2) Laboratory for Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China;
3) Institute of Evolution & Marine Biodiversity, Ocean University of China, Qingdao 266003, China
Bacteria regulate the expression of the related genes by quorum sensing (QS) system, which is also an important mechanism of information exchange among bacterial cells. QS system involves the production, release, and detection of extracellular signal molecules called autoin-ducers (AIs). These molecules allow cells to coordinate gene expression based on cell density and play an important role in many physiological metabolic processes, such as bioluminescence, sporulation, motility, antibiotic production, the formation process of sea-snow, biofilm formation and bacterial virulence factor secretion (Nealson and Hastings, 1979; Jatt et al., 2015; Hmelo, 2017). Quorum sensing is closely related to human healthcare, agricultural production and environmental protection. For a given QS system, the QS signal molecules can be sensed and the expression of the target genes can be regulated to adapt to (i.e., a threshold population density) is reached the complex environment once a threshold concentration (Defoirdt, 2018). Quorum quenching (QQ) can attenuate the pathogenicity of pathogens by destroying the QS system, which is crucial to the prevention and control of pathogens. The mechanism is to prevent bacteria from expressing virulence factors through degrading specific signal molecules to control the concentration of signal molecules (Kalaiarasan et al., 2017). Meanwhile, QQ is considered as an environment-friendly disease prevention and control measure. This measure postpones the evolution of drug resistance and reduces the production of super bacteria because it only targets virulence factors or the expression of virulence factors without killing the pathogens. Microorganisms contain abundant quorum quenching substances, including enzymes and natural product small molecules (Tang et al., 2015). With the development and utilization of marine resources in recent years, a large number of ocean-derived quorum sensing quenchers play an increasingly important role. The emergence of QQ has provided new ideas for healthcare, agriculture, aquaculture and the environmental protection.
2 Diversity of Quorum Sensing Systems and Signaling MoleculesQS is a microbial communication mechanism that regulates gene expression and coordinates microbial group behavior. Bacteria use specific enzymes to synthesize a class of signal molecules and release them into the environment. When the concentration of signal molecules in the environment accumulates to a certain threshold, signal molecules can be recognized by the receptor proteins, and then regulate the expression of the target genes directly or indirectly to initiate the 'cooperative' behavior to benefit the whole population.
QS is commonly found not only in bacteria but also in fungi (Table 1). QS phenomenon in fungi was revealed by the inhibitory effect of farnesol on pathogenic polymorphic fungus Candida albicans. The lipids (oxylipins), peptides (pheromones), alcohols (tyrosol, farnesol, tryptophol, and 1-phenyl-ethanol) and acetaldehydes are involved in fungal QS system and regulate their various physiological behaviors. Although the study of fungal QS system is still in the infancy, population density behaviors similar to QS have been found in several fungal species (Padder et al., 2018). Furthermore, several remarkable results have shown that bacterial QS signals can also be perceived by plants and animals. An example is that plants treated with 3OC14-HSL have increased resistance to the fungal pathogen Blumeria graminis. Another example is that when Arabidopsis roots were treated with N-acylhomoserine lactone (AHL), they could produce systemic resistance to the biotrophic fungus Golovinomyces. The protection system in plants may be closely related to AHL-mediated QS system (Schenk et al., 2014). The animal studies have shown that azithromycin (AZM) can improve the infection of Pseudomonas aeruginosa. In the experiments of mice, treatment with AZM reduced bacterial load in the lungs of mice, and the QS-regulated lasB gene expression was down-regulated in vivo. AZM treatment also downregulated the production and polymerization of alginate, which, combined with reducing QS responses, was an important reason for reduced survival (LaSarre and Federle, 2013). The perceptions of QS signals by animals and plants have some differences: plants respond differentially to various AHLs, while animals only respond to 3OC12-HSL.
|
|
Table 1 Quorum sensing systems of microorganisms |
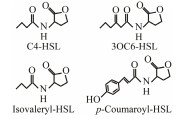
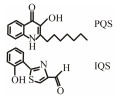
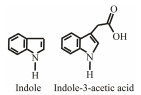
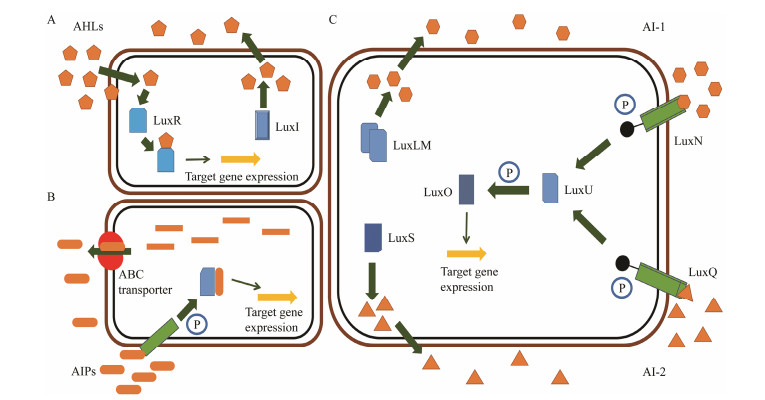
The discovery of bacterial QS was featured by the report of luminescence phenomenon of gram-negative bacteria Vibrio fischeri (Nealson and Hastings, 1979). AHLs (N-acyl-homoserine lactones) is the most commonly used autoinducer for gram-negative bacteria, and there are more than 25 kinds of gram-negative bacteria regulated by AHLs. Marine snow is the product of the interaction of organic and inorganic matters mediated by microorganisms, which plays a crucial role in the transport of materials from the sea surface to the deep sea. Research has shown that marine snow particles contain AHL-type signal molecules, and the N-(3-oxo-hexanoyl)-1-homoserine lactone (3OC6-HSL) and C8-HSL were identified directly from marine snow particles (Jatt et al., 2015). The AHLs signal molecules consist of a hydrophobic head, a highly conserved serine lactone ring, a hydrophilic tail and variable amide side chain with its tail determining its diversity (Dong et al., 2001). The typical AHLs-regulated QS system plays a regulatory role by binding the LuxI-type synthase to the LuxR-type receptor, in which LuxI encodes AHLs signal synthase and LuxR encodes AHLs signal receptor regulatory protein. AHLs are membrane-permeable molecules and can diffuse to the exterior of the cell membrane randomly. When AHLs accumulated to a certain concentration in the environment, the molecules diffused through the cell membrane and bound to the amino terminus of LuxR receptor proteins in the cytoplasm to form the LuxI/LuxR protein complexes and regulate the expressions of certain functional genes. At the same time, the LuxI/LuxR protein complexes also have a feedback regulation effect on the production of AHLs signal molecules and their receptor proteins. The LuxI/LuxR two-component system of V. fischeri is considered as a model system for gram-negative bacterial QS (Fig. 1A). The luminescence phenomenon of V. fischeri was regulated by QS, which had provided protection for the symbiotic host and enabled V. fischeri to obtain suitable habitats (Nealson and Hastings, 1979). In addition to AHLs, many signal molecules with different chemical structures are discovered in gram-negative pathogens, including AI-2, PQS, indole, pyrones, DARs and CHDs (Diggle et al., 2006; Brachmann et al., 2013; Pereira et al., 2013; Brameyer et al., 2015; Lee et al., 2015).
|
Fig. 1 Three typical QS pathways in different bacteria. A, LuxI/R pathway of Vibrio fischeri; B, AIPs-mediated QS system in gram-positive bacteria; C, The QS pathway of Vibrio harveyi. Different types of orange patterns represent different signaling molecules. |
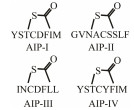
In gram-positive bacteria, the QS pathway is essentially the same as the gram-negative bacteria. The difference between the two types of bacteria is the gram-positive bacteria mainly use AIPs to achieve the information exchange among cells. The immature AIPs molecules enter and exit cells through a specific transportation system and are modified into mature AIPs molecules in the transport process. Gram-positive bacteria encode the synthesis of AIPs precursor peptides in the growth process, and these peptides can be modified to be stable and active AIPs. AIPs cannot cross the cell membrane freely, but they can cross the cell membrane with the help of ABC transporter (ATP-binding cassette transporter) or other membrane proteins (Singh and Ray, 2014). When the concentration of AIPs in environment reaches a threshold, the AIPs can bind to receptors located on the cell surface and activate the two-component phospho-kinase system (TCS) to initiate the corresponding signal transduction and finally initiate gene transcription (Fig. 1B). The AIPs are sensed by the two-component signal transduction system (TCSTS) comprising of transmembrane sensor kinase AgrC and response regulatory protein AgrA.
The release of virulence factors of Staphylococcus aureus is regulated by AIPs-mediated QS system. AgrC could be activated by AIPs to inhibit the expression of agr-regulated virulence factors in S. aureus. In addition, the AgrC was also found in other Staphylococcus species, such as Staphylococcus epidermidis (Kleerebezem et al., 1997).
