2) Key Laboratory of Submarine Geosciences and Technology, MOE, Ocean University of China, Qingdao 266100, China;
3) Shandong Provincial No.4 Institute of Geological and Mineral Survey, Weifang 261021, China;
4) Key Laboratory of Marine Geology and Metallogeny, Ministry of Natural Resources, Qingdao 266061, China;
5) Laboratory for Marine Geology, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266061, China
Marine barite particles are widely distributed throughout oceans (Sun et al., 2015). It is well established that barite is the major carrier of barium in suspended matters and the formation, dissolution, sinking, and preservation of the former restricts the biogeochemical cycle of the latter in the marine environment (Hoppema et al., 2010; Griffith and Paytan, 2012; Singh et al., 2013). The processes that control the formation of barite in the marine environment are poorly understood (Bishop, 1988; Rushdi et al., 2000); however, studies on the biogeochemical processes, actions, and mechanisms of suspended barites in ocean water indicated that the majority of marine barite is formed in organic-rich microenvironments during the decay of biogenic debris (Dehairs et al., 1980; Bishop, 1988; Gingele and Dahmke, 1994; Rushdi et al., 2000). High barite concentrations in sediments or water column have been found to have a strongly correlation with the highly productivity in upper seawater and are closely related to the high organic carbon contents (Paytan et al., 1993, 1996; Eagle et al., 2003; Sternberg et al., 2007). Therefore, the barite concentration is considered one of the most ideal indicators for reconstructing the primary production in the ocean (Paytan et al., 1996; Jeandel et al., 2000; Paytan and Griffith, 2007; Sternberg et al., 2008).
Barite generally forms in the mesopelagic zone (100 – 1000 m) within aggregates of decomposing organic detritus (Dehairs et al., 1990; Stroobants et al., 1991; Ganeshram et al., 2003; Beek et al., 2009), below which the ocean water is typically undersaturated with regard to barites (Monnin et al., 1999; Rushdi et al., 2000; Hoppema et al., 2010). There are a series of changes in the content and particle morphology of barite crystals as they sink through thousands of meters of the water column; it has been suggested that some barite crystals dissolve before reaching the bottom of the sea, directly affecting the geochemical cycle of barium and the buried flux of barite in sediments (Paytan and Griffith, 2007; Griffith and Paytan, 2012; Sun et al., 2015). Therefore, it is essential to study the content, morphology, and distribution of barite in the oceanic water column to better understand the biogeochemical process of barite and accurately evaluate the significance of barite as an indicator of oceanic productivity.
The northeastern Indian Ocean is one of the most important sedimentary units in the world because of its special geographical position and timely response to global environmental changes since the Cenozoic era (Hovan and Rea, 1992; Klootwijk et al., 1992; Burton and Vance, 2000; Fang et al., 2002; Wei et al., 2007). The largest abyssal fan in the world (the Bengal abyssal fan) and an important ridge (the 90°E Ridge) both developed in the northeastern Indian Ocean. The sediments making up the Bengal Fan are derived primarily from terrigenous detritus from the denudation of the Qinghai-Tibet Plateau and are dominated by turbidity current deposits and hemipelagic deposits (Stow et al., 1990; Fagel et al., 1994). The monsoons in this region cause changes in the surface currents; in winter they run counterclockwise while in summer they move clockwise (Wyrtkik, 1973; Qiao et al., 2014). The northern movements of the Antarctic Intermediate Water (AAIW) and Antarctic Bottom Water (AABW) that occur in the middle and bottom layers are the most important deep water masses affecting this region (Gorsline, 1984; Alexander et al., 2009). The 90°E Ridge is 4000 km long and extends from 5°N to 31°S along the longitude line, with the central Indian Ocean Basin and Wharton Basin located to the west and east, respectively (Fig.1). Previous studies have demonstrated that the unique structural units and towering terrains inhibit the direct influence of turbidity currents and the pelagic sedimentary sequences occur in this region (Wei et al., 2007). The primary productivity of surface water in this area is high due to the influence of upwelling and the local climate (Fagel et al., 1994; Liu, 2002). However, there have been no studies of suspended barite particles in the water in this area. This study, therefore, examined the morphology, particle size, and spatial distribution of suspended marine barite in the waters from the surface to the depth of approximately 5000 m at four stations near the 90°E Ridge in the Indian Ocean using scanning electron microscopy (SEM) and energy dispersive X-ray spectrometry (EDS). The origin, settling, and preservation of barite particles near the 90°E Ridge are discussed systematically, with the aim of improving the understanding of biogeochemical processes related to marine barite.
|
Fig. 1 Geographical setting and hydrography of the study area and the location of sampling sites. The blue arrows show the currents of Antarctic Bottom Water (AABW) and Deep Western Boundary Current (DWBC). |
Water samples were collected at four stations located on both sides of the 90°E ridge in northeastern Indian Ocean by using Niskin bottles coupled to a conductivity-temperature-depth (CTD) instrument (Sea-Bird 911plus) throughout the water column during the DY52-1 cruise of the R/V Dayangyihao from December 2018 to January 2019. Water samples were collected from 9 to 13 different water depths at each station, and a total of 45 samples were obtained. Specific sampling information is given in Table 1. Temperature, salinity, and chlorophyll-a concentrations were measured simultaneously by using the CTD instrument.
|
|
Table 1 The sampling information |
The suspended particulate matter was concentrated by filtering the water samples (3 – 5 L) through a double acetate fiber microporous filter membrane (47 mm in diameter, micropore diameter of 0.45 μm) in the shipboard chemistry laboratory. The filter membranes were rinsed 3 to 5 times with deionized water to remove salts, dried in the oven at 40℃, stored in tin foil, and refrigerated at 4℃ until analysis.
The filter membrane obtained was dried in an oven at 40℃ for 24 h again, and an approximately 3 mm × 5 mm piece was cut from the center of the filter and glued onto the sample platform of the SEM. The sample was gold-coated under high vacuum to conduct electricity and then placed in a sample chamber for crystal morphology, surface structure, and chemical composition analyses via SEM/EDS in the laboratory.
Barite crystals were identified by using a backscattered electron detector combined with composition analysis based on the EDS technology. The particle sizes were measured by using the length measuring tool of the SEM, and images of each barite particle were acquired to document its morphology. Point determination and surface scanning were performed to determine the characteristics of the chemical composition of the barite crystals based on their shapes.
The SEM used in this study was a TESCAN VEGA3 SBH, and the working regimen for the SEM/EDS analysis was as follows: an accelerating voltage of 25 kV, emissions current of 100 μA, and working distance of 10 mm. The X-ray spectra collection time was greater than 60 s until the counts/second (c/s) were stabilized.
