at C3 migrates to C2 via ts19, generating int19. A ring-open step takes place, just like the one in channel 6a, which leads to the formation of int20. Finally, the C2H5+ (p6) and a neutral part C3H3O2 (bf6b) are produced by the directly fission of the C2-C3 bond and no transition state is found at the G3B3 level. As shown in Table 2, the calculated ΔHf, 298(C2H5+) value is 215.577 kcal/mol, which is basically consistent with the value of 218.93 kcal/mol reported by Maccoll[28]. The energy difference between channel 6a and channel 6b is so small that our experiment can not distinguish between the two isomers.
|
Download:
|
|
Fig. 9 Formation pathways of C2H5+: C2H5++CH2=C-COOH (a) and C2H5++CH=CH-COOH (b) |
|
In this study, VUV-TOF-PIMS coupled with technics of supersonic expansion molecular beam was used to investigate the photoionization and dissociation of methyl crotonate in the photon energy region 9.0~14.5 eV. IE of Methyl crotonate and AEs of major fragments C4H5O2+, C4H5O+, C4H4O+, C2H3O2+, C3H5+, and C2H5+ were obtained from the PIE curves. The fragment ions were identified, and possible formation channels were proposed and discussed in detail based on experimental AEs and energies predicted by ab initio G3B3 calculations. To sum up, hydrogen shift dominates most of the fragment channels of the MC cation, and ring closing/opening also plays an important role. The present study is of great importance for understanding the photoionization and dissociation processes of methyl crotonate and similar α, β-unsaturated esters in the photon energy region 9.0~14.5 eV.
| [1] |
Graedel T. Chemical compounds in the atmosphere[M]. Orlando: Academic Press Orlando FL, 2012.
|
| [2] |
Ryou M, Thompson C C. Tissue adhesives: a review[J]. Techniques in Gastrointestinal Endoscopy, 2006, 8(1): 33-37. DOI:10.1016/j.tgie.2005.12.007 |
| [3] |
Srivastava S. Co-polymerization of acrylates[J]. Designed Monomers and Polymers, 2009, 12(1): 1-18. DOI:10.1163/156855508X391103 |
| [4] |
Teruel M A, Benitez-Villalba J, Caballero N, et al. Gas-phase oxidation of methyl crotonate and ethyl crotonate. kinetic study of their reactions toward OH radicals and Cl atoms[J]. The Journal of Physical Chemistry A,, 2012, 116(24): 6127-6133. DOI:10.1021/jp2113889 |
| [5] |
Zhao C X, Liu B L, Peng G, et al. Synthesis and properties of flame retardant vinyl acetate copolymer emulsion[J]. Journal of the Graduate School of the Chinese Academy of Sciences, 2011, 28(5): 591-596. |
| [6] |
Du Q, Shan X B, Liu F Y, et al. Vacuum ultraviolet photoionization mass spectrometric study of methyl acrylate[J]. Journal of the Graduate School of the Chinese Academy of Sciences, 2009, 26(6): 749-758. |
| [7] |
Ma S M, Yu Z Q. Synthesis of butenolides via transition metal-catalyzed cyclization reaction of 2, 3-allenoic acids[J]. Journal of the Graduate School of the Chinese Academy of Sciences, 2005, 22(1): 110-121. |
| [8] |
Canosa-Mas C E, Flugge M L, King M D, et al. An experimental study of the gas-phase reaction of the NO3 radical with α, β-unsaturated carbonyl compounds[J]. Physical Chemistry Chemical Physics, 2005, 7(4): 643-650. DOI:10.1039/B416574H |
| [9] |
Grosjean D, Grosjean E, Williams E L. Rate constants for the gas-phase reactions of ozone with unsaturated alcohols, esters, and carbonyls[J]. International Journal of Chemical Kinetics, 1993, 25(9): 783-794. DOI:10.1002/kin.550250909 |
| [10] |
Grosjean D. Atmospheric chemistry of toxic contaminants 1. Reaction rates and atmospheric persistence[J]. Journal of the Air and Waste Management Association, 1990, 40(10): 1397-1140. DOI:10.1080/10473289.1990.10466792 |