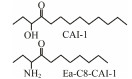
2.3 Quorum Sensing in Interspecies CommunicationAI-2 is a kind of signal molecule mediated by LuxS protein, which widely exists in gram-negative and grampositive bacteria and can sense the amounts of different types of microorganisms in the surrounding environment to regulate their metabolic behaviors (Pereira et al., 2013). LuxS/AI-2-mediated QS system was first found in the bioluminescence of Vibrio harveyi, which can produce signal molecules AI-1 and AI-2. AI-1 is encoded by luxLM gene, and LuxN is the corresponding receptor protein. AI-2 is a new signal molecule, whose receptor proteins are LuxP and LuxQ. LuxN and LuxQ can transmit signals through LuxU which is a phosphotransferase. The phosphorylated LuxU can transmit signals to the regulatory protein LuxO and activate the expression of certain genes with the help of protein LuxR (Fig. 1C). The pathogenicity of Edwardsiella tarda is related to the QS system mediated by LuxS/AI-2 (Zhang et al., 2008).
2.4 Other Types of Quorum SensingAI-3 is a less polar molecule that mediates the bacterial QS system, which can interact with the host adrenergic signaling system. The AI-3 system was first found in the intestinal tissues of gram-negative bacteria infected animals, involved in the pathogenic process of Enterohemorrhagic Escherichia coli (EHEC) and Shigella Castellani (Moreira and Sperandio, 2010).
In pathogenic bacteria, several different QS systems coexist in the same cells, and they act together to regulate the expression of target genes. In P. aeruginosa, additional QS systems are present using DKPs and PQS as signal molecules besides AHL mediated QS systems. At the same time, homologous genes encoding DKPs- and PQS-related proteins were also found in Pseudomonas and Burkholderia.
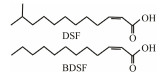
Most DSF and DF are isolated from agricultural biological control bacteria and agricultural pathogens. Among them, DF signal molecules can regulate the production of yellow pigment (xanthomycin) and extracellular polysaccharides (exopolysaccharides, EPS) in Xanthomonas campestris pv. campestris. These two products are essential for the colonization and pathogenicity of pathogens that parasitize in plant hosts. Previous research has shown that DF, a butyrolactone, is used as a signal molecule by Streptomyces (Poplawsky et al., 2005).
The physiological functions of fungi are also regulated by some chemical factors. Kugler et al. (2000) studied the parasitic fungus Histoplasma capsulatum and found that it had yeast-like form and filamentous form. H. capsulatum can adapt two morphological transformations by detecting the concentrations of cells in the environment. Farnesol (C15H26O) is a chemical signal secreted by C. albicans. It can not only be used as a virulence factor to prevent the transformation of C. albicans from yeast morphology to mycelia morphology, but also be used to inhibit the formation of biofilm during the early stage of cell adhesion. Tyrosine is another kind of QS signal molecule found in C. albicans, which can promote the transformation of yeast morphology into mycelia morphology. Farnesol and Tyrosine in C. albicans regulate the existence of microorganisms in fungi by antagonistic action (Ramage et al., 2002). QS plays an important role in the formation of mycelium and biofilm during fungal growth, but its specific signaling pathways and receptor proteins need to be further studied.
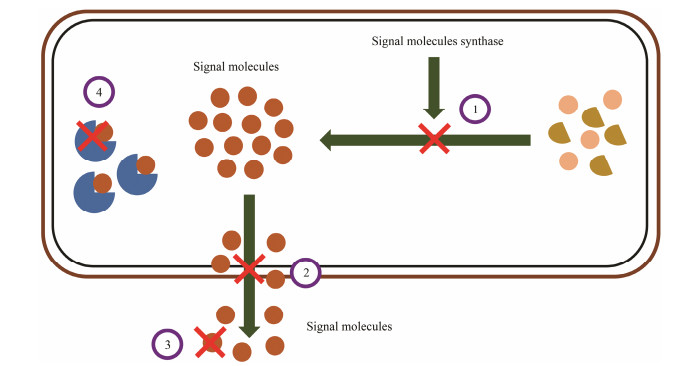
3 Quorum Quenching (QQ) in Nature and as a TherapyQQ is a mechanism to prevent pathogenic bacteria infection by interfering the QS system between microbial cells and preventing the expression of QS-dependent genes. In recent years, it has been found that bacteria can produce biofilm, a kind of membrane complex, to protect themselves. According to the statistics, there are more than 60 kinds of microbial infections caused by bacterial biofilm. QS system is involved in regulating bacteria to produce a large amount of extracellular mucopolysaccharides to form biofilm. Bacteria can be protected from the action of antibiotics and eliminate the host immune function by forming biofilms. Previous studies have shown that the pathogenicity of P. aeruginosa is closely related to the biofilm. Bauer et al. (2002) successfully blocked the bacterial QS system and further prevented the formation of biofilm by interfering with AI signal molecules, which provided a breakthrough for biological control of diseases caused by biofilm. QQ effect can occur at different stages of the QS pathway, which mainly includes four mechanisms: 1) Direct inhibition of the synthesis of signal molecules; 2) Inhibition of the transport of signal molecules; 3) Chemical or biological degradation of signal molecules; 4) Competitive inhibition of the combination of signal molecules and receptor (Fig. 2). An attractive aspect of QQ is that it does not kill pathogens and does not cause harsh selective pressures, thereby minimizing the production of drug resistance (von Bodman et al., 2008). Therefore, QQ is regarded as a promising biological control strategy, and is expected to become a new approach for antibacterial treatment and biological control.
|
Fig. 2 Inhibition mechanisms of quorum sensing system. 1, Inhibition of signal molecule synthesis; 2, Inhibition of signal molecule transport; 3, Degradation of signal molecules; 4, Competitive inhibition in the combination of signal molecules and receptors. |
Usually, substances with QQ activity can be classified into two categories according to their molecular weight: small molecule QS inhibitors (QSIs) and macromolecular QQ substances. In general, QSIs and macromolecular QQ substances can affect the bacterial QS system independently. However, when multiple QS systems exist in a single pathogen to jointly control their pathogenicity, they pose a challenge to biological control. Previous research has shown that the combination of QSIs and QQ enzymes almost completely blocked the QS system mediated by las and rhl in P. aeruginosa (Fong et al., 2018).
3.1 Small Molecule QS InhibitorsQSIs can interfere with the microbial QS system through competitive inhibition, disrupting the signaling pathway used for intra- and inter-species coordination for the expression of virulence factors, so as to reverse the regulation in the expression of target genes. Quite a few studies have indicated that marine organisms are a potential source of QSIs. These results are supported in the metagenomics studies that many high-abundance of marine bacteria have high abundance of QSIs and QQ genes, such as α-D-galactopyranosyl-(1→2)-glycerol (Floridoside) produced by Ahnfeltiopsis flabelliformis, a variety of compounds secreted by Chlamydomonas reinhardtii (Kim et al., 2007). In addition, the natural halogenated peroxides produced by algae (Laminaria digitata) can also be exploited as QSI to interfere with the microbial QS system. Saurav et al. (2016) found that most of the 14 sponge extracts collected in the red sea and Mediterranean region had QQ biological activity, and these QQ bioactive substances can be used to inhibit the expression of virulence factors in pathogenic bacteria. The andrographolide has been proved to be one kind of QS inhibitors, which can interfere with the AI-2-mediated QS system and reduce cell damage caused by avian pathogenic E. coli (Guo et al., 2014). However, QSIs may have certain limitations in practical applications due to the weak stability or low efficiency of natural QSIs in the environment, or potential toxicity to higher organisms. The way to overcome these limitations may be to design and synthesize new biomolecules based on natural QSIs.
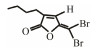
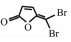
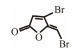
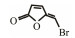
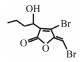
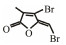
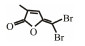
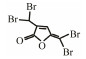
3.1.1 Natural QSIsStudies have shown that various substances extracted from different species exhibited QQ activity (Table 2). At present, many reports indicate that QSIs can be isolated from natural plants to reduce the pathogenicity of infecting bacteria. The brominated furanone produced by Delisea pulchra is the first natural product to be found to have QS inhibitory activity. The structure of furanone is similar to AHLs, which is able to interfere with the binding of AHLs to LuxR and then affect the QS system. Meanwhile, furanone can also inhibit the AI-2-mediated QS system (Defoirdt et al., 2007). Previous reports have highlighted the potential role of coumarin as an alternative therapeutic method based on its ability to block the QS signaling system and inhibit the formation of clinically relevant pathogen biofilms. Meanwhile, it was also found that coumarins can effectively control plant pathogens, aquaculture infection, food corruption and reduce the biological pollution caused by eukaryotes (Reen et al., 2018). In addition, the witch hazel tannin, isolated from the witch hazel, can inhibit the QS system of Staphylococcus epidermidis and Methicillin-resistant Stphylococcus aureus (Kiran et al., 2008). Flavonoids contained in Combretum albiflorum can inhibit the production of pyocyanin, elastase and biofilm formation in P. aeruginosa (Vandeputte et al., 2010). The ajoene extracted from Allium sativum can reduce the mortality of mice caused by P. aeruginosa (von Bodman et al., 2008).