3 Results 3.1 Morphology of the Barite CrystalsBarite crystals are rhombic, biconical and typically exist as plate-like, granular, and fibrous aggregates. The morphologies of barites observed in the study area are complex and various, and can be classified into four evident morphological types: euhedral-subhedral, oval or round, rhombic crystals, and irregular. For each type of barite crystal, the complete crystal morphology has been observed in some particles, while many crystals possessed dissolution features at their edges or as cavities in their central portions.
1) Euhedral-subhedral barite crystals
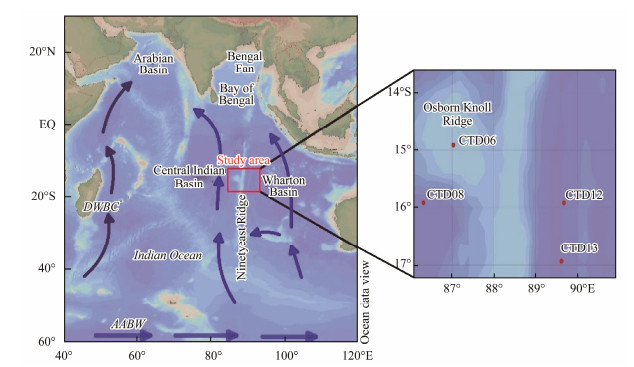
The outline of this type of barite crystal was clear, and the crystal surface was smooth with obvious edges and corners. However, many crystals were observed to possess dissolution features at their edges or on their faces. A subhedral barite crystal with a rhombic column shape, clear edge, and smooth crystal surface is shown in Fig.2a; however, a dissolution phenomenon appears at the local edges. Fig.2b presents a euhedral barite with clear edges and no obvious dissolution features.
|
Fig. 2 Barite crystals with subhedral (a) and euhedral (b) morphologies. |
2) Ovoid or rounded barite crystals
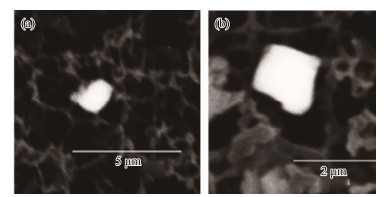
Two crystal morphologies (intact and dissolved) were also observed in this type of barite crystal. Barite crystals with intact morphologies are predominantly ovoid, elongated-ovoid, or rounded (Figs.3a, b), and have been reported briefly in other studies as suspended particulates in the Atlantic and Pacific oceans (Dehairs et al., 1980; Bertram and Cowen, 1997; Sun et al., 2015). Dissolved barites of this type showed dissolution etch pits on different portions of the crystal edges, which occasionally affected the crystal surface or appeared as a thinned etched pattern on particle faces (Figs.3c, d).
|
Fig. 3 Barite crystals. (a) – (b), intact and ovoid or round; (c) –(d), oval or round with dissolution features. |
3) Rhombic barite crystals
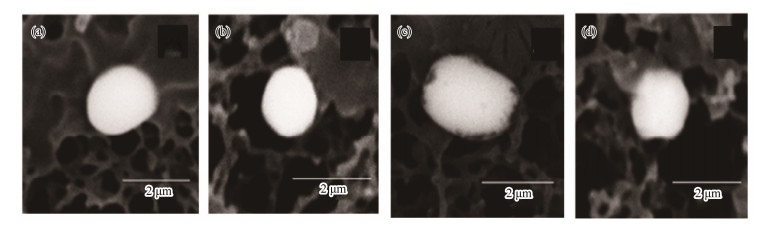
This type of barite exhibits hexagonal, rhomboid, or octagonal shapes with clear crystal edges and smooth crystal surfaces. Several such crystals also have dissolution features on their surfaces (Figs. 4a, b).
|
Fig. 4 Rhombic barite crystals. |
4) Irregular barite crystals
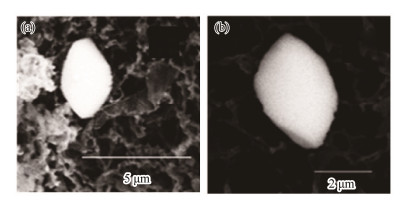
Barite crystals of this type possess clear crystal edges, almost without dissolution features (Fig.5a). Several crystals are arrow-like in shape, with an 'arrowhead' at one end of the crystal that may have been formed via dissolution (Fig.5b). Intensive dissolution may also pass through the entire particle so that it almost altered the original crystal profile (Figs.5c, d). Most barite crystals with strong dissolution features contained strontium as part of their chemical composition.
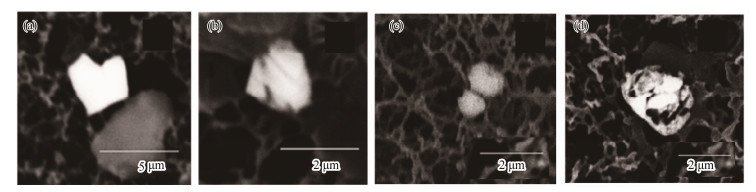
|
Fig. 5 Irregular barite crystals. (a), irregular polygonal; (b), arrowhead-shaped; (c) – (d), strongly dissolved. |
A total of 211 suspended barite crystals from the water column at four stations throughout the study area were analyzed. The particle sizes of barite crystals were typically fine and ranged from 0.5 μm to 7 μm, with a dominant size of 1 – 3 μm (Fig.6a). As presented in Fig.6b, the particle sizes of barite crystals from different stations were similar; however, the distribution of barite sizes varied with regard to the groupings of different sizes.
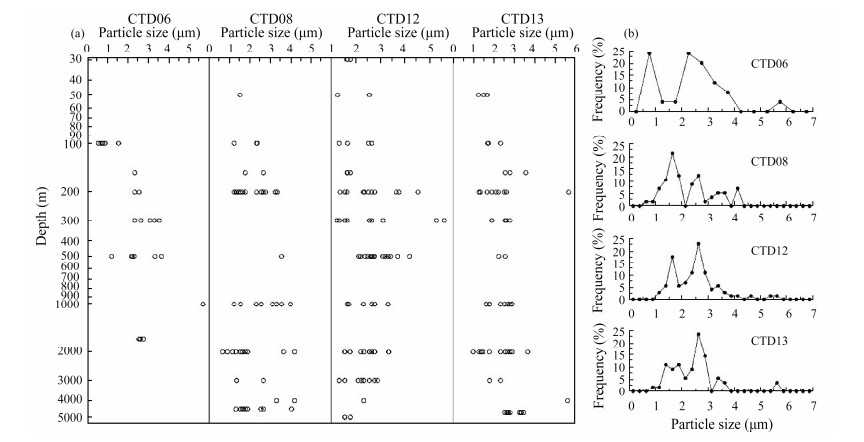
|
Fig. 6 Vertical distribution in the water column (a) and the frequency distribution (b) of barite crystal particle size. |
The barite crystals in the water column from the surface to the depth of 200 m were relatively fine, most of which are less than 3 μm in length. Barite particles at depths of 200 – 2000 m were coarser, and their sizes peaked at the depth of 200 m or 300 m. The particle size of barites then typically decreased as the water depth increased. However, large barite particles also appeared in the bottom of water column at stations CTD08 and CTD13, at depths of approximately 4500 – 4700 m.
