2) Laboratory for Marine Drugs and Bioproducts of Qingdao National Laboratory for Marine Science and Technology, Qingdao, 266237, China
Brown algae is widely distributed throughout various seas around the world. At present, there are approximately 2000 species according to statistical reports (Thompson et al., 2019). It is well known for its richness in bioactive compounds, such as polysaccharides, polyphenols, omega-3 fatty acids, and carotenoids, which have considerable economic values (Wijesekara et al., 2011). Laminarin, the storage carbohydrate in brown algae, is also known as brown algal starch and is mainly formed by glucose monomers connected by β-1, 3-glucosidic bonds on its backbone and partial β-1, 6-glucosidic bonds on its branches (Kadam et al., 2015). It belongs to the family of β-glucans because of its single structure and connection method (Rioux et al., 2010). The laminarin content of brown algae is related to the species, growth environment, and harvest season. Generally, Saccharina, Laminarina, and Fucus spp. are the main sources of laminarin, and their laminarin content can be up to 62% of total dry weight at the highest. It has been reported that during the summer and autumn, laminarin accumulates and its content in Laminaria digitata increase, while during the winter, the content decreases due to the consumption of energy for new tissue growth (Adams et al., 2011). As a kind of functional marine algal polysaccharide, laminarin possesses a wide range of biological activities, including antitumor, antioxidant, anti-inflammatory, prebiotic, and other biofunctional activities (Kim et al., 2006; Kadam et al., 2015). In addition, it also plays an important role in the marine carbon cycle (Becker et al., 2020). As a relatively underexploited algal polysaccharide, laminarin also can be transformed into bioethanol through the bioprocess of fermentation (Lee and Lee, 2016). Therefore, laminarin possess an array of important applications in the fields of food, medicine, cosmetics, and energy.
Marine algal oligosaccharides can be obtained from marine algal polysaccharides through physical or chemical and enzymatic methods. Currently, marine algal oligosaccharides, such as alginate oligosaccharides, agar oligosaccharides, and carrageenan oligosaccharides, have received increasing attention due to their excellent solubility, bioavailability and prominent biological activities. Similarly, laminarin oligosaccharides, the degradation products of laminarin, can also be prepared by these strategies of physical hydrolysis, chemical hydrolysis, and enzymatic degradation. It has been reported that laminarin oligosaccharides have a significant effect on improving immunity and antitumor activity, and regulating the intestinal flora (Yvin et al., 1999; Kim et al., 2006; Kadam et al., 2015).
The present article reviews the preparation strategies, biofunctional activities, and potential applications of laminarin and laminarin oligosaccharides.
2 Preparation of LaminarinThe process of laminarin preparation can be divided into pre-treatment of raw materials, extraction, and purification. Generally, the raw materials need to be washed several times to remove epiphytes and sands, and dried thoroughly (Imbs et al., 2016). Traditionally, laminarin is extracted from dried brown algae with high temperature and mild acidic conditions. Nowadays, some environment-friendly green methods, including enzymatic extraction and microwave extraction, can also be exploited for laminarin preparation.
2.1 Traditional Laminarin Extraction StrategyDuring the traditional solvent extraction process, the appropriate concentration of ethanol can precipitate laminarin and separate some impurities. After ultrafiltration and dialysis, laminarin can be isolated (O'Shea et al., 2014). Most laminarin extraction methods use mild acidic or basic solvents. The type of acid solution and extraction temperature can affect the extraction efficiency of laminarin. Devillé et al. (2004) investigated the extraction of laminarin by different acid solutions. The results showed that HCL produces a higher extraction yield than H2SO4. Meanwhile, a high temperature can facilitate laminarin extraction and improve the yield. In fact, a high temperature was beneficial to the extraction of laminarin. Zha et al. (2012) extracted laminarin from Laminaria japonica at 4, 20, 40, 60, and 80℃ using water as the extraction solvent. The results showed that when the temperature was increased from 4 to 60℃, there was a corresponding increase in the quantity of laminarin. When the temperature was increased sequentially, the total sugar amount remained unchanged, and some extracted laminarin was degraded into laminarin oligosaccharides. Following the initial extraction, the obtained solution contained mixed polysaccharides such as laminarin, fucoidan, and alginate. CaCl2 or MnCl2 can be added to the solution to eliminate the alginate because alginate exhibits gel properties in the Ca2+ or Mn2+ solution (Cong et al., 2016). Rioux et al. (2010) used CaCl2 solution as a solvent to extract laminarin at 85℃ for 4 h. The same volume of sodium chloride solution (2%) and twice the volume of anhydrous ethanol were added to the extraction solution to precipitate the laminarin for 1 h at room temperature. Finally, laminarin can be further purified from the crude extract following the purification procedures described below (Cong et al., 2016).