|
|
Table 2 Natural QS inhibitors |
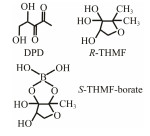
The competitive signal molecule inhibitors which are synthesized based on the chemical structure of natural signal molecules can be employed for obtaining QSIs. AI-2 homologues have potential applications in controlling bacterial infection and biofilm formation. The luminescence experiments of V. harveyi confirm that some AI-2 homologues can interfere with AI-2 activity, thereby block QS system (Nealson and Hastings, 1979). Tedder et al. (2004) reported several homologues of AI-2, which can inhibit the activity of MTA nucleosidase involved in the synthesis of AI-2. Some boronic acid compounds and homologues of DPD (DPD is the synthetic substrate for AI-2) have proved to be an antagonist of AI-2. In addition, some studies have found that 4-[(anilino) thiomethyl] amino-N-phenylben-zenesulfonamide (LED209) can effectively interfere with the QS regulatory system of E. coli, Salmonella and Francisella, and reduce their pathogenicity (Rasko et al., 2008). Based on the structure of natural brominated furanone, a library of chemical molecules with different substituents on the side chain and furan ring and with different side chain lengths was designed, while the QSI activity of some of these compounds was confirmed (Table 3). AIPs is a type of short peptide molecule and a signal molecule of gram-positive bacteria. According to the molecular structure of AIPs, some structural analogs of AIPs have been designed as QSIs. For example, the AIPs peptide containing only the sulfur lactone ring structure can simultaneously serve as QSI in the S. aureus and between the S. aureus populations. RNAIII-inhibiting peptide (RIP) is a linear AIPs peptide which can reduce the virulence of S. aureus and has good therapeutic effects. At the same time, researchers also found that the RIPs could inhibit bacterial resistance and biofilm formation (Giacometti et al., 2005).
|
|
Table 3 Several representative brominated furanones |
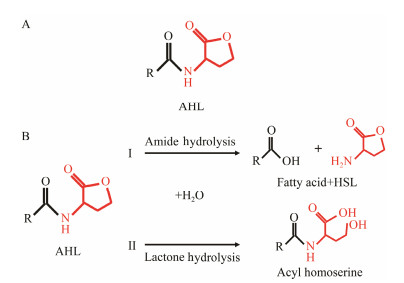
In addition to QSIs, a variety of macromolecular QQ substances were discovered. Unlike the competitive inhibition mechanism of QS small molecule repressor, the macromolecular QQ substances mainly blocked the QS pathway by degrading the QS signal molecules. The macromolecular QQ substances have been discovered mainly to inhibit the QS system that depends on AHLs-type signal molecules, but some studies have also reported the enzymatic hydrolysis of DSF, PQS and AI-2. For example, DSF molecules can be degraded in various bacteria hosts, such as Bacillus, Staphylococcus, and Pseudomonas (Newman et al., 2008). At present, the studies on macromolecular QQ substances are mainly focused on the QQ enzymes of degradable AHLs signal molecules. Some studies have also found that some antibodies can block QS by isolating or degrading AHLs signal molecules (LaSarre and Federle, 2013). AHLs degrading enzymes can be classified into three types according to their degradation mechanisms: AHL lactonases, AHL acylases and AHL oxidoreductases (Bzdrenga et al., 2017). The general structure of AHL signals and two mechanisms by which AHLs can be inactivated are showed in Fig. 3.
|
Fig. 3 Structure of AHL signal molecule and two mechanisms of degradation of AHL. A. The structure of AHL. B. Two mechanisms for degradation of AHL signal molecule. Ⅰ: AHL acylase cleaves amide bond and produces HSL and the corresponding fatty acids; Ⅱ: AHL lactase cleaves the lactone ring to obtain the corresponding acyl-homoserine. |
AiiA isolated from Bacillus sp. 240B1 was the first AHL lactonase to be found. AiiA can interfere with the QS system by degrading signal molecules, for example, it can degrade the AHLs signal molecules in Erwinia carotovora to attenuate the degree of decay (Dong et al., 2001). Muricauda olearia Th120, isolated from the gill of Paralichthys olivaceus, has obvious AHLs degradation activity. It can effectively inhibit biofilm formation and virulence factor release of P. aeruginosa PAO1 (Tang et al., 2013). Tang et al. (2013) isolated a novel AHL lactonase MomL from M. olearia Th120. MomL belongs to the metallo-β-lactamase family and shares 24.5% identity with AiiA. According to liquid chromatography mass spectrometry, MomL had significant degradation capability on AHLs molecules from N-hexanoyl-homoserine lactone (C6-HSL) to 3OC14-HSL. And the degradation activity of C6-HSL was 10 times higher than that of AiiA. In addition, MomL can significantly reduce the extracellular protease activity of P. aeruginosa PAO1 (Tang et al., 2015). However, AHL lactone enzyme also has certain structural defects. AHL lactonase can cleave the lactone ring to form N-acyl homoserine, but the product was unstable and could automatically close the loop under acidic conditions to restore AHL signal molecules activity. Therefore, AHL lactonase is not an excellent quorum sensing quencher. Recent studies have found that in the QQ system of prokaryotes, a group of 'lactoneases', encoded by the bpiB gene, is responsible for inhibiting the formation of the biofilm. At the same time, three homologues encoding BpiB01, BpiB04 and BpiB07 were detected in Nitrobacter sp. strain Nb-311A, Pseudomonas fluorescens and X. campestris (Schipper et al., 2009).
3.2.2 AHL acylaseAHL acylase is a class of QQ enzymes that can catalyze the degradation of amide bonds in AHLs molecules and release homoserine lactones and the corresponding fatty acids. The AHLs degradation catalyzed by AHL acylase is an irreversible reaction. Compared with AHL lactones, it is a quorum sensing quencher with great applicability. Variovorax paradoxus is the earliest discovered bacteria with AHL acylase activity. It can use AHLs signal molecules as its sole source of energy and nitrogen to sustain survival, but its related genes have not yet been identified (Leadbetter and Greenberg, 2000). In addition, the AhlM in Streptomyces bacteria and AiiD in Ralstonia XJ12B also belong to the acylase family. In vitro experiments showed that AiiD and AhlM could greatly reduce the swimming of P. aeruginosa, extracellular elastase activity, secretion of pyocyanin, and the pathogenicity of nematodes (Tang et al., 2015).
3.2.3 AHL oxidoreductaseAHL oxidoreductase is the third group of QQ enzymes, which is the same as the AHL lactonase, AHL oxidoreductase can catalyze the cleavage of lactone ring in AHLs signal molecules to form homoserine (Bzdrenga et al., 2017). AHL oxidoreductase activity was firstly found in human epithelial cells and was later found to be widespread in mammalian cells. There are three isozymes of AHL oxidoreductase, which usually have high hydrolysis activity for long-chain AHLs signal molecules and low activity for short-chain AHLs molecules.
3.3 Quorum Quenching AntibodiesQS signal molecules cannot be used as immunogens because they are unstable small molecules. However, recent studies have found that bacterial AHLs signal molecules can induce apoptosis in mammalian cells and regulate the activity of NF-κB, which is the key regulatory factor of innate immunity (Kravchenko et al., 2008). Thus the use of antibodies to neutralize QS signal molecules has also become a promising strategy to quench the QS system of bacteria.
Kaufmann et al. (2006) made the first attempt to apply immunological drugs to treat QS-mediated bacterial infections, using monoclonal antibody RS2-1G9 produced by the 3-oxo-AHL homologue RS2, which could inhibit QS system based on the AHLs in P. aeruginosa. RS2-1G9 showed good specificity and affinity for 3-oxo-C12-HSL. The effect of active immunization with 3-oxo-C12-HSL-carrier protein conjugate on acute pulmonary infection caused by P. aeruginosa in mice were investigated, and the result showed that 3-oxo-C12-HSL had protective effect in acute pulmonary infection. Along with the in creasing interest in AHL-targeting antibodies, a novel series of targeted antibodies directed against different AHL molecules have recently been developed. The active antibodies are able to efficiently recognize OC10-HSL and C8-HSL, as well as cross-react with other AHL molecules. These antibodies are more effective in recognizing the forms of AHL open lactone ring, such as OC10-HL (homoserine lactone), C10-HL and C8-HL (Chen et al., 2011). The other QS signals such as PQS analog coupled to BSA as the antigen is another development strategy for antibody-based quorum quenching strategies. In addition, sulfone compounds with structure similar to the hydrolysis products of AHL lactone ring can inhibit the activity of lactonase, and the active antibodies produced by this compound can reduce the toxicity of bacteria (De Lamo Marin et al., 2007). The above data demonstrate the effectiveness of the antibody-based QQ strategy, which has been verified in animal models. In the future, this method aiming at interfering with cell communication and eliminating the control of key pathogenic functions is expected to become a new therapeutic strategy for animal and human pathogens.
4 QQ Applications in Agriculture, Aquaculture, Environmental Protection, Medicine and Food Processing and Safety 4.1 Application of Quorum Quenching in AgricultureWith the rapid growth of the population, the global demand for food and agricultural crops is increasing at a rapid pace. However, plant pathogens cause huge economic losses to agriculture every year. Traditionally, antibiotics are recognized as effective agents to control bacterial pathogens. However, the widespread use of antibiotics has created a series of problems, such as environmental pollution, ecological balance destruction and drug resistance. Therefore, more and more attentions have been paid to biological control of plant diseases. QQ can effectively control plant diseases by regulating the expression of genes related to plant pathogens to improve the efficiency of agricultural production. For these reasons, QQ is considered to be a possible alternative or complementary strategy for antibiotics.