The frequency distribution for different intervals of barite particle sizes shows that the peak typically occurred within the ranges of 1 – 2 μm and 2 – 3 μm; while it was within the ranges of 0 – 1 μm and 2 – 4 μm at station CTD06. Approximately 60% – 70% of the barite particles were within these dominant size ranges.
3.3 Spatial Distribution of Barite Crystal ConcentrationsThe concentration of barite crystals in the water column can reflect their spatial distribution. Therefore, the number density of barite particles was calculated based on the proportion of the observed filter membrane area to the whole filter membrane area and the volume of filtered water to reflect the concentration of barite particles in the study area. The number density of barite crystals is expressed as follows:
| $ C = n{S_2}/({S_1} \cdot V), $ | (1) |
where C is the concentration of barite, n is the number of barite crystals observed under the SEM, V is the volume of filtered water, S1 is the statistical area of the filter membrane observed under the microscope, and S2 is the area of the filter membrane covered with suspended particulate matter.
The barite particle concentration calculated and the temperature, salinity, and chlorophyll-a concentration measured by CTD instrument at each station are given in Fig.7. The variation trends of the hydrological parameters were consistent at all stations within the study area. The halocline occurred between 200 and 400 m with a maximum salinity of approximately 35.4, while the thermocline appeared at 200 – 500 m, characterized by high surface water temperatures and relatively low water depths. The chlorophyll-a content peaked near the depth of 80 – 100 m with a maximum value of 1.5 mg L−1.
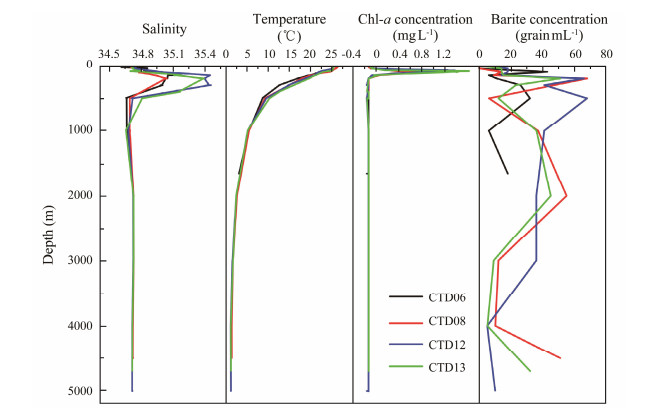
|
Fig. 7 Variation in barite particle concentration and hydrological parameters. |
The vertical distribution pattern of barite crystal concentration at each station in the water column exhibited its own distinct characteristics while adhering to a consistent law. Because of the shallow water depth, approximately 1800 m, at the CTD06 station, which is located at the Osborn Knoll Ridge, a total of 25 barite crystals were found, and most of the crystals were distributed within the water column at 100 – 500 m and 1500 m. The concentrations of barite at the other three stations exhibited similar distribution characteristics, with low concentrations in the surface water from the depth of 30 m. The barite particles were abundant in the water column at the depth of 200 – 300 m, with the maximum value at 200 m. The concentration of barites then decreased rapidly, followed by an increase at approximately 1000 – 2000 m. The concentration then decreased again with a downward oscillation in the water column below 2000 m. However, higher concentrations of barites were also detected close to the bottom of the water column at approximately 4500 m and at 4700 m at stations CTD08 and CTD13, respectively.
In terms of horizontal distribution, there was no significant difference in barite concentration between the stations located on both sides of the 90°E Ridge. The particle size, concentration, morphology and vertical distribution of barite crystals at most of the stations were consistent, except the station CTD06. The barite crystals exhibited characteristics of rapid enrichment in the water column from the surface to depth of approximately 200 – 300 m, followed by gradual enrichment in the range of 1000 – 2000 m, and remained in settlement and dissolution states after they formed below 2000 m. The vertical distribution patterns of barite at the station CTD08 and CTD13, on two sides of the 90°E Ridge, are similar. However, at CTD12 the distribution pattern of oceanic barite represents a typical one in water column.
4 Discussion 4.1 Vertical Distribution Pattern of Barite Crystals and Its Relationship with Key Environmental InterfacesBecause the water is shallow at station CTD06, the distribution pattern of barite there did not represent the main characteristics of barites in the study area. However, the patterns of barite distribution at stations CTD08, CTD12, and CTD13 are consistent; and they better reflect the development and evolution characteristics of barites in the water column of the study area. Fig.8 presents a statistical assessment of the characteristics of barite distribution at these three stations. In this study, there is a little controversy on the depth of the water samples collected, especially in deep water, according to the depth of the study stations to adjust the sample water depth. When calculating the average concentration of barite, if there is only one sampling layer researched at one station, we took this data as the average to reflect the real distribution characteristics of barite concentrations.