2.2 Innovative Extraction Strategy of LaminarinIn addition to the traditional extraction methods, some environmentally friendly and efficient extraction methods, such as enzyme-assisted extraction (EAE), microwave-assisted extraction (MAE), and ultrasound-assisted extraction (UAE), have been used for the gradual extraction of laminarin (Chen et al., 2013).
EAE is a promising extraction strategy (Zhu et al., 2014). Charoensiddhi et al. (2016) used three commercial cell wall carbohydrate hydrolytic enzymes and three proteases to assist in the extraction of laminarin from the brown seaweed Ecklonia radiata at 50℃ for 24 h. During this process, the cell walls of the algae were broken up, and the polysaccharides containing laminarin were released. Subsequently, lyophilization and fractionation were employed for the isolation and purification of laminarin, and the molecular weight (Mw) of the purified laminarin was less than 65 kDa.
Microwaves have good penetrability and are effective for the extraction of active, heat sensitive substances (Pap et al., 2013). It was found that the amount of laminarin extracted increased with increasing microwave power during the MAE process (Gao et al., 2006). However, when the microwave power exceeded 400 W, the amount of laminarin extracted gradually decreased. It was speculated that excessive power can cause the degradation of laminarin. Therefore, it is necessary to control the power level in the MAE process to keep the yield and activity of laminarin. Many algal polysaccharides have been extracted with MAE up to now, such as fucoidan from Fucus vesiculosus, Undaria pinnatifida, and Ascophyllum nodosum (Quitain et al., 2013). Therefore, MAE shows good prospects for application in laminarin extraction.
UAE (ultrasound-assisted extraction) is another cost-effective laminarin extraction technology with great potential for large-scale industrialization (García-Vaquero et al., 2017). In addition to polysaccharide extraction, UAE has been used to extract functional components such as phycoerythrin, amino acids, and lipids from a variety of algae sources (Adam et al., 2012). Kadam et al. (2015) used UAE with an ultrasonic power amplitude of 69% to extract laminarin from Ascophyllum nodosum. The extracts were treated with 0.1 mol L−1 HCl for 15 min followed by solid-liquid extraction. Finally, the amount of laminarin obtained was estimated to be 5.82% of the dry weight of A. nodosum. The 1, 1-diphenyl-2-picryl-hydrazyl (DPPH) radical-scavenging activity of the extracted laminarin reached as high as 93.23%, demonstrating a good antioxidant activity.
These new extraction strategies lay an industrial foundation for the efficient extraction of polysaccharides and other active substances from marine algae. A comparison of these extraction strategies is outlined in Table 1.
|
|
Table 1 Comparison of several strategies for extracting laminarin |
In order to remove impurities such as other polysaccharides, proteins, and phenolic compounds from the crude laminarin extract, it is necessary to further purify laminarin (Ale et al., 2011). In fact, the procedures of laminarin extraction and laminarin purification are sometimes not strictly distinguishable, because some extraction processes include certain purification steps. For example, the fractional precipitation of laminarin described in the extraction section can also be considered as a step of laminarin purification. The laminarin purification process described in this section mainly refers to the next step of separation and purification of pure laminarin to detect its biological activity. At present, the common methods of pure laminarin purification include size-exclusion chromatography (SEC) and affinity chromatography (AC).
SEC can be used to separate and purify polysaccharides according to their molecular weights (Mw). Thus, SEC is an efficient and convenient method for separating and purifying laminarin. Zhang et al. (2015) used this method with a Waters UltrahydrogelTM WATO 11530 size-exclusion column (300 mm × 7.8 mm) to separate algal polysaccharides and obtained 383.8 mg g−1 of laminarin, which showed a good purification effect. In addition, SEC can be used as an effective method to determine the Mw of polysaccharides (Gaborieau and Castignolles, 2011).