Dong et al. (2000) transferred the plasmid carrying aiiA gene into Erwinia carotovora strain SCG1 and found that the expression of aiiA could interfere with QS system and inhibit the production of virulence factors. Several plants including Chinese cabbage, eggplant and potatoes were infected with the recombinant pathogens without getting soft rot symptoms. This is the first application of QQ in the area of biological disease control. Pseudomonas aureofaciens 30–84 is a symbiotic bacterium that can regulate the production of phenazine antibiotics by AHLs-mediated QS system. At the same time, it can protect wheat against Gaeumannomyces graminis var. tritici and improve the resistance of wheat to fungal infection. The exchange of signals between bacteria allows them to coordinate many different physiological activities. In legume rhizobia, the establishment and regulation of the symbiotic interactions between nitrogen-fixing bacteria and plant hosts are closely related to the QS system. This symbiotic interaction can enhance the nitrogen fixation by stimulating the QS system in these bacteria and reducing the demand for fertilizer and financial investment for crop hosts. It can protect the environment and maintain the ecological balance (Cao et al., 2009).
4.2 Application of Quorum Quenching in AquacultureAquaculture is one of the fastest growing food production systems in the world. However, the frequent occurrences of diseaseshave hindered its further development. Antibiotics are considered to be an effective treatment for bacterial diseases. The widespread and frequent use of antibiotics in aquaculture has resulted in the rapid propagation of antibiotic-resistance bacteria and has seen the situation becoming even more precarious in the near future if no adequate measures are undertaken. Finally, it may even threaten human health and safety (Harms et al., 2016). In order to promote sustainable development of the aquaculture industry and reduce the threat to human health, novel strategies are needed to control bacterial infections. QQ technology has shown potential application values in the prevention and treatment of harmful aquatic pathogens.
Bacterial diseases are one of the most critical problems in commercial aquaculture. Vibrio caused high mortality rates in almost every type of aquaculture organisms, such as mollusks, crustaceans and fishes. Research has shown that the virulence of V. harveyi towards different host organisms depends on the QS system in vivo. We can reduce its pathogenicity and improve the survival of fish and shrimp larvae by interfering with its QS system (Defoirdt et al., 2008). Some QQ enzymes are considered to be an economically-friendly alternative. Research has showed that AHL lactonase (AiiAB546) from Bacillus sp. B546 produced by Pichia pastoris reduced Aeromonas hydrophila infection in zebrafish. A. hydrophila and Vibrio parahaemolyticus are common aquatic pathogens in aquaculture to use AHL-mediated QS system to regulate the release of virulence factors (Defoirdt et al., 2004). V. parahaemolyticus is responsible for significant infections in shrimp and contributes to gastroenteritis in people who consume the infected shrimp. QQ enzymes play a potential role in inhibiting the formation of V. parahaemolyticus virulence and reducing its pathogenicity.
Another strategy for disease control using organisms or extracts with autoinducer degradation capacities has been applied. For example, Bacillus sp. QSI-1 with AHLase activity, isolated from the intestines of the Carassius auratus, can increase the survival rate of infected zebra fishes (Chu et al., 2014). The ability to incorporate biocontrol bacteria in the rearing water or through bio-encapsulation in raw materials (e.g., Artemia nauplii) is an advantage of this strategy, suggesting that the QQ is particularly attractive to restrict bacterial infections in aquaculture industry and is conducive to the development of new technology for the prevention and control of aquatic diseases. However, the use of AHLs-degrading bacteria to fight bacterial fish disease may be harmful to invertebrates. There-fore, other strategies may be proposed, such as the use of probiotic bacteria. The relevant reports suggest that the combination of probiotics and QQ strategy may be beneficial because the inhibitory activity of Phaeobacter on Vibrio anguillarum is independent of the QS system in aquaculture environments (García et al., 2013).
4.3 Application of Quorum Quenching in Preventing Environmental PollutionMarine environmental pollution has caused tremendous damage to marine organisms and posed a serious threat to people's lives. Biofouling is a serious problem for marine industries and marine environment (Dobretsov et al., 2011). In the past, we used efficient antifouling molecules such as tributyltin (TBT) to control biofouling, but it was forbidden due to its high toxicity and pollution. In view of the fact that QQ is an environmentally friendly control strategy, it has become an important technology for inhibiting the biological pollution at early stages.
AHLs-degrading enzymes or QSIs can reduce the biological pollution. It has been reported that the use of QSIs in coatings can prevent biofouling. Kojic acid has been reported as a non-toxic QSI. When added to the paints, it has the ability to inhibit the contamination of bacteria and diatom granules within a month to reduce marine biofouling (Dobretsov et al., 2011). Relevant reports have described the QQ effect of Piper Betle, which can reduce biofouling in membrane bioreactors. At the same time, the use of QQ enzymes also serve as a manner to disrupt information exchange between bacteria and restrict the formation of biofilms to reduce damage to the filtration system (Siddiqui et al., 2012). Lee et al. (2014) immobilized the acylase and applied it to water treatment and the results showed that immobilized acylase could effectively reduce biofilm formation. Overall, further study on the role of bacterial QS in biofouling is a new direction of developing novel and less toxic biofouling control agents.
4.4 Application of Quorum Quenching in MedicineDue to the extensive development of drug-resistant bacteria, antimicrobial resistance is a new major threat to the field of public healthcare. It has become an urgent research topic to find new methods to inhibit bacterial infection and solve the problem of bacterial drug resistance (Saurav et al., 2016). QQ-based new type therapeutic strategy is one of these anti-virulence approaches which aims at reducing virulence functions and behaviors (including biofilm formation) rather than killing pathogens. Therefore, this method provides less selective pressure for evolving new resistance mechanism towards antibiotics treatment.
Current research suggests that more than 80% of the bacteria can form biofilms and therefore contribute to many infectious diseases. Compared with free cells, cells living within biofilm have higher resistance to antibiotics with the minimum inhibitory concentration being increased for 100–1000 times. The study also found that the resistance of new biofilms to antibiotics was not as good as the aged biofilms (Stoodley et al., 2002). Treatments of lung infected with P. aeruginosa in the form of biofilms require much higher quantity of antibiotics than in vitro sensitive experiments, and P. aeruginosa has high intrinsic resistance to many antibiotics (Singh et al., 2000). Learning the mechanism of drug-resistance and pathogenicity of P. aeruginosa to develop new anti-P. aeruginosa drugs are important topics in current microbiology and medicine. Study suggested that the destruction of QS system directly affect the formation and variation of biofilm. For example, the wild-type biofilms are typically mushroom-shaped, while mutant strains lacking AHLs can prevent premature biofilm development or form the biofilm with a loose structure or missing effects. In addition, QS also plays a major role in the regulation of virulence factor production by Staphylococci. Chemical inhibitors that block this process and prevent the production of exotoxins and extracellular enzymes could have medical utility for infection prophylaxis and therapy (Quave and Horswill, 2018). The rapid spread of antibiotic-resistance strains and the ineffective treatment of biofilmassociated infections urge people to discover more effective treatment modalities than antibiotics. Among them, the way of interfering with the process of pathogen QS system to prevent its pathogenicity is the most promising new strategy for drug development.
QS-targeted compounds can attenuate virulence and prevent microbial infections by interfering with QS system among microbial populations. Therefore, QS system can be used as a new target for drugs development. Serratia marcescens can cause widespread infection by producing C6-HSL and N-(3-ketohexanoyl) homoserine lactone, and regulate prodigiosin production and biofilm formation. When the furanone derivatives were used to treat P. aeruginosa infection, the number of P. aeruginosa cells in infected lung tissue was significantly reduced and the symptoms of disease were alleviated in mice (Wu et al., 2004). In laboratory studies, some QSIs have provided encouraging results, but the use of QSIs in clinical practice still require a significant amount of time. One reason is that a lot of problems need to be overcome before the new drug can be released on the market. Another reason is that although many compounds are considered QSIs, only a few compounds have the molecular target been identified (Suneby et al., 2017).
4.5 Application of Quorum Quenching in Food Processing and SafetyFood-borne microorganisms, including production-type food microorganisms, food-borne pathogenic microorganisms and rot-inducing food microorganisms, are closely related to food safety. A variety of food-borne pathogenic microorganisms can form the corresponding biofilm. This phenomenon is widespread in a variety of food-borne pathogenic microorganisms and it has an important effect on food processing and safety. QS is a key factor in the regulation of biofilm formation.
QS plays an important role in the formation of biofilms while the formation of biofilms also affects the regulation of QS, casting synergistic effects in between. Food-borne disease caused by Salmonella is one of the most important public health problems in the world, which seriously threatens human health. Listeria monocytogenes can easily form biofilms on food processing facilities and containers. This protection mechanism can be used to form more powerful mixed biofilms with other microorganisms, which are difficult to remove and often cause food-borne diseases (Daneshvar Alavi and Truelstrup Hansen, 2013). V. parahaemolyticu is widely distributed in food-borne pathogen that can cause gastroenteritis or food poisoning when they are ingested together with uncooked or improperly cooked food. Studies have shown that brominated furanone can inhibit the activity of QS signal molecule AI-2 and the expression of luxS gene in V. parahaemolyticus. Its biofilms and extracellular enzymatic activity are also affected similarly (Phuvasate et al., 2012). Therefore, QQ strategy can inhibit the pathogenicity of bacteria by affecting the formation of biofilms.