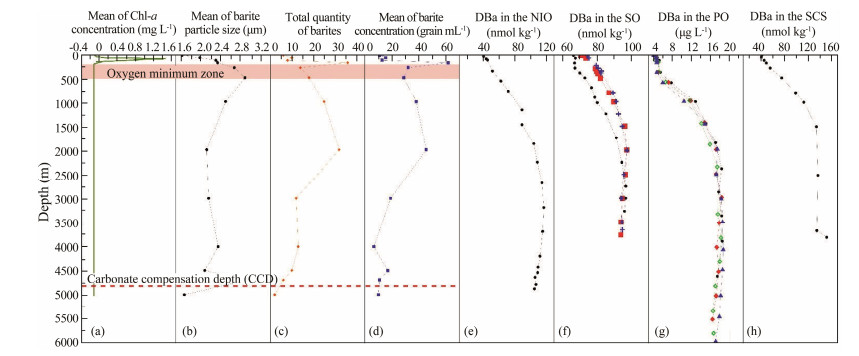
|
Fig. 8 Vertical distribution patterns of barite crystals and their relationships with key environmental interfaces. (a) – (d), the mean concentration of Chl-a, mean barite particle size, total number of barite particles, and mean concentration of barites detected at stations CTD08, CTD12, and CTD13, respectively. (e) – (h), dissolved barium (DBa) concentrations in the Northwest Indian Ocean (NIO; Jeandel et al., 1996), Southern Ocean (SO; Pyle et al., 2018), Pacific Ocean (PO; Sugiyama et al., 1984) and South China Sea (SCS; Cao et al., 2016), respectively. Shaded areas represent the oxygen minimum zone, the red dash line represents the carbonate compensation depth (a – d), and different symbols represent different research stations (f – g). |
Barite crystals begin to appear at a depth of 30 m, but their particle sizes are small and the quantity and concentration are low. This indicates that barite is formed in nearsurface water; however, the amount of barite was low and the particles were fine. The concentration of chlorophyll-a was high at the depth range of 85 – 100 m and peaked in this zone, accompanied by a gradual increase in the barite crystal concentration and particle size, which reached a peak value of the entire vertical profile in the water layer at approximately 200 m. According to data from studies of the oxygen minimum zone (OMZ) in the Bay of Bengal (Paulmier and Ruiz, 2009; Wang et al., 2018), the OMZ in our study area was roughly distributed at depths of 200 – 500 m. The formation and development of barite was clearly affected by the presence of the OMZ, which resulted in a decrease in the amount and concentration of barite (Fig.8). However, the mean particle sizes of the barites reached their maximum values at a depth of 500 m due to their postformation settlement. Yet, as the dissolved oxygen (DO) contents in the water recovered, the amount and concentration of barites increased until a depth of 2000 m while the particle size continued to decrease. This suggests that new barites are likely formed in the water below 500 m up to a depth of 2000 m. The amount and concentration of barite then decreased at water depths below 2000 m; however, their particle sizes tended to remain stable. The particle size and concentration of barite did peak again at approximately 4500 – 4700 m.
The distribution of barites in the water column suggests that they are formed primarily at water depths from the surface to 2000 m. However, their formation is clearly divided into two stages: rapid formation in the water column at 0 – 500 m resulting in a rapid increase in barite concentration and particle size, and gradual formation in the water column at 500 – 2000 m. At the same time, however, barites are also dissolved; this resulted in an increase in barite concentrations and a reduction in their particle sizes. The formation of barite is closely related to the primary productivity of the ocean (represented here by chlorophyll-a concentration) and restricted by the presence of OMZs in the ocean. Barite crystals may gradually settle after formation in the water column, accompanied by a continuous decrease in the particle size. Barite crystals may not form in the water below 2000 m, and the barites in deep water primarily come from the sedimentation of the upper water particles. Therefore, the amount and concentration of barites continue to decrease while the particle size tends to remain stable. The particle size and concentration of barites in the water at approximately 4500 – 4700 m increased primarily because of the accumulation in this layer of barites settled from the upper water. Carbonate minerals may dissolve in deep water, at depths of more than 4800 m, that's below the carbonate compensation depth, in the Indian Ocean (Campbell et al., 2018). The particle size and quantity of barite particles also had a similar decrease, especially for the mean particle size, but the average concentration did not change significantly. This layer has only been studied at Station CTD12, so its changes can be analyzed from the data of CTD12. Obviously, the particle size of barite reduces significantly (Fig.6), but the concentration donot significantly change compared with the overlying layer (Fig.7), which indicates that barite may also be dissolved at this stage, but the concentration does not decrease.
4.2 Formation of Marine Barite in the Indian OceanMarine barites are not saturated in ocean water, and there is no consensus regarding their origins. However, there are two main views regarding the formation mechanism of marine barite in water (Sun, 2011). The first is the celestite model, which holds that the formation of barite crystals is related to biological processes of barium-rich skeletons in decaying organic matter. For example, radiolarians, fibrous cyanobacteria, and bryozoans can enrich barium in their skeletons. Although the barium contents in their carbonate skeletons are low, these organisms are present in the oceans in high densities. This can remove barium from in the surface layer of the ocean. After the death of these organisms, their bones decompose in the water; and barium is bound to transparent exopolymer particles, cell wall-associated polysaccharides, or extracellular polymeric substances from bacterial biofilm in microenvironments formed by the cell walls and shell material of phytoplankton (Martinez-Ruiz et al., 2018) before reacting with sulfate derived largely from seawater to form barite (Gooday and Nott, 1982; Bernstein et al., 1992; Dymond and Collier, 1996; Bertram and Cowen, 1997; Bernstein et al., 1998; Pyle et al., 2018). The second viewpoint, the organic aggregate model, suggests that marine organisms with carbonate skeletons rich in elemental barium will gradually decompose during the sedimentation process after their deaths. As part of this, the carbonate dissolution will release barium into deep seawater, where it would combine with sulfate from rotten organic matters, leading finally to the crystallization and precipitation of barite crystals. This suggests that biogenic barite, in particular, is formed during the process of organic matter migrating from surface water to the bottom of the ocean in conjunction with the decomposition of organic matters. Therefore, the amount of barite increases toward the deep water and reaches its maximum value at the bottom of the ocean (Dymond et al., 1992; Francois et al., 1995; Klump et al., 2000, 2001; Esser and Volpe, 2002).
In the study area, sunlight was sufficient, the chlorophyll-a content of surface water was high (Fig.7), and there were many phytoplankton in the surface water (Liu, 2002). There were also numerous algal particles among the suspended particles, as SEM analysis showed. The maximum barite crystal concentration is just below the layer with the maximum concentration of chlorophyll-a (Fig.8). Therefore, we inferred that a large quantity of plankton with enriched barium in their bones lived in the surface water, and when they died and decomposed with other organic matters, the barium is released and the barite crystals are formed. Therefore, the formation of marine barite crystals in the study area follows the celestite model.
As shown in Figs.7 and 8, barite crystals begin to appear at approximately 30 m in the water column; however, their concentration is low in the shallow water above 200 m, which indicates that they began to form in the euphotic layer of the water (Paytan and Griffith, 2007; Sun et al., 2015). The high temperature of seawater in this layer also makes it more suitable for the plankton growth, and several dead organisms also begin decomposing in this area. All of these features and processes promote the development of barite. The concentration and size of the barite crystals also increased significantly at the water depth of 200 m, indicating that a large number of barite crystals were formed in this zone – exactly the depth at which plankton died and began to decay and sink. We also found, by SEM, that many barite crystals were wrapped in or attached to biological debris or organic films. Additionally, the barite crystals observed in this layer were relatively thick and the crystal morphologies were relatively intact. Therefore, barite crystals are mainly formed in the water from the surface to the depth of 200 m.