AC (affinity chromatography) is a powerful method to separate, purify, and analyze target compounds in a sample (Pohleven et al., 2012). Affinity columns can be used alone or together with other purification methods to purify laminarin (Hirabayashi et al., 2002). At present, AC has been used to successfully separate a variety of active substances, such as proteins, enzymes, antibodies, and polysaccharides (Hahn et al., 2016). This method is commonly used in the purification of sulfated polysaccharides (Mak et al., 2013). Labourel et al. (2015) combined both AC and SEC for the analysis of enzymatic products of laminarin and found that the laminarinase ZgLamCGH16 preferentially hydrolyzed branched laminarin.
SEC can be combined with many purification methods for further separation and purification of the target products. AC is mainly used to separate biomacromolecules such as proteins according to their different chemical structures. SEC and AC have been applied successfully in the purification of many polysaccharides (García-Vaquero et al., 2017). However, high cost remains the main factor limiting their large-scale application. In addition to the above chromatography methods, there are other purification methods, such as ultrafiltration and membrane filtration (Oda et al., 2016). Ultrafiltration and membrane filtration can be used to separate diverse solutions and are suitable for polysaccharide purification on an industrial scale, but the product purity is relatively low. Therefore, the appropriate purification methods are usually selected according to actual needs.
These methods can be chosen to further characterize the biological activity of laminarin. The processes of laminarin extraction and purification are presented in Fig. 1.

|
Fig. 1 Laminarin extraction and purification processes. |
In general, laminarin oligosaccharides can be prepared
by depolymerization of laminarin (chemical, physical, and enzymatic hydrolysis) (Iji and Tivey, 1998). Furthermore, laminarin oligosaccharides can also be produced by synthesis from disaccharide or monosaccharide substrates.
3.1 Laminarin Oligosaccharide Preparation by Chemical and Physical HydrolysisMany algal polysaccharides, such as fucoidan, carrageenan, and agarose, can be degraded into oligosaccharides by acidic hydrolysis at high temperatures due to the cleavage of glycosidic bonds (Chen et al., 2005). Laminarin oligosaccharides can also be produced by partial acid hydrolysis and then separated by size-fractionation and reversed-phase high-performance liquid chromatography (Natsuka et al., 2018). As an effective method of degrading polysaccharides, acid hydrolysis can help us to understand the specific structure of polysaccharides. Graiff et al. (2016) explored the preparation of laminarin oligosaccharides by using 0.5 mol L−1 H2SO4 to hydrolyze commercial laminarin. The hydrolysis temperature was 121℃, and the ionexclusion chromatography results showed that when the hydrolysis time was 5 min, there were oligosaccharides in the hydrolytic products. When the hydrolysis time was extended to 180 min, the chromatogram showed only glucose and sulfuric acid without oligosaccharides in the products (Renard et al., 1997).
Radiolysis, such as ultraviolet light and gamma rays, can induce the degradation of polysaccharides by breaking glycosidic bonds and is the main physical treatment for oligosaccharide preparation (Ramani and Ranganathaiah, 2000). Gamma irradiation has been applied to the acquisition of oligosaccharides (Nagasawa et al., 2000). Choi et al. (2011) used gamma irradiation to obtain laminarin oligosaccharides, and the results of Nuclear Magnetic Resonance (NMR) analysis indicated that the glycosidic bonds of laminarin were randomly broken. The antioxidant activity of the prepared laminarin oligosaccharides was higher than that of laminarin. Unlike chemical methods, physical hydrolysis for laminarin oligosaccharide production is eco-friendly. However, laminarin oligosaccharides with the desired degree of polymerization (DP) cannot be produced by physical irradiation of polysaccharides, and long exposure times to physical radiation may cause damage to the structures of oligosaccharides (Delattre et al., 2005).