On the other hand, microorganisms in biofilms can degrade wastes such as waste residue in the food industry, remediate the polluted environment, and promote the adaptability of beneficial microorganisms towards better stability (Singh et al., 2006). Therefore, it is expected to solve practical problems in the food industry such as leavening agent instability. In addition to controlling the formation of biofilm by regulating QS system and reducing the resistance of microorganisms, it is also possible to promote the formation of certain probiotic biofilms through the regulation of the QS system to accelerate the synthesis of certain human metabolites. Simultaneously, biofilm can also be used to improve the environmental adaptability and stability of probiotics. Lactobacillus is a kind of probiotic and can metabolize lactic acid and other antibacterial substances. It is widely used in yogurt, kimchi and other products. Some researches found that when Lac- tobacillus plantarum HE-1 produces bacteriostatic substances, the synthesis of AI-2 and biofilm almost reached the peak at the same time during the metabolic process, indicating that the metabolism of lactic acid bacteria to bacteriostatic substances may be dependent on QS system and biofilm formation. However, the detailed regulatory mechanisms have not been fully characterized (Risoen et al., 2000). It is considered to enhance the formation of biofilm through population induction to increase the production of beneficial metabolites and to provide new ideas for food production.
5 ProspectsDuring recent decades, we start appreciating that in the bacterial pathogens of plants, animals and humans, the production of certain virulence factors is controlled by the quorum sensing system. Traditional antibiotics are regarded as potent agents in controlling bacterial pathogens, but the abuse of antibiotics at current time has caused serious drug-resistance problem and accelerated the emergence of many antibiotic-resistant superbacteria (Harms et al., 2016; Defoirdt, 2018). Therefore, new antibiotic substitution strategies are needed to reduce the emergence of superbacteria. The anti-virulence therapy developed by using agents that interfere with QS system of bacterial pathogens is an intensively studied strategy at present time. However, disruption of QS system may have a sequential of unexpected side effects, such as affecting the nutrition and metabolism of the bacteria, interfering with host immune regulation and beneficial bacterial colonization. Pseudomonas strains are able to produce antibiotics and antifungal agents under the control of AHLs-mediated QS. However, the use of QQ strategy may prevent their beneficial effects. Another example is the AI-2, which has a significant effect on growth and biofilm formation of beneficial bacteria such as Lactobacillus spp. and Bifidobacterium spp. (Lebeer et al., 2007). As a consequence, QS disruption might have a negative effect on the adaptability of these bacteria in the intestinal tract, though the specific mechanism is yet to be determined. Further study on the QS mechanism between individual bacteria, between bacteria and their colonizing hosts will become a major research focus in the field of biological control to build a new ecological prevention and control of pathogens.
Quorum quenching, especially QQ enzymes application, is a new alternative method for QS destruction and antifouling. Enzymes are generally non-toxic and may be integrated into various matrices without being released. It can be further developed and applied specifically to medical devices, paintings, coatings and other fields to solve the problems of bacterial virulence and biofouling. However, there are limitations in practical application, in particular when the stability of QQ enzymes is concerned. Stability is usually the main constraint to reduce enzyme utilization, and high activity enzyme resources are also scarce. Therefore, the researchers are committed to separating highly stable enzymes from extreme environments. Furthermore, the application of QQ enzymes is focused on the AHL mediated QS mechanism. There are a few studies on the degrading enzymes of AI-2, AI-3 and AIPs. These studies are of great significance in extending the potential of QQ strategy to a wider range of gram-negative and gram-positive bacteria (Bzdrenga et al., 2017). Although the QQ strategy has research significance in theoretical studies and in application prospects, the drawbacks mentioned above cannot be ignored.
A recent study by Tian et al. (2018) found that Qsp 1, a quorum sensing peptide, is an important signal molecule in two forms of sexual reproduction. Qsp 1 orchestrates various differentiation and molecular processes, including meiosis. Qsp 1 also plays an important role in the initiation of parthenogenesis and the coordination of intercellular communication. At the same time, it was also found that the atypical zinc finger regulator Cqs 2 is an important part of the Qsp 1 signaling cascade during bisexual and parthenogenesis. These findings extend the range of quorum sensing behavior to sexual development and meiosis (Tian et al., 2018). Meanwhile, with the in-depth study on the molecular regulation mechanism of QS system, the ecological significance of bacterial QS system becomes increasingly obvious. In the ecological environment, a variety of relationships and effects occur between bacteria and other microorganisms, responding to different signal control mechanisms, and maintaining a dynamic balance among microbial communities. In addition, research has shown that the QS system regulated by signal molecules such as AHLs or AI-2 participates in marine carbon cycle, and plays an important role in maintaining the health of the coral reef ecosystem and in the interaction between eukaryotes and their bacteria (Hmelo, 2017). Further exploration of the significance of QS in evolution and ecology will provide a new direction for elucidating the mechanism of marine carbon cycle and maintaining global ecological balance.
AcknowledgementsWe are very grateful to Dr. Yunxuan Xie in Tianjin University for his suggestions and language modification. This work was supported by the Young Elite Scientists Sponsorship Program by China Association for Science and Technology (CAST) (No. YESS20160009), and the National Natural Science Foundation of China (Nos. 31870023, 31571970 and 41506160).
Ahlgren, N. A., Harwood, C. S., Schaefer, A. L., Giraud, E. and Greenberg, E. P., 2011. Aryl-homoserine lactone quorum sensing in stem-nodulating photosynthetic bradyrhizobia. Proceedings of the National Academy of Sciences of the United States of America, 108: 7183-7188. DOI:10.1073/pnas.1103821108 (  0) 0) |
Albuquerque, P. and Casadevall, A., 2012. Quorum sensing in fungi–A review. Medical Mycology, 50: 337-345. DOI:10.3109/13693786.2011.652201 (  0) 0) |
Amaya, S., Pereira, J. A., Borkosky, S. A., Valdez, J. C., Bardón, A. and Arena, M. E., 2012. Inhibition of quorum sensing in Pseudomonas aeruginosa by sesquiterpene lactones. Phytomedicine, 19: 1173-1177. DOI:10.1016/j.phymed.2012.07.003 (  0) 0) |
Bauer, W. D. and Robinson, J. B., 2002. Disruption of bacterial quorum sensing by other organisms. Current Opinion in Biotechnology, 13: 234-237. DOI:10.1016/S0958-1669(02)00310-5 (  0) 0) |
Biswa, P. and Doble, M., 2013. Production of acylated homoserine lactone by gram-positive bacteria isolated from marine water. FEMS Microbiology Letters, 343: 34-41. DOI:10.1111/1574-6968.12123 (  0) 0) |
Borthwick, A. D., 2012. 2, 5-Diketopiperazines: Synthesis, reactions, medicinal chemistry, and bioactive natural products. Chemical Reviews, 112: 3641-3716. DOI:10.1021/cr200398y (  0) 0) |
Brachmann, A. O., Brameyer, S., Kresovic, K., Hitkova, I., Kopp, Y., Manske, C., Schubert, C., Bode, H. B. and Heermann, R., 2013. Pyrones as bacterial signaling molecules. Nature Chemical Biology, 9: 573-578. DOI:10.1038/nchembio.1295 (  0) 0) |