However, the DO concentration of seawater decreases below 200 m, and OMZs are located at depths of approximately 200 – 500 m in the study area (Paulmier and Ruiz, 2009; Wang et al., 2018). This was not conducive to the decomposition of organic organisms and the release of barium, thus limited the formation of barite in water. As a result, the concentration of barite crystals decreased sharply in the water layers at 300 m and 500 m (Figs.7 and 8). At depths ranging from 500 m to 2000 m, influenced by the oxygen-rich AAIW, DO increased again (Alexander et al., 2009; Wang et al., 2018). Therefore, residual organic matters began decomposing again, leading to a subsequent increase in the concentration of barite crystals.
Barite particles in the water column below 2000 m are primarily derived from the settling of the upper water particles, accompanied by the continuous dissolution. In the near-bottom water, sedimentary processes as well as the partition of the bottom current from the AABW (Alexander et al., 2009; Wang et al., 2018) bring more barite particles from the upper water, thus resulting in the increase in both the concentration and particle size of barite crystals.
4.3 Dissolution and Preservation of Barite CrystalsBarite in marine sediments can be used as an important indicator of the primary productivity (Dymond et al., 1992; Paytan and Griffith, 2007), so it is of great significance to understand how marine barite is dissolved and preserved after its formation in the water column. The ocean is typically unsaturated in terms of pure marine barite (Monnin et al., 1999), and previous studies have demonstrated that its crystals are subject to dissolution after they are formed in ocean water. It is therefore a common for marine barite to possess a dissolution morphology in addition for some of it to have an intact morphology (Putnis et al., 1995; Bosbach et al., 1998; Kai et al., 1999; Wang et al., 1999).
In the open ocean, the concentration of dissolved barium (DBa) is low in the surface water and increases with depth (Wolgemut and Broecker, 1970; Bacon and Edmond, 1972; Bernat et al., 1972; Chan et al., 1976). The concentration of DBa in seawater is controlled predominantly by the solubility of barite (Church and Wolgemuth, 1972; Dehairs et al., 1980; Sugiyama et al., 1984). The elemental barium is either taken up by suspended substances in the surface water, or combined with sulfate to form particulate barite.
Some of these substances are destroyed as they settle to the bottom of the ocean, and barium is finally released into the deep water again (Sugiyama et al., 1984). There is no database on the DBa concentrations in the water of our study area yet; however, the DBa concentrations in the Pacific Ocean, the Southern Ocean, the Indian Ocean and the South China Sea all gradually increase from the surface to the deep water and become stable at depths below 2000 m (Fig.8). The coordinated changes in the DBa concentrations in global ocean water confirm that barite is primarily formed in the water column above 2000 m, and that the dissolution begins as it settles after formation. This can explain the increase in the DBa concentrations in water above 2000 m. As there is no new generation of barite in the water column below 2000 m, the amount and concentration of barite crystals gradually decrease and the dissolution-compensation equilibrium tends to be stable.
The morphology of barite always changes, indicating its crystal shape is destroyed due to the dissolving process in the water column. Etch pits were observed around the edges or on the surface of the barite crystals unevenly, which may make crystals irregular in shape (Fig.5). Barite particles with dissolution features around the edges of the crystals were widely distributed within the study area (Figs.9a, b, c) and observed at all depths of all stations. Additionally, they were more likely to be found in deeper water. However, a notable dissolution characteristic is that the etch pits were not homogeneously distributed on the crystal surfaces, and the intensive dissolution occurred in the middle or center of the crystal surfaces rather than just along their edges. The inner etch pits were detected at the center of the particle surfaces or in inner cavities that occurred at the core of the crystals (Figs.9d, e). The proportion of barite particles with strong dissolution features is low; however, an X-ray energy spectrum study incorporating the use of SEM showed that they all had high strontium contents (Figs.9e).
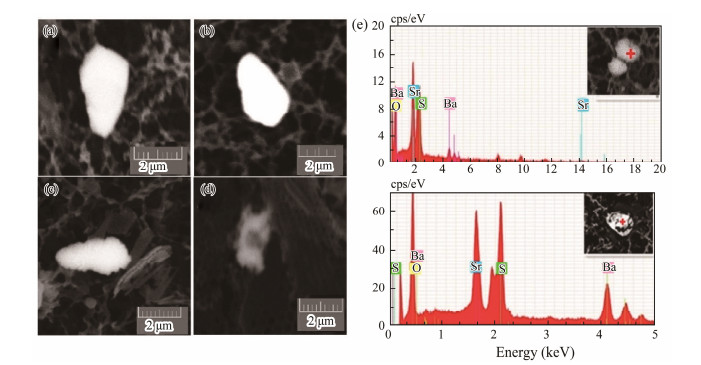
|
Fig. 9 Morphology of dissolved barite crystals (a) – (d) and the chemical composition of strongly dissolved barites determined by the X-ray energy spectrum (e). |
The presence of several barite crystals in seawater may be related to the dissolution system of barite and celestite coexisting in a certain proportion in the marine environment, and a considerable amount of strontium and barium exists in solid solution. This may alter the saturation state of barite in seawater to a value that can be as high as 30% (Rushdi et al., 2000). Sun et al. (2015) proposed that the differential dissolution of barite crystals in the eastern equatorial Pacific Ocean is a consequence of the heterogeneous distribution of strontium in barite crystals, exerting an important effect on the morphology of the latter. The elemental strontium in barite plays a unique role in its dissolution; the solubility of marine barite containing strontium is 1013 times higher than that of pure barite (Putnis et al., 1995; Prieto et al., 1997). Previous studies have demonstrated that the composition of crystals may change from the core to the edge in a complete series of celestite and barite solid solution systems in the process of forming the (Ba, Sr)SO4 crystal. The barite crystals tends to be rich in strontium in their center and are almost pure barite at the edge (Prieto et al., 1993). The selective dissolution of barite particles is caused by the non-homogeneous participation of strontium during the barite formation, and the Sr-enriched portion dissolves first and the barium-enriched portion is preserved. Therefore, the inner etch pits or cavities in the barite crystals were likely caused by the selective dissolution of crystal nuclei that is enriched in elemental strontium.
The barite particles in the study area with a dominant size of 1 – 3 μm remained in the water for a long period of time, until they eventually settled on the bottom of the ocean after formation in the upper water. According to the distribution characteristics of barite in the water column at stations CTD08, CTD12, and CTD13, approximately 33% of the total barite and 22% of the barite concentration settled to the bottom water of the stable area below a depth of 4000 m, compared to the former in the main barite formation area in water at depths of 0 – 500 m (Fig.8). A large proportion of barite in the underlying water will be deposited and buried in seafloor sediment; this is an important indicator of the primary productivity of water in this area.