3.2 Laminarin Oligosaccharide Preparation by Enzymatic DegradationSome enzymes can efficiently and specifically hydrolyze laminarin into laminarin oligosaccharides under mild conditions. Enzymes that can specifically hydrolyze laminarin include endo-β-1, 3-glucanase (laminarinase, EC 3.2.1.39) and exo-β-1, 3-glucanase (EC 3.2.1.58) (Santos et al., 1979). Laminarinase can randomly cleave (1→3)-β-linkages within laminarin glycoside bonds to release oligosaccharides, while exo-β-1, 3-glucanases can hydrolyze laminarin by sequentially cleaving glucose residues from the non-reducing end and release glucose (Bara et al., 2003). There are many reports about laminarinases from bacteria, fungi, higher plants, and archaea isolated from soil and marine environments (Tschiggerl et al., 2008). These glycoside hydrolases can be used not only to prepare oligosaccharides, but also to accurately quantify laminarin in marine organic matter. Becker et al. (2017) used laminarinase to digest glycans selectively and quantify laminarin in particulate organic matter.
The main sources of laminarinase include bacteria (such as Bacillus circulans, Flavobacterium johnsoniae, Paenibacillus sp., Rhodothermus marinus, and Thermotoga maritima), fungi (such as Candida albicans, Saccharomyces cerevisiae, and Yarrowia lipolytica), and algae (such as L. digitata) (Sandini et al., 2007; Li et al., 2009; Kim et al., 2011; Kusaykin et al., 2017). According to sequence information, endo-β-1, 3-glucanases can be grouped into six glycoside hydrolase (GH) families including GH16, GH17, GH55, GH64, GH81, and GH128 in the CAZy database (http://www.cazy.org/) (Sakamoto et al., 2011). Similarly, exo-β-1, 3-glucanases can be classified into GH3, GH5, GH17, GH55, and GH132 in the CAZy database (Kumar et al., 2018). Based on amino acid sequence divergence, most laminarinases derived from bacteria are categorized into GH16, while laminarinases originating from plants are all assigned to GH17 (Sandini et al., 2007). Diverse laminarinases have been employed for the preparation of laminarin oligosaccharides. The characteristics and hydrolysis products of laminarinases from different GH families are listed in Table 2.
|
|
Table 2 Action patterns, hydrolysis products, optimum pH, and temperature of laminarinases |
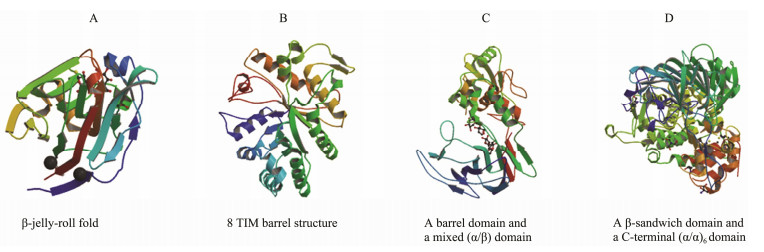
Most of the characterized laminarinases belonging to the GH16 family have conserved sequences. For example, the conserved sequences of the laminarinase from the marine bacterium Formosa algae KMM 3553 include the sequences WPAXWXL (substrate binding site) and EIDXXE (catalytic active site) (Kusaykin et al., 2017). Until now, crystal structures of different laminarinases from the GH16 family have been analyzed, and their crystal structures share a β-jelly-roll fold, and the inner β-fold bends outward to form a long catalytic groove (Dong et al., 2015) (Fig. 2A). The laminarinase from P. rolfsii c3-2(1) IBRL belongs to the GH16 family, and it is a thermostable laminarinase that can hydrolyze laminarin to laminaribiose and glucose at 70℃ (Lee et al., 2014). The heat resistance of this laminarinase and its product characteristics indicate its application in the preparation of laminarin oligosaccharides. Recently, Badur et al. (2020) reported three laminarinases from Vibrio breoganii 1C10 belong to the GH16 family. As a member of these laminarinases, laminarinase VbGH16C can hydrolyze laminarin to oligosaccharides of DP8 and DP9. This characteristic of the hydrolysate can be applied in the preparation of relatively large laminarin oligosaccharides.