Brackman, G., Celen, S., Hillaert, U., Calenbergh, S. V., Cos, P., Maes, L., Nelis, H. J. and Coenye, T., 2011. Structure-activity relationship of cinnamaldehyde analogs as inhibitors of AI-2 based quorum sensing and their effect on virulence of Vibrio spp. PLoS One, 6: e16084. DOI:10.1371/journal.pone.0016084 (  0) 0) |
Brameyer, S., Kresovic, D., Bode, H. B. and Heermann, R., 2015. Dialkylresorcinols as bacterial signaling molecules. Proceedings of the National Academy of Sciences of the United States of America, 112: 572-577. DOI:10.1073/pnas.1417685112 (  0) 0) |
Bzdrenga, J., Daude, D., Remy, B., Jacquet, P., Plener, L., Elias, M. and Chabriere, E., 2017. Biotechnological applications of quorum quenching enzymes. Chemico-Biological Interactions, 267: 104-115. DOI:10.1016/j.cbi.2016.05.028 (  0) 0) |
Cao, H., Yang, M., Zheng, H., Zhang, J., Zhong, Z. and Zhu, J., 2009. Complex quorum-sensing regulatory systems regulate bacterial growth and symbiotic nodulation in Mesorhizobium tianshanense. Archives of Microbiology, 191: 283-289. DOI:10.1007/s00203-008-0454-7 (  0) 0) |
Chen, G., Swem, L. R., Swem, D. L., Stauff, D. L., O'Loughlin, C. T., Jeffrey, P. D., Bassler, B. L. and Hughson, F. M., 2011. A strategy for antagonizing quorum sensing. Molecular Cell, 42: 199-209. DOI:10.1016/j.molcel.2011.04.003 (  0) 0) |
Chu, W., Zhou, S., Zhu, W. and Zhuang, X., 2014. Quorum quenching bacteria Bacillus sp. QSI-1 protect zebrafish (Danio rerio) from Aeromonas hydrophila infection. Scientific Reports, 4: 5446. DOI:10.1038/srep05446 (  0) 0) |
Daneshvar Alavi, H. E. and Truelstrup Hansen, L., 2013. Kinetics of biofilm formation and desiccation survival of Listeria monocytogenes in single and dual species biofilms with Pseudomonas fluorescens, Serratia proteamaculans or Shewanella baltica on food-grade stainless steel surfaces. Biofouling, 29: 1253-1268. DOI:10.1080/08927014.2013.835805 (  0) 0) |
De Lamo Marin, S., Xu, Y., Meijler, M. M. and Janda, K. D., 2007. Antibody catalyzed hydrolysis of a quorum sensing signal found in gram-negative bacteria. Bioorganic & Medicinal Chemistry Letters, 17: 1549-1552. DOI:10.1016/j.bmcl.2006.12.118 (  0) 0) |
Defoirdt, T., 2018. Quorum-sensing systems as targets for antivirulence therapy. Trends in Microbiology, 26: 313-328. DOI:10.1016/j.tim.2017.10.005 (  0) 0) |
Defoirdt, T., Boon, N., Bossier, P. and Verstraete, W., 2004. Disruption of bacterial quorum sensing: An unexplored strategy to fight infections in aquaculture. Aquaculture, 240: 69-88. DOI:10.1016/j.aquaculture.2004.06.031 (  0) 0) |
Defoirdt, T., Boon, N., Sorgeloos, P., Verstraete, W. and Bossier, P., 2008. Quorum sensing and quorum quenching in Vibrio harveyi: Lessons learned from in vivo work. The ISME Journal, 2: 19-26. DOI:10.1038/ismej.2007.92 (  0) 0) |
Defoirdt, T., Miyamoto, C. M., Wood, T. K., Meighen, E. A., Sorgeloos, P., Verstraete, W. and Bossier, P., 2007. The natural furanone (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2 (5H)-furanone disrupts quorum sensing-regulated gene expression in Vibrio harveyi by decreasing the DNA-binding activity of the transcriptional regulator protein LuxR. Environmental Microbiology, 9: 2486-2495. DOI:10.1111/j.1462-2920.2007.01367.x (  0) 0) |
Diggle, S. P., Cornelis, P., Williams, P. and Cámara, M., 2006. 4-Quinolone signalling in Pseudomonas aeruginosa: Old molecules, new perspectives. International Journal of Medical Microbiology, 296: 83-91. (  0) 0) |
Dobretsov, S., Teplitski, M., Bayer, M., Gunasekera, S., Proksch, P. and Paul, V. J., 2011. Inhibition of marine biofouling by bacterial quorum sensing inhibitors. Biofouling, 27: 893-905. (  0) 0) |
Dong, Y. H., Wang, L. H., Xu, J. L., Zhang, H. B., Zhang, X. F. and Zhang, L. H., 2001. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature, 411: 813-817. DOI:10.1038/35081101 (  0) 0) |
Dong, Y. H., Xu, J. L., Li, X. Z. and Zhang, L. H., 2000. AiiA, an enzyme that inactivates the acylhomoserine lactone quorumsensing signal and attenuates the virulence of Erwinia carotovora. Proceedings of the National Academy of Sciences of the United States of America, 97: 3526-3531. DOI:10.1073/pnas.060023897 (  0) 0) |
Fong, J., Zhang, C., Yang, R., Boo, Z. Z., Tan, S. K., Nielsen, T. E., Givskov, M., Liu, X. W., Bin, W., Su, H. and Yang, L., 2018. Combination therapy strategy of quorum quenching enzyme and quorum sensing inhibitor in suppressing multiple quorum sensing pathways of Pseudomonas aeruginosa. Scientific Reports, 8: 1155. DOI:10.1038/s41598-018-19504-w (  0) 0) |
Fuchs, S. W., Bozhüyük, K. A., Kresovic, D., Grundmann, F., Dill, V., Brachmann, A. O., Waterfield, N. R. and Bode, H. B., 2013. Formation of 1, 3-cyclohexanediones and resorcinols catalyzed by a widely occurring ketosynthase. Angewandte Chemie, International Edition in English, 52: 4108-4112. DOI:10.1002/anie.201210116 (  0) 0) |
Ganin, H., Rayo, J., Amara, N., Levy, N., Krief, P. and Meijler, M. M., 2013. Sulforaphane and erucin, natural isothiocyanates from broccoli, inhibit bacterial quorum sensing. MedChemComm, 4: 175-179. DOI:10.1039/C2MD20196H (  0) 0) |
García, M. P., D'Alvise, P. and Gram, L., 2013. Disruption of cellto-cell signaling does not abolish the antagonism of Phaeobacter gallaeciensis toward the fish pathogen Vibrio anguillarum in algal systems. Applied and Environmental Microbiology, 79: 5414-5417. DOI:10.1128/AEM.01436-13 (  0) 0) |
Giacometti, A., Cirioni, O., Ghiselli, R., Dell'Acqua, G., Orlando, F., D'Amato, G., Mocchegiani, F., Silvestri, C., DelPrete, S. M., Rocchi, M., Balaban, N., Saba, V. and Scalise, G., 2005. RNAIII-inhibiting peptide improves efficacy of clinically used antibiotics in a murine model of Staphylococcal sepsis. Peptides, 26: 169-175. DOI:10.1016/j.peptides.2004.09.018 (  0) 0) |
Guo, X., Zhang, L. Y., Wu, S. C., Xia, F., Fu, Y. X., Wu, Y. L., Leng, C. Q., Yi, P. F., Shen, H. Q., Wei, X. B. and Fu, B. D., 2014. Andrographolide interferes quorum sensing to reduce cell damage caused by avian pathogenic Escherichia coli. Veterinary Microbiology, 174: 496-503. DOI:10.1016/j.vetmic.2014.09.021 (  0) 0) |
Han, Y., Hou, S., Simon, K. A., Ren, D. and Luk, Y-Y., 2008. Identifying the important structural elements of brominated furanones for inhibiting biofilm formation by Escherichia coli. Bioorganic & Medicinal Chemistry Letters, 18: 1006-1010. (  0) 0) |
Harms, A., Maisonneuve, E. and Gerdes, K., 2016. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science, 354: f4268. DOI:10.1126/science.aaf4268 (  0) 0) |
Hmelo, L. R., 2017. Quorum sensing in marine microbial environments. Annual Review of Marine Science, 9: 257-281. (  0) 0) |
Hume, E., Baveja, J., Muir, B., Schubert, T. L., Kumar, N., Kjelleberg, S., Griesser, H. J., Thissen, H., Read, R., Poole-Warren, L. A., Schindhelm, K. and Willcox, M., 2004. The control of Staphylococcus epidermidis biofilm formation and in vivo infection rates by covalently bound furanones. Biomaterials, 25: 5023-5030. DOI:10.1016/j.biomaterials.2004.01.048 (  0) 0) |
Jakobsen, T. H., Bragason, S. K., Phipps, R. K., Christensen, L. D., van Gennip, M., Alhede, M., Skindersoe, M., Larsen, T. O., Høiby, N., Bjarnsholt, T. and Givskov, M., 2012. Food as a source for quorum sensing inhibitors: iberin from horseradish revealed as a quorum sensing inhibitor of Pseudomonas aeruginosa. Applied and Environmental Microbiology, 78: 2410-2421. DOI:10.1128/AEM.05992-11 (  0) 0) |
Jatt, A. N., Tang, K., Liu, J., Zhang, Z. and Zhang, X-H., 2015. Quorum sensing in marine snow and its possible influence on production of extracellular hydrolytic enzymes in marine snow bacterium Pantoea ananatis B9. FEMS Microbiology Ecology, 91: 1-13. (  0) 0) |
Jones, M. B., Jani, R., Ren, D., Wood, T. K. and Blaser, M. J., 2005. Inhibition of Bacillus anthracis growth and virulence-gene expression by inhibitors of quorum-sensing. The Journal of Infectious Diseases, 191: 1881-1888. DOI:10.1086/429696 (  0) 0) |
Jones, S. M., Dang, T. T. and Martinuzzi, R., 2009. Use of quorum sensing antagonists to deter the formation of crystalline Proteus mirabilis biofilms. International Journal of Antimicrobial Agents, 34: 360-364. DOI:10.1016/j.ijantimicag.2009.06.011 (  0) 0) |