| [11] |
Grosjean D, Williams E L Ⅱ. Environmental persistence of organic compounds estimated from structure-reactivity and linear free-energy relationships. Unsaturated aliphatics[J]. Atmospheric Environment. Part A. General Topics, 1992, 26(8): 1395-1405. DOI:10.1016/0960-1686(92)90124-4 |
| [12] |
Blanco M B, Barnes I, Teruel M A. FTIR gas-phase kinetic study of the reactions of Cl atoms with (CH3)2C=CHC(O)H and CH3CH=CHC(O)OCH3[J]. Chemical Physics Letters, 2010, 488(4-6): 135-139. DOI:10.1016/j.cplett.2010.02.023 |
| [13] |
Wang J, Yang B, Cool T A, et al. Absolute cross-sections for dissociative photoionization of some small esters[J]. International Journal of Mass Spectrometry, 2010, 292(1-3): 14-22. DOI:10.1016/j.ijms.2010.02.010 |
| [14] |
Wang S S, Kong R H, Shan X B, et al. Performance of the atomic and molecular physics beamline at the National Synchrotron Radiation Laboratory[J]. Journal of Synchrotron Radiation, 2006, 13(6): 415-420. DOI:10.1107/S0909049506030536 |
| [15] |
Chen J, Cao M Q, Wei B, et al. Vacuum ultraviolet photoionization mass spectrometric study of cyclohexene[J]. Journal of Mass Spectrometry, 2016, 51(2): 169-181. DOI:10.1002/jms.3743 |
| [16] |
Fang W Z, Gong L, Zhang Q, et al. Dissociative photoionization of 1, 3-butadiene: experimental and theoretical insights[J]. The Journal of Chemical Physics, 2011, 134(17): 174306. DOI:10.1063/1.3575401 |
| [17] |
Sun Y, Zhao Y J, Fang W Z, et al. Dissociative photoionization of methylbutenol experimental and computational investigations[J]. Journal of the Graduate School of the Chinese Academy of Sciences, 2011, 28(2): 161-168. |
| [18] |
Sun R R, Meng Q H, Wang M, et al. Experimental and theoretical study on the dissociative photoionization of methyl methacrylate[J]. Journal of Physics B: Atomic, Molecular and Optical Physics, 2017, 50(23): 235101. DOI:10.1088/1361-6455/aa92d4 |
| [19] |
Frisch M J, Trucks G, Schlegel H B, et al. Gaussian 09, Revision A[CP]. Gaussian: Wallingford, CT, 2009.
|
| [20] |
Jensen F. Introduction to computational chemistry[M]. 2nd ed. Chichester: John Wiley & Sons, 2007.
|
| [21] |
Foresman J B, Frisch A. Exploring chemistry with electronic structure methods: a guide to using Gaussian[M]. Pittsburgh: Gaussian Incorporated, 1996.
|
| [22] |
Fukui K. The path of chemical reactions-the IRC approach[J]. Accounts of Chemical Research, 1981, 14(12): 363-368. DOI:10.1021/ar00072a001 |
| [23] |
Baboul A G, Curtiss L A, Redfern P C, et al. Gaussian-3 theory using density functional geometries and zero-point energies[J]. The Journal of chemical physics, 1999, 110(16): 7650-7657. DOI:10.1063/1.478676 |
| [24] |
Lias S G, Bartmess J, Liebman J F, et al. Gas-phase ion and neutral thermochemistry[J]. Journal of Physical and Chemical Reference Data, 1988, 17(1): 1-861. DOI:10.1063/1.555819 |
| [25] |
NIST. 2-Butenoic acid, methyl ester, (E)-[S/OL]. (2009-07-31)[2019-03-19]. https://webbook.nist.gov/cgi/cbook.cgi?ID=C623438&Units=SI&Mask=221.
|
| [26] |
Liu X Y, Zhang W J, Wang Z Y, et al. Dissociative photoionization of isoprene: experiments and calculations[J]. Journal of Mass Spectrometry, 2009, 44(3): 404-409. DOI:10.1002/jms.1518 |
| [27] |
Bowen R D, Williams D H, Schwarz H, et al. Energy barriers for isomerization of gaseous C3H5+ ions[J]. Journal of the American Chemical Society, 1979, 101(16): 4681-4683. DOI:10.1021/ja00510a040 |
| [28] |
Maccoll A. Plenary lecture: ion enthalpies and their application in mass spectrometry[J]. Journal of Mass Spectrometry, 1982, 17(1): 1-9. |
 2021, Vol. 38
2021, Vol. 38 