|
Fig. 2 Three-dimensional structures of laminarinases, including laminarinase ZgLamC of the GH16 family (PDB: 4CTE), laminarinase of the GH17 family (PDB: 2CYG), laminarinase LPHase of the GH64 family (PDB: 3GD9), and laminarinase ZgLamC of the GH81 family (PDB: 4K35). |
The laminarinases derived from plants that have been reported so far belong to GH17 (Badur et al., 2020). Their crystal structures have a typical (roll) 8 TIM-barrel structure, which consists of eight α-helices and eight β-sheets. Laminarinases of GH17 share many conserved sequences, which encode many α-helices, η-helices, β-sheets, and strict β-turns (Ezzine et al., 2016). Receveur-Bréchot et al. (2006) revealed a crystal structure at 1.45-Å resolution of the β-1, 3-glucanase Ban-Gluc from banana. Its three-dimensional structure consists of an internal crown of eight β-strands connected by extended loops to an outer crown of eight α-helices, which exhibit the typical (α/β) 8 TIM-barrel motif (Fig. 2B). There are a few reports on these laminarinases about the preparation of laminarin oligosaccharides, some of which even lack the degradation activity of laminarin (Menu-Bouaouiche et al., 2003). Recently, the laminarinase VbGH17A belonging to GH17 family was identified from V. breoganii 1C10 by Badur et al. (2020). It can hydrolyze laminarin into a series of laminarin oligosaccharides (DP4-DP9). In contrast, complex laminarin oligosaccharide products with different DP may cause difficulties in the separation and purification of laminarin oligosaccharides at a later stage, which is not conducive to the production of laminarin oligosaccharides.
Compared with laminarinases belonging to GH16 and GH17, there have been fewer studies on laminarinases of GH55, GH64, and GH81. Until now, only the crystal structures of laminarinases belonging to GH64 and GH81 have been reported (Ezzine et al., 2016; Badur et al., 2020).
Wu et al. (2009) revealed the essential amino acid residues and crystal structure at 1.80 Å resolution of the laminarinase LPHase of GH64 from S. matensis DIC-108. Its conserved sequences include two strictly conserved carboxylates (Glu154 and Asp170) and several saccharidelinked residues (Thr156, Thr167, Trp163, Asn165, and Val169). The LPHase structure consists of a barrel domain and a mixed (α/β) domain, and its main hydrolysate is laminaripentaose (Fig. 2C). The homogeneous product of enzymatic hydrolysis is conducive to the preparation of laminarin oligosaccharides.
Zhou et al. (2013) reported essential residues and a crystal structure to resolutions of 2.3 and 2.0 Å of the laminarinase as a member of GH81 from Rhizomucor miehei. The conserved amino acid residues (251–343 aa) may be involved in the stabilization of the whole structure. The overall structure of the laminarinase mainly consists of a β-sandwich domain and a C-terminal (α/α)6 domain (as shown Fig. 2D). Kumar et al. (2018) reported a thermostable laminarinase belonging to GH81 from C. thermocellum, and its hydrolysates are a series of oligosaccharides (DP2 to DP7).
3.3 Laminarin Oligosaccharide Preparation by Synthesis StrategiesSeveral algal oligosaccharides, such as alginate oligosaccharides and fucose oligosaccharides, can be produced by organic synthesis and biosynthesis (Mong et al., 2003). These synthesis strategies have also been applied in the preparation of laminarin oligosaccharides. He et al. (2003) reported an organic synthesis strategy. He used a 4, 6-Obenzylidenated acceptor for β-(1→3) bond formation to avoid the predominant generation of α glycosides. Eventually, a homogeneous laminarin oligosaccharide (DP4) with α-(1→3) and α-(1→6) bonds was prepared, and the yield was up to 43%. However, the operational steps were cumbersome and costly, and the synthesis of oligosaccharides was accompanied by the introduction of other glycosidic bonds. In contrast, the biosynthesis of laminarin oligosaccharides can be accomplished with other methods at a low cost. Sun et al. (2019) designed an in vitro multienzyme catalytic system consisting of α-glucan phosphorylase and laminaribiose phosphorylase. This catalytic system could convert low-value starch and glucose into highvalue laminaribiose, which can be used to synthesize hyaluronic acid.