Kügler, S., Sebghati, T. S., Eissenberg, L. G. and Goldman, W. E., 2000. Phenotypic variation and intracellular parasitism by Histoplasma Capsulatum. Proceedings of the National Academy of Sciences of the United States of America, 97: 8794-8798. DOI:10.1073/pnas.97.16.8794 (  0) 0) |
Kalaiarasan, E., Kottha, T., Harish, B. N., Gnanasambandam, V., Sali, V. K. and John, J., 2017. Inhibition of quorum sensing-controlled biofilm formation in Pseudomonas aeruginosa by quorum-sensing inhibitors. Microbial Pathogenesis, 111: 99-107. DOI:10.1016/j.micpath.2017.08.017 (  0) 0) |
Kaufmann, G. F., Sartorio, R., Lee, S. H., Mee, J. M., Altobell, L. J., Kujawa, D. P., Jeffries, E., Clapham, B., Meijler, M. M. and Janda, K. D., 2006. Antibody interference with N-acyl homoserine lactone-mediated bacterial quorum sensing. Journal or the American Chemical Society, 128: 2802-2803. DOI:10.1021/ja0578698 (  0) 0) |
Kelly, R. C., Bolitho, M. B., Higgins, D. A., Lu, W., Ng, W. L., Jeffrey, P. D., Rabinowitz, J. D., Semmelhack, M. F., Hughson, F. M. and Bassler, B. L., 2009. The Vibrio cholerae quorum-sensing autoinducer CAI-1: Analysis of the biosynthetic enzyme CqsA. Nature Chemical Biology, 5: 891-895. DOI:10.1038/nchembio.237 (  0) 0) |
Kim, J. S., Kim, Y. H., Seo, Y. W. and Park, S., 2007. Quorum sensing inhibitors from the red alga, Ahnfeltiopsis flabelliformis. Biotechnology and Bioprocess Engineering, 12: 308. DOI:10.1007/BF02931109 (  0) 0) |
Kiran, M. D., Adikesavan, N. V., Cirioni, O., Giacometti, A., Silvestri, C., Scalise, G., Ghiselli, R., Saba, V., Orlando, F., Shoham, M. and Balaban, N., 2008. Discovery of a quorumsensing inhibitor of drug-resistant Staphylococcal infections by structure-based virtual screening. Molecular Pharmacology, 73: 1578-1586. DOI:10.1124/mol.107.044164 (  0) 0) |
Kleerebezem, M., Quadri, L. E., Kuipers, O. P. and De Vos, W. M., 1997. Quorum sensing by peptide pheromones and twocomponent signal-transduction systems in gram-positive bacteria. Molecular Microbiology, 24: 895-904. DOI:10.1046/j.1365-2958.1997.4251782.x (  0) 0) |
Kravchenko, V. V., Kaufmann, G. F., Mathison, J. C., Scott, D. A., Katz, A. Z. and Grauer, D. C., 2008. Modulation of gene expression via disruption of NF-κB signaling by a bacterial small molecule. Science, 321: 259-263. DOI:10.1126/science.1156499 (  0) 0) |
Kwan, J. C., Meickle, T., Ladwa, D., Teplitski, M., Paul, V. and Luesch, H., 2011. Lyngbyoic acid, a 'tagged' fatty acid from a marine Cyanobacterium, disrupts quorum sensing in Pseudomonas aeruginosa. Molecular BioSystems, 7: 1205-1216. DOI:10.1039/c0mb00180e (  0) 0) |
LaSarre, B. and Federle, M. J., 2013. Exploiting quorum sensing to confuse bacterial pathogens. Microbiology and Molecular Biology Reviews, 77: 73-111. DOI:10.1128/MMBR.00046-12 (  0) 0) |
Leadbetter, J. R. and Greenberg, E. P., 2000. Metabolism of acylhomoserine lactone quorum-sensing signals by Variovorax paradoxus. Journal of Bacteriology, 182: 6921-6926. DOI:10.1128/JB.182.24.6921-6926.2000 (  0) 0) |
Lebeer, S., De Keersmaecker, S. C., Verhoeven, T. L., Fadda, A. A., Marchal, K. and Vanderleyden, J., 2007. Functional analysis of luxS in the probiotic strain Lactobacillus rhamnosus GG reveals a central metabolic role important for growth and biofilm formation. Journal of Bacteriology, 189: 860-871. DOI:10.1128/JB.01394-06 (  0) 0) |
Lee, B., Yeon, K. M., Shim, J., Kim, S. R., Lee, C. H., Lee, J. and Kim, J., 2014. Effective antifouling using quorum-quenching acylase stabilized in magnetically-separable mesoporous silica. Biomacromolecules, 15: 1153-1159. DOI:10.1021/bm401595q (  0) 0) |
Lee, H., Chang, C. Y., Nardone, G. and Kwon-Chung, K. J., 2007. TUP1 disruption in Cryptococcus neoformans uncovers a peptide-mediated density-dependent growth phenomenon that mimics quorum sensing. Molecular Microbiology, 64: 591-601. DOI:10.1111/j.1365-2958.2007.05666.x (  0) 0) |
Lee, J. H., Wood, T. K. and Lee, J., 2015. Roles of indole as an interspecies and interkingdom signaling molecule. Trends in Microbiology, 23: 707-718. DOI:10.1016/j.tim.2015.08.001 (  0) 0) |
Lindemann, A., Pessi, G., Schaefer, A. L., Mattmann, M. E., Christensen, Q. H., Kessler, A., Hennecke, H., Blackwell, H. E., Greenberg, E. P. and Harwood, C. S., 2011. Isovaleryl-homoserine lactone, an unusual branched-chain quorum-sensing signal from the soybean symbiont Bradyrhizobium japonicum. Proceedings of the National Academy of Sciences of the United States of America, 108: 16765-16770. DOI:10.1073/pnas.1114125108 (  0) 0) |
Manefield, M., Rasmussen, T. B., Henzter, M., Andersen, J. B., Steinberg, P., Kjelleberg, S. and Givskov, M., 2002. Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology, 148: 1119-1127. DOI:10.1099/00221287-148-4-1119 (  0) 0) |
Mansson, M., Nielsen, A., Kjærulff, L., Gotfredsen, C. H., Wietz, M., Ingmer, H., Gram, L. and Larsen, T. O., 2011. Inhibition of virulence gene expression in Staphylococcus aureus by novel depsipeptides from a marine Photobacterium. Marine Drugs, 9: 2537-2552. DOI:10.3390/md9122537 (  0) 0) |
Martins, M. B. and Carvalho, I., 2007. Diketopiperazines: Biological activity and synthesis. Tetrahedron, 63: 9923-9932. DOI:10.1016/j.tet.2007.04.105 (  0) 0) |
Moreira, C. G. and Sperandio, V., 2010. The Epinephrine/ Norepinephrine/Autoinducer-3 interkingdom signaling system in Escherichia coli O157:H7. Advances in Experimental Medicine & Biology, 874: 213-227. DOI:10.1007/978-1-4419-5576-0_12 (  0) 0) |
Murzyn, A., Krasowska, A., Stefanowicz, P., Dziadkowiec, D. and Lukaszewicz, M., 2010. Capric acid secreted by Saccharomyces boulardii inhibits Cancida albicans filamentous growth, adhesion and biofilm formation. PLoS One, 5: e12050. DOI:10.1371/journal.pone.0012050 (  0) 0) |
Nealson, K. H. and Hastings, J. W., 1979. Bacterial bioluminescence: Its control and ecological significance. Microbiological Reviews, 43: 496-518. (  0) 0) |
Newman, K. L., Chatterjee, S., Ho, K. A. and Lindow, S. E., 2008. Virulence of plant pathogenic bacteria attenuated by degradation of fatty acid cell-to-cell signaling factors. Molecular Plant-Microbe Interactions, 21: 326-334. DOI:10.1094/MPMI-21-3-0326 (  0) 0) |
Ng, W. L. and Bassler, B. L., 2009. Bacterial quorum-sensing network architectures. Annual Review of Genetics, 43: 197-222. DOI:10.1146/annurev-genet-102108-134304 (  0) 0) |
Stoodley, P., Sauer, K., Davies, D. G. and Costerton, J. W., 2002. Biofilms as complex differentiated communities. Annual Review of Microbiology, 56: 187-209. DOI:10.1146/annurev.micro.56.012302.160705 (  0) 0) |
Packiavathy, I. A. S. V., Priya, S., Pandian, S. K. and Ravi, A. V., 2014. Inhibition of biofilm development of uropathogens by curcumin–An anti-quorum sensing agent from Curcuma longa. Food Chemistry, 148: 453-460. DOI:10.1016/j.foodchem.2012.08.002 (  0) 0) |
Padder, S. A., Prasad, R. and Shah, A. H., 2018. Quorum sensing: A less known mode of communication among fungi. Microbiological Research, 210: 51-58. DOI:10.1016/j.micres.2018.03.007 (  0) 0) |
Pereira, C. S., Thompson, J. A. and Xavier, K. B., 2013. AI-2-mediated signalling in bacteria. FEMS Microbiology Reviews, 37: 156-181. DOI:10.1111/j.1574-6976.2012.00345.x (  0) 0) |
Phuvasate, S., Chen, M. H. and Su, Y. C., 2012. Reductions of Vibrio parahaemolyticus in Pacific oysters (Crassostrea gigas) by depuration at various temperatures. Food Microbiology, 31: 51-56. DOI:10.1016/j.fm.2012.02.004 (  0) 0) |
Poplawsky, A. R., Walters, D. M., Rouviere, P. E. and Chun, W., 2005. A gene for a dioxygenase-like protein determines the production of the DF signal in Xanthomonas campestris pv. campestris. Molecular Plant Pathology, 6: 653-657. DOI:10.1111/j.1364-3703.2005.00307.x (  0) 0) |
Quave, C. L. and Horswill, A. R., 2018. Identification of Staphylococcal quorum sensing inhibitors by quantification of õ-hemolysin with high performance liquid chromatography. Methods in Molecular Biology, 1673: 363-370. (  0) 0) |