5 ConclusionsMarine barite crystals were observed as suspended particles in the water columns at four stations near the 90°E Ridge of the Indian Ocean. The crystals came in four morphological types: euhedral-subhedral, oval or round, rhombic, and irregular. The barite crystals in the study area were typically fine and ranged in size from 0.5 – 7 μm, with a dominant particle size of 1 – 3 μm.
The horizontal distribution of barite crystals was not crucially influenced by the presence of the 90°E Ridge; however, its vertical distribution was significantly affected by its formation and sedimentation process. The surface to a depth of 2000 m is a formation zone, with explosive formation occurring at approximately 200 m; at this depth, barite particles are coarse. Areas below 2000 m are sedimentation and dissolution zones, where barite particles are fine and their concentration decreases downward. Barite is formed primarily based on the celestite model, with the decomposition of barium-rich carbonate skeletons of organisms in the decaying microenvironment. The process is affected by hydrological conditions especially primary productivity and DO content in the water column.
Barite did not form below 2000 m; however, continuous dissolution occurred during the settlement process, exhibiting a consistent relationship with the concentration of dissolved barium in ocean water. The dissolution of barites caused a decrease in their particle size and a significant change in their morphology. The substitution and non-uniform distribution of strontium in barite crystals promoted the dissolution of barite, leading to the selective dissolution of the nucleus of the barite crystal and exerting an important impact on its morphology. Once formed, barite took a long time to settle to the ocean floor; approximately 33% of the total and a concentration of 22% achieved this compared to the levels of barite present in the main formation zone.
AcknowledgementsThe authors would like to thank the captain and the crew of the R/V Dayangyihao for field sampling assistance and Prof. Tan Jinshan of Qingdao University for assistance in SEM observations. This study was supported by the COMRA Major Project (No. DY135-S1-01-09), and the Opening Foundation of Key Laboratory of Submarine Geosciences and Prospecting Techniques, Ocean University of China (No. SGPT-2019OF-02).
Alexander, M. P., Virupaxa, K. B., Adam, E. S., Elderfield, H., Galy, A., and Dennis, A., 2009. Indian Ocean circulation and productivity during the last glacial cycle. Earth and Planetary Science Letters, 285: 179-189. DOI:10.1016/j.epsl.2009.06.007 (  0) 0) |
Bacon, M. P., and Edmond, J. M., 1972. Barium at GEOSECS Ⅲ in the Southwest Pacific. Earth and Planetary Science Letters, 16: 66-74. DOI:10.1016/0012-821X(72)90237-3 (  0) 0) |
Beek, P. V., Sternberg, E., Reys, J. L., Souhaut, M., Robin, E., and Jeandel, C., 2009. 228Ra/226Ra and 226Ra/Ba ratios in the western Mediterranean Sea: Barite formation and transport in the water column. Geochimica et Cosmochimica Acta, 73: 4720-4737. DOI:10.1016/j.gca.2009.05.063 (  0) 0) |
Bernat, M., Church, T., and Allegre, C. J., 1972. Barium and strontium concentrations in Pacific and Mediterranean Sea water profiles by direct isotope dilution mass spectrometry. Earth and Planetary Science Letters, 16: 75-80. DOI:10.1016/0012-821X(72)90238-5 (  0) 0) |
Bernstein, R., Byrne, R. H., and Schijf, J., 1998. Acantharians: A missing link in the oceanic biogeochemistry of barium. Deep Sea Research Part I: Oceanographic Research Papers, 45: 491-505. DOI:10.1016/S0967-0637(97)00095-2 (  0) 0) |
Bernstein, R., Byrne, R., Betzer, P., and Greco, A. M., 1992. Morphologies and transformations of celestite in seawater: The role of acantharians in strontium and barium geochemistry. Geochimica et Cosmochimica Acta, 56: 3273-3279. DOI:10.1016/0016-7037(92)90304-2 (  0) 0) |
Bertram, M., and Cowen, J., 1997. Morphological and compositional evidence for biotic precipitation of marine barite. Journal of Marine Research, 55: 577-593. DOI:10.1357/0022240973224292 (  0) 0) |
Bishop, J. K., 1988. The barite-opal-organic carbon association in oceanic particulate matter. Nature, 332: 341-343. DOI:10.1038/332341a0 (  0) 0) |
Bosbach, D., Hall, C., and Putnis, A., 1998. Mineral precipitation and dissolution in aqueous solution: In-situ microscopic observations on barite (001) with atomic force microscopy. Chemical Geology, 151: 143-160. DOI:10.1016/S0009-2541(98)00076-X (  0) 0) |
Burton, K. W., and Vance, D., 2000. Glacial-interglacial variations in the neodymium isotope composition of seawater in the Bay of Bengal recorded by planktonic foraminifera. Earth and Planetary Science Letters, 176: 425-441. DOI:10.1016/S0012-821X(00)00011-X (  0) 0) |
Campbell, S. M., Moucha, R., Derry, L. A., and Raymo, M. E., 2018. Effects of dynamic topography on the Cenozoic carbonate compensation depth. Geochemistry, Geophysics, Geosystems, 19(4): 1025-1034. DOI:10.1002/2017GC007386 (  0) 0) |
Cao, Z., Siebert, C., Hathorne, E. C., Dai, M., and Frank, M., 2016. Constraining the oceanic barium cycle with stable barium isotopes. Earth and Planetary Science Letters, 434: 1-9. DOI:10.1016/j.epsl.2015.11.017 (  0) 0) |
Chan, L. H., Edmond, J. M., Stallard, R. F., Broecker, W. S., and Ku, T. L., 1976. Radium and barium at GEOSECS stations in the Atlantic and Pacific. Earth and Planetary Science Letters, 32: 258-267. DOI:10.1016/0012-821X(76)90066-2 (  0) 0) |