In contrast to the degradation of polysaccharides, oligosaccharide synthesis is based on oligosaccharides with smaller Mw (Boons, 1996). However, there are only a few studies on the biosynthesis of laminarin oligosaccharides, and only a few laminarin oligosaccharides with a specific DP can be synthesized. Table 3 presents strategies for laminarin oligosaccharide preparation, specific methods, production, advantages, and disadvantages of each strategy.
|
|
Table 3 Strategies for laminarin oligosaccharides preparation, specific methods, production, advantages and disadvantages of each strategy |
Laminarin and laminarin oligosaccharides have been widely reported for their biological functions. Like other algal polysaccharides, laminarin has prebiotic, antioxidant, and anti-inflammatory activities (Kim et al., 2006; Kadam et al., 2015). In addition, due to its unique triple helical structure, laminarin also exhibits excellent antitumor and anticancer activities, which can be applied to drug development (Novak and Vetvicka, 2008). The biological activities of laminarin and laminarin oligosaccharides are considered to depend on their molecular structure, such as the DP, the introduction of sulfate groups, and the side chain branches (Pang et al., 2005; Menshova et al., 2014; Zargarzadeh et al., 2020). Therefore, appropriate structural modification of laminarin can significantly improve its biological activities.
4.1 Antitumor and Anticancer ActivitiesTo date, many studies have shown that laminarin and laminarin oligosaccharides have significant antitumor and anticancer activities (Park et al., 2012). The mechanisms of antitumor and anticancer activities of laminarin and laminarin oligosaccharides include apoptosis and inhibition of cancer cell colony formation (Zargarzadeh et al., 2020). For instance, the capacity of laminarin to induce apoptosis in HT-29 colon cancer cells was investigated by Park et al. (2013). The results demonstrated that laminarin extracted from Laminaria digitate could not only induce apoptosis in HT-29 colon cancer cells through an apoptotic pathway involving growth factors, but also regulate the ErbB signaling pathway. Ji et al. (2012) used different concentrations of laminarin to treat human colon cancer LOVO cells at different times. The intracellular reactive oxygen species (ROS), pH, intracellular calcium ion concentration, mitochondrion permeability transition pore, mitochondrial membrane potential, and expression levels of Cyt-C, Caspase-9, and Caspase-3 were detected and analyzed. It was found that laminarin could induce human colon cancer LOVO cell apoptosis through a mitochondrial pathway. Subsequently, the relationship between laminarin-induced apoptosis and the death receptor-mediated (DR-mediated) pathway in human colon cancer LOVO cells was illuminated by Ji and Ji (2014). The structural characteristics and antitumor activity of laminarin from Eisenia bicyclis were determined and analyzed by Ermakova et al. (2013). Laminarin exhibited no direct cytotoxicity and showed significant antitumor activity against SK-MEL-28 human melanoma cells.
Similarly, Usoltseva et al. (2016) also found that laminarin extracted from Alaria angusta and A. angusta had no cytotoxicity in vitro and could effectively inhibit colony formation in HT-29 cells. Tian et al. (2020) elucidated the anticancer effect of laminarin from L. japonica on Human HCC cell lines (Bel-7404 and HepG2). The results revealed that laminarin could inhibit the proliferation of Bel-7404 and HepG2 cells in a dose-dependent manner, and the expression levels of SMP-30 in laminarin-treated cells were lower than those in the control LO2 cells. Thus, it is speculated that laminarin can inhibit cancer cells by regulating the expression levels of SMP-30.
Laminarin oligosaccharides also showed obvious anticancer activity (Pang et al., 2005; Menshova et al., 2014). The human tissue lymphoma cell line (U937 cells) was treated with the hydrolytic products of laminarin oligosaccharides LB and LI, respectively (Pang et al., 2005). The results suggested that LB can inhibit the proliferation of U937 cells by stimulating monocytes to produce cytokines. Moreover, it was revealed that specific enzymatic products (laminarin oligosaccharides with DP 9-23) with a high content of 1, 6-linked glucose residues showed significant anticancer activity, and they could inhibit colony formation in melanoma and colon cancer cells (Menshova et al., 2014). This also provides new ideas to produce laminarin products with high antitumor activity through enzymatic hydrolysis.
Additionally, laminarin can be modified to enhance its antitumor and anticancer activities. Ji et al. (2013) applied the chlorosulfonic acid-pyridine method for laminarinsulfated modification and obtained a sulfated laminarin (LAMS) with a sulfate content of 45.92%. The main sulfate substitution site was at the hydroxyl groups of C2 and C6. The MTT assay (MTT) results showed that LAMS had more obvious inhibition effects than laminarin on LoVo cell growth, which suggested that LAMS had better antitumor activity. It was hypothesized that the introduction of sulfate groups can not only change the molecular structure and spatial conformation of laminarin, but also enhance its anion repulsion to stretch the sugar chains and form hydrogen bonds. These changes lead to the formation of a helical structure and adoption of an active conformation, which enhances the antitumor activity of laminarin (Zargarzadeh et al., 2020). This means that some specific structural modifications such as sulfated modification is an effective method to enhance the antitumor activity of laminarin. Nanoparticle modification of polysaccharide structure is another strategy to improve its physical and chemical properties. Laminarin decorated with selenium nanoparticles (LP-SeNPs) was prepared by Cui et al. (2019). Laminarin with nano modification can induce mitochondria-mediated apoptosis of tumor cells (HepG2 cells) by promoting the expression of Bax and decreasing the expression of Bcl-2.