Ramage, G., Saville, S. P., Wickes, B. L. and Lopez-Ribot, J. L., 2002. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Applied and Environmental Microbiology, 68: 5459-5463. DOI:10.1128/aem.68.11.5459-5463.2002 (  0) 0) |
Rasko, D. A., Moreira, C. G., Li, D. R., Reading, N. C., Ritchie, J. M., Waldor, M. K., Williams, N., Taussig, R., Wei, S., Roth, M., Hughes, D. T., Huntley, J. F., Fina, M. W., Falck, J. R. and Sperandio, V., 2008. Targeting QseC signaling and virulence for antibiotic development. Science, 321: 1078-1080. DOI:10.1126/science.1160354 (  0) 0) |
Rasmussen, T. B., Skindersoe, M. E., Bjarnsholt, T., Phipps, R. K., Christensen, K. B., Jensen, P. O., Andersen, J. B., Koch, B., Larsen, T. O., Hentzer, M., Eberl, L., Hoiby, N. and Givskov, M., 2005. Identity and effects of quorum-sensing inhibitors produced by Penicillium species. Microbiology, 151: 1325-1340. DOI:10.1099/mic.0.27715-0 (  0) 0) |
Reen, F. J., Gutiérrez-Barranquero, J. A. and Parages, M. L., 2018. Coumarin: A novel player in microbial quorum sensing and biofilm formation inhibition. Applied Microbiology and Biotechnology, 102: 2063-2073. DOI:10.1007/s00253-018-8787-x (  0) 0) |
Risoen, P. A., Brurberg, M. B., Eijsink, V. G. and Nes, I. F., 2000. Functional analysis of promoters involved in quorum sensing-based regulation of bacteriocin production in Lactobacillus. Molecular Microbiology, 37: 619-628. DOI:10.1046/j.1365-2958.2000.02029.x (  0) 0) |
Saenz, H. L., Augsburger, V., Vuong, C., Jack, R. W., Götz, F. and Otto, M., 2000. Inducible expression and cellular location of AgrB, a protein involved in the maturation of the Staphylococcal quorum-sensing pheromone. Archives of Microbiology, 174: 452-455. DOI:10.1007/s002030000223 (  0) 0) |
Saurav, K., Bar-Shalom, R., Haber, M., Burgsdorf, I., Oliviero, G., Costantino, V., Morgenstern, D. and Steindler, L., 2016. In search of alternative antibiotic drugs: Quorum-quenching activity in sponges and their bacterial isolates. Frontiers in Microbiology, 7: 416. (  0) 0) |
Schaefer, A. L., Greenberg, E. P., Oliver, C. M., Oda, Y., Huang, J. J., Bittan-Banin, G., Peres, C. M., Schmidt, S., Juhaszova, K., Sufrin, J. R. and Harwood, C. S., 2008. A new class of homoserine lactone quorum-sensing signals. Nature, 454: 595-599. DOI:10.1038/nature07088 (  0) 0) |
Schenk, S. T., Hernández-Reyes, C., Samans, B., Stein, E., Neumann, C., Schikora, M., Reichelt, M., Mithöfer, A., Becker, A., Kogel, K. H. and Schikora, A., 2014. N-acyl-homoserine lactone primes plants for cell wall reinforcement and induces resistance to bacterial pathogens via the salicylic acid/oxylipin pathway. Plant Cell, 26: 2708-2723. DOI:10.1105/tpc.114.126763 (  0) 0) |
Schipper, C., Hornung, C., Bijtenhoorn, P., Quitschau, M., Grond, S. and Streit, W., 2009. Metagenome-derived clones encoding two novel lactonase family proteins involved in biofilm inhibition in Pseudomonas aeruginosa. Applied and Environmental Microbiology, 75: 224-233. DOI:10.1128/AEM.01389-08 (  0) 0) |
Siddiqui, M. F., Sakinah, M., Singh, L. and Zularisam, A. W., 2012. Targeting N-acyl-homoserine-lactones to mitigate membrane biofouling based on quorum sensing using a biofouling reducer. Journal of Biotechnology, 161: 190-197. DOI:10.1016/j.jbiotec.2012.06.029 (  0) 0) |
Singh, P. K., Schaefer, A. L., Parsek, M. R., Moninger, T. O., Welsh, M. J. and Greenberg, E. P., 2000. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature, 407: 762-764. DOI:10.1038/35037627 (  0) 0) |
Singh, R., Paul, D. and Jain, R. K., 2006. Biofilms: Implications in bioremediation. Trends in Microbiology, 14: 389-397. DOI:10.1016/j.tim.2006.07.001 (  0) 0) |
Singh, R. and Ray, P., 2014. Quorum sensing-mediated regulation of Staphylococcal virulence and antibiotic resistance. Future Microbiology, 9: 669-681. DOI:10.2217/fmb.14.31 (  0) 0) |
Skindersoe, M. E., Ettinger-Epstein, P., Rasmussen, T. B., Bjarnsholt, T., de Nys, R. and Givskov, M., 2008. Quorum sensing antagonism from marine organisms. Marine Biotechnology, 10: 56-63. DOI:10.1007/s10126-007-9036-y (  0) 0) |
Suneby, E. G., Herndon, L. R. and Schneider, T. L., 2017. Pseudomonas aeruginosa LasR DNA binding is directly inhibited by quorum sensing antagonists. ACS Infectious Diseases, 3: 183-189. DOI:10.1021/acsinfecdis.6b00163 (  0) 0) |
Tang, K., Su, Y., Brackman, G., Cui, F., Zhang, Y., Shi, X., Coenye, T. and Zhang, X-H., 2015. MomL, a novel marinederived N-acyl homoserine lactonase from Muricauda olearia. Applied and Environmental Microbiology, 81: 774-782. DOI:10.1128/AEM.02805-14 (  0) 0) |
Tang, K., Zhang, Y., Yu, M., Shi, X., Coenye, T., Bossier, P. and Zhang, X-H., 2013. Evaluation of a new high-throughput method for identifying quorum quenching bacteria. Scientific Reports, 3: 2935. DOI:10.1038/srep02935 (  0) 0) |
Teasdale, M. E., Donovan, K. A., Forschner-Dancause, S. R. and Rowley, D. C., 2011. Gram-positive marine bacteria as a potential resource for the discovery of quorum sensing inhibitors. Marine Biotechnology, 13: 722-732. DOI:10.1007/s10126-010-9334-7 (  0) 0) |
Teasdale, M. E., Liu, J., Wallace, J., Akhlaghi, F. and Rowley, D. C., 2009. Secondary metabolites produced by the marine bacterium Halobacillus salinus that inhibit quorum sensing-controlled phenotypes in gram-negative bacteria. Applied and Environmental Microbiology, 75: 567-572. DOI:10.1128/AEM.00632-08 (  0) 0) |
Tedder, M. E., Nie, Z., Margosiak, S., Chu, S., Feher, V. A., Almassy, R., Appelt, K. and Yager, K. M., 2004. Structure-based design, synthesis, and antimicrobial activity of purine derived SAH/MTA nucleosidase inhibitors. Bioorganic & Medicinal Chemistry Letters, 14: 3165-3168. DOI:10.1016/j.bmcl.2004.04.006 (  0) 0) |
Tian, X., He, G-J., Hu, P., Chen, L., Tao, C., Cui, Y. L., Shen, L., Ke, W., Xu, H., Zhao, Y., Xu, Q., Bai, F., Wu, B., Yang, E., Lin, X. and Wang, L., 2018. Cryptococcus neoformans sexual reproduction is controlled by a quorum sensing peptide. Nature Microbiology, 3: 698-707. DOI:10.1038/s41564-018-0160-4 (  0) 0) |
Vandeputte, O. M., Kiendrebeogo, M., Rajaonson, S., Diallo, B., Mol, A., Jaziri, M. E. and Baucher, M., 2010. Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorumsensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Applied and Environmental Microbiology, 76: 243-253. DOI:10.1128/AEM.01059-09 (  0) 0) |
von Bodman, S. B., Willey, J. M. and Diggle, S. P., 2008. Cell-cell communication in bacteria: United we stand. Journal of Bacteriology, 190: 4377-4391. DOI:10.1128/JB.00486-08 (  0) 0) |
Walters, M. and Sperandio, V., 2006. Quorum sensing in Escherichia coli and Salmonella. International Journal of Medical Microbiology, 296: 125-131. DOI:10.1016/j.ijmm.2006.01.041 (  0) 0) |
Wongsuk, T., Pumeesat, P. and Luplertlop, N., 2016. Fungal quorum sensing molecules: Role in fungal morphogenesis and pathogenicity. Journal of Basic Microbiology, 56: 440-447. DOI:10.1002/jobm.201500759 (  0) 0) |
Wu, D., Huang, W., Duan, Q., Li, F. and Cheng, H., 2014. Sodium houttuyfonate affects production of N-acyl homoserine lactone and quorum sensing-regulated genes expression in Pseudomonas aeruginosa. Frontiers in Microbiology, 5: 635. DOI:10.3389/fmicb.2014.00635 (  0) 0) |
Wu, H., Song, Z., Hentzer, M., Andersen, J. B., Molin, S., Givskov, M. and Hoiby, N., 2004. Synthetic furanones inhibit quorum-sensing and enhance bacterial clearance in Pseudomonas aeruginosa lung infection in mice. Journal of Antimicrobial Chemotherapy, 53: 1054-1061. DOI:10.1093/jac/dkh223 (  0) 0) |
Xavier, K. B. and Bassler, B. L., 2005. Interference with AI-2-mediated bacterial cell-cell communication. Nature, 437: 750-753. DOI:10.1038/nature03960 (  0) 0) |
Zhang, G., Zhang, F., Ding, G., Li, J., Guo, X., Zhu, J., Zhou, L., Cai, S., Liu, X., Luo, Y., Zhang, G., Shi, W. and Dong, X., 2012. Acyl homoserine lactone-based quorum sensing in a methanogenic archaeon. The ISME Journal, 6: 1336-1344. DOI:10.1038/ismej.2011.203 (  0) 0) |
Zhang, M., Sun, K. and Sun, L., 2008. Regulation of autoinducer 2 production and luxS expression in a pathogenic Edwardsiella tarda strain. Microbiology, 154: 2060-2069. DOI:10.1099/mic.0.2008/017343-0 (  0) 0) |
 2019, Vol. 18
2019, Vol. 18