Church, T. M., and Wolgemuth, K., 1972. Marine barite saturation. Earth and Planetary Science Letters, 15: 35-44. DOI:10.1016/0012-821X(72)90026-X (  0) 0) |
Dehairs, R., Chesselet, R., and Jedwab, J., 1980. Discrete suspended particles of barite and the barium cycle in the open ocean. Earth and Planetary Science Letters, 49: 529-550. (  0) 0) |
Dehairs, F., Goeyens, L., Stroobants, N., Bernard, P., Goyet, C., Poisson, A., et al., 1990. On suspended barite and the oxygen minimum in the Southern Ocean. Global Biogeochemical Cycles, 4: 85-102. DOI:10.1029/GB004i001p00085 (  0) 0) |
Dymond, J., and Collier, R., 1996. Particulate barium fluxes and their relationships to biological productivity. Deep Sea Research Part Ⅱ: Topical Studies in Oceanography, 43: 1283-1308. DOI:10.1016/0967-0645(96)00011-2 (  0) 0) |
Dymond, J., Suess, E., and Lyle, M., 1992. Barium in deep-sea sediment: A geochemical proxy for paleoproductivity. Paleoceanography, 7: 163-181. DOI:10.1029/92PA00181 (  0) 0) |
Eagle, M., Paytan, A., Arrigo, K. R., Dijken, G. V., and Murray, R. W., 2003. A comparison between excess barium and barite as indicators of carbon export. Paleoceanography, 18: 1021-1033. (  0) 0) |
Esser, B. K., and Volpe, A., 2002. At-sea high-resolution trace element mapping: San Diego Bay and its plume in the adjacent coastal ocean. Environmental Science & Technology, 36: 2826-2832. (  0) 0) |
Fagel, N., Debrabant, P., and Andre, L., 1994. Clay supplies in the central Indian Basin since the late Miocene: Climatic or tectonic control?. Marine Geology, 122: 151-172. DOI:10.1016/0025-3227(94)90209-7 (  0) 0) |
Fang, N., Ding, X., Liu, Y., Hu, C., Chen, X., and Zhang, Z., 2002. Pelagic sedimentary records of the Ninetyeast Ridge and the late Cenozoic important tectono-environment events. Earth Science Frontiers, 9(1): 103-111 (in Chinese with English abstract). DOI:10.3321/j.issn:1005-2321.2002.01.013 (  0) 0) |
Francois, R., Honjo, S., Manganini, S. J., and Ravizza, G. E., 1995. Biogenic barium fluxes to the deep sea: Implications for paleoproductivity reconstruction. Global Biogeochemistry Cycles, 9: 289-303. DOI:10.1029/95GB00021 (  0) 0) |
Ganeshram, R. S., François, R., Commeau, J., and Brown-Leger, S. L., 2003. An experimental investigation of barite formation in seawater. Geochimica et Cosmochimica Acta, 67: 2599-2605. DOI:10.1016/S0016-7037(03)00164-9 (  0) 0) |
Gingele, F., and Dahmke, A., 1994. Discrete barite particles and barium as tracers of paleoproductivity in South Atlantic sediments. Paleoceanography, 9: 151-168. DOI:10.1029/93PA02559 (  0) 0) |
Gooday, A., and Nott, J., 1982. Intracellular barite crystals in two Xenophyophores, Aschemonella ramuliformis and Galahteammina sp. with comments on the taxomony of A. ramuliformis. Journal of the Marine Biological Association of the United Kingdom, 62: 595-605. (  0) 0) |
Gorsline, D. S., 1984. A review of fine-grained sediment origins, characteristics, transport and deposition. In: Fine Grained Sediments: Deep-Water Processes and Facies. Stow, D., and Piper, D., eds., Blackwell Scientific, Oxford, 17-34.
(  0) 0) |
Griffith, E. M., and Paytan, A., 2012. Barite in the ocean-occurrence, geochemistry and palaeoceanographic applications. Sedimentology, 59(6): 1817-1835. DOI:10.1111/j.1365-3091.2012.01327.x (  0) 0) |
Hoppema, M., Dehairs, F., Navez, J., Monnin, C., and Baar, H. J. W. D., 2010. Distribution of barium in the Weddell Gyre: Impact of circulation and biogeochemical processes. Marine Chemistry, 122(1-4): 118-129. DOI:10.1016/j.marchem.2010.07.005 (  0) 0) |
Hovan, S. A., and Rea, D. K., 1992. The Cenozoic record of continental mineral deposition on Broken and Ninetyeast Ridges, Indian Ocean: Southern African aridity and sediment delivery from the Himalayas. Paleoceanography, 7: 833-860. DOI:10.1029/92PA02176 (  0) 0) |
Jeandel, C., Dupré, B., Lebaron, G., Monnin, C., and Minster, J. F., 1996. Longitudinal distributions of dissolved barium, silica and alkalinity in the western and southern Indian Ocean. Deep Sea Research Part I: Oceanographic Research Papers, 43: 1-31. DOI:10.1016/0967-0637(95)00098-4 (  0) 0) |
Jeandel, C., Tachikawa, K., Bory, A., and Dehairs, F., 2000. Biogenic barium in suspended and trapped material as a tracer of export production in the tropical NE Atlantic (EUMELI sites). Marine Chemistry, 71: 125-142. DOI:10.1016/S0304-4203(00)00045-1 (  0) 0) |
Kai, D., Daniel, E., Shuler, P. J., Chen, H. J., Tang, Y., and Yen, T. F., 1999. Mechanisms of surface precipitation and dissolution of barite: A morphology approach. Journal of Colloid and Interface Science, 214(2): 427-437. DOI:10.1006/jcis.1999.6224 (  0) 0) |
Klootwijk, C. T., Gee, J. S., Peirce, J. W., and Smith, G. M., 1992. Neogene evolution of the Himalayan-Tibetan region: Constraints from ODP758, northern Ninetyeast Ridge; bearing on climatic change. Palaeogeography, Palaeoclimatology, Palaeoecology, 95: 95-110. DOI:10.1016/0031-0182(92)90167-4 (  0) 0) |
Klump, J., Hebbeln, D., and Wefer, G., 2000. The impact of sediment provenance on barium-based productivity estimates. Marine Geology, 169(3-4): 259-271. DOI:10.1016/S0025-3227(00)00092-X (  0) 0) |
Klump, J., Hebbeln, D., and Wefer, G., 2001. High concentrations of biogenic barium in Pacific sediments after Termination I – A signal of changes in productivity and deep water chemistry. Marine Geology, 177(1): 1-11. (  0) 0) |
Liu, Q. Y., 2002. Pelagic sedimentary records and its palaeoenvironmental implication in Ninetyeast Ridge if the NE Indian Ocean since middle Miocene. Master thesis. China University of Geosciences.
(  0) 0) |
Martinez-Ruiz, F., Jroundi, F., Paytan, A., Guerra-Tschuschke, I., Abad, M., and González-MuOz, M. T., 2018. Barium bioaccumulation by bacterial biofilms and implications for Ba cycling and use of Ba proxies. Nature Communications, 9(1): 1619. DOI:10.1038/s41467-018-04069-z (  0) 0) |
Monnin, C., Jeandel, C., Cattaldo, T., and Dehairs, F., 1999. The marine barite saturation state of the world's oceans. Marine Chemistry, 65: 253-261. DOI:10.1016/S0304-4203(99)00016-X (  0) 0) |
Paulmier, A., and Ruiz-Pino, D., 2009. Oxygen minimum zones (OMZs) in the modern ocean. Progress in Oceanography, 80: 113-128. DOI:10.1016/j.pocean.2008.08.001 (  0) 0) |
Paytan, A., and Griffith, E., 2007. Marine barite: Recorder of variations in ocean export productivity. Deep-Sea Research Part Ⅱ: Topical Studies in Oceanography, 54: 687-705. DOI:10.1016/j.dsr2.2007.01.007 (  0) 0) |
Paytan, A., Kastner, M., and Chavez, F. P., 1996. Glacial to interglacial fluctuations in productivity in the equatorial Pacific as indicated by marine barite. Science, 274: 1355-1357. DOI:10.1126/science.274.5291.1355 (  0) 0) |
Paytan, A., Kastner, M., Martin, E. E., Macdougall, J. D., and Herbert, T., 1993. Marine barite as a monitor of seawater strontium isotope composition. Nature, 366: 445-448. DOI:10.1038/366445a0 (  0) 0) |
Prieto, M., Fernández-González, A., Putnis, A., and Fernández-Díaz, L., 1997. Nucleation, growth, and zoning phenomena in crystallizing (Ba, Sr)CO3, Ba(SO4, CrO4), (Ba, Sr) SO4, and (Cd, Ca)CO3 solid solutions from aqueous solutions. Geochimica et Cosmochimica Acta, 61: 3383-3397. DOI:10.1016/S0016-7037(97)00160-9 (  0) 0) |
Prieto, M., Putnis, A., and Fernandez-Diaz, L., 1993. Crystallization of solid solution from aqueous solutions in a porous medium: Zoning in (Ba, Sr)SO4. Geological Magazine, 130: 289-299. DOI:10.1017/S0016756800019981 (  0) 0) |
Putnis, A., Junta-Rosso, J. L., and Hochella, M. F., 1995. Dissolution of barite by a chelating ligand: An atomic force microscopy study. Geochimica et Cosmochimica Acta, 59: 4623-4632. DOI:10.1016/0016-7037(95)00324-X (  0) 0) |
Pyle, K. M., Hendry, K. R., Sherrell, R. M., Legge, O., Hind, A. J., Bakker, D., et al., 2018. Oceanic fronts control the distribution of dissolved barium in the Southern Ocean. Marine Chemistry, 204: 95-106. DOI:10.1016/j.marchem.2018.07.002 (  0) 0) |
Qiao, B., Liu, Z., Zhang, S., Liu, C., and Li, P., 2014. Equatorial current system structure and hydrologic characteristics in monsoonal wind transition period. Advances in Marine Science, 32(3): 301-305 (in Chinese with English abstract). DOI:10.3969/j.issn.1671-6647.2014.03.001 (  0) 0) |
Rushdi, A. L., McManus, J., and Collier, R. W., 2000. Marine barite and celestite saturation in seawater. Marine Chemistry, 69: 19-31. DOI:10.1016/S0304-4203(99)00089-4 (  0) 0) |
Singh, S. P., Singh, S. K., and Bhushan, R., 2013. Internal cycling of dissolved barium in water column of the Bay of Bengal. Marine Chemistry, 154: 12-23. DOI:10.1016/j.marchem.2013.04.013 (  0) 0) |
Sternberg, E., Jeandel, C., Miquel, J. C., Gasser, B., Souhaut, M., Arraes-Mescoff, R., et al., 2007. Particulate barium fluxes and export production in the northwestern Mediterranean. Marine Chemistry, 105: 281-295. DOI:10.1016/j.marchem.2007.03.003 (  0) 0) |
Stow, D. A. V., Amano, K., Balson, P. S., and Wijayananda, N. P., 1990. Sediment facies and processes on the distal Bengal Fan, Leg 116. Proceedings of the Ocean Drilling Program, Scientific Results, 116: 377-395. (  0) 0) |
Sugiyama, M., Matsuil, M., and Nakayama, E., 1984. Direct determination of barium in sea water by inductively coupled plasma emission spectrometry. Journal of the Oceanographical Society of Japan, 40: 295-302. DOI:10.1007/BF02302522 (  0) 0) |
Sun, X., 2011. Study on the suspended particulate minerals in the water column in the eastern equatorial Pacific Ocean and hydrothermal active areas in the Southwest Indian Ocean. PhD thesis. Ocean University of China.
(  0) 0) |
Sun, X., Yang, Z., Fan, D., and Li, Y., 2015. Crystals of suspended marine barite in the eastern equatorial Pacific: Processes of dissolution and effects on crystal morphology. Chinese Journal of Oceanology and Limnology, 33(1): 194-203. DOI:10.1007/s00343-015-3353-1 (  0) 0) |
Stroobants, N., Dehairs, F., Goeyens, L., Vanderheijden, N., and Grieken, R. V., 1991. Barite formation in the Southern Ocean water column. Marine Chemistry, 35: 411-421. DOI:10.1016/S0304-4203(09)90033-0 (  0) 0) |
Wang, K., Resch, R., Dunn, K., Shuler, P., Tang, Y., Koel, B. E., et al., 1999. Dissolution of the barite (001) surface by the chelating agent DTPA as studied with non-contact atomic force microscopy. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 160: 217-227. (  0) 0) |
Wang, Y., Wang, B., Wei, Q., Sun, X., Xin, M., and Liu, L., 2018. Seasonal variation of hypoxic zone in the central eastern Indian Ocean. Advances in Marine Science, 36(2): 262-271 (in Chinese with English abstract). DOI:10.3969/j.issn.1671-6647.2018.02.011 (  0) 0) |
Wei, H., Fang, N., Ding, X., Nie, L., and Liu, X., 2007. Major environmental events reflected by pelagic records since 3.5 Ma BP in the Ninetyeast Ridge at the equator. Geological Bulletin of China, 26(12): 1627-1632. (  0) 0) |
Wolgemuth, K., and Broecker, W. S., 1970. Barium in sea water. Earth and Planetary Science Letters, 8: 372-378. DOI:10.1016/0012-821X(70)90110-X (  0) 0) |
Wyrtkik, 1973. An equatorial jet in the Indian Ocean. Science, 181(4096): 262-264. DOI:10.1126/science.181.4096.262 (  0) 0) |
 2023, Vol. 22
2023, Vol. 22