4.2 Prebiotic ActivityIt has been well documented that many algal polysaccharides and their functional oligosaccharides play an important role in regulating human intestinal health, which is considered as a prebiotic activity (Ramnani et al., 2012). The prebiotic activities of laminarin and laminarin oligosaccharides have also aroused the interest of many scholars. It was confirmed that neither in vitro hydrochloric acid under physiological conditions nor in vitro homogenates of the human digestive system can hydrolyze laminarin and laminarin oligosaccharides (Walsh et al., 2013; Kadam et al., 2015). Laminarin and laminarin oligosaccharides are resistant to hydrolytic enzymes in the human upper gastrointestinal tract (Devillé et al., 2004). However, the intestinal microflora can utilize laminarin and laminarin oligosaccharides (Devillé et al., 2007). Therefore, laminarin and laminarin oligosaccharides cannot be degraded and absorbed by host. Thus they can become prebiotics potentially. This beneficial effects of laminarin on intestinal microorganisms were demonstrated by Nguyen et al. (2016). With the supplementation of laminarin in the diets of mice, a significant increase in Bacteroides and a significant decrease in Firmicutes were observed. The results suggested that laminarin could enhance the high energy metabolism of the gut microbiota to reduce the side effects of a high-fat diet. Zaporozhets et al. (2014) proposed the prebiotic properties of laminarin. Moreover, it was shown that laminarin could regulate intestinal metabolism via its effect on mucus composition, the intestinal pH, and short-chain fatty acids (SCFA) (Devillé et al., 2007). Leal et al. (2017) found that laminarin oligosaccharides were beneficial to the growth of Bifidobacterium animalis and Lactobacillus casei, and could increase their production of SCFA, such as lactic acid and acetic acid.
4.3 Antioxidant ActivityLaminarin and laminarin oligosaccharides present good antioxidant activity (Zhou et al., 2009). Jia et al. (2010) separated and purified laminarin from L. Japonica by gel chromatography and obtained two components with Mw of 5.5 × 104 and 2.7 × 104 Da. The results indicated that the laminarin with 5.5 × 104 Da had a good scavenging effect on the hydroxyl free radical, superoxide anion free radical, and diphenyl generation of free radical.
When laminarin oligosaccharides were prepared by gamma irradiation of laminarin (Choi et al., 2012), they showed higher ferric reducing antioxidant potential values and greater DPPH radical-scavenging than that of nonirradiated laminarin. This means that proper reduction of the DP of laminarin can enhance its antioxidant activity. However, the mechanism remains to be further explored.
4.4 Anti-Inflammatory ActivityStudies have confirmed that several algal polysaccharides and their oligosaccharides exhibited excellent antiinflammatory activity (Dore et al., 2013). Dectin-1 is a receptor related to extracellular pathogen recognition. Previous studies found it was associated with anti-inflammatory activity (Karsten et al., 2012). Smith et al. (2018) tested the biological activity of five different laminarin preparations and identified some of them as either Dectin-1 antagonists or agonists. Kim et al. (2006) prepared laminarin with Mw between 5 and 10 kDa and laminarin oligosaccharides derived by enzymatic hydrolysis. Both laminarin and laminarin oligosaccharides showed good results for suppression of apoptosis and extension of cell survival in culture. A mouse cDNA microarray indicated that the genes coding immune response proteins were induced in the process, which showed potential for application as new immunopotentiating substances of laminarin and laminarin oligosaccharides.
In addition, laminarin and laminarin oligosaccharides can also be prepared as a drug for treating inflammatory diseases induced by non-specific inflammatory responses (Yvin et al., 2006).
4.5 Applications in CosmeticsSkin protection and repair functions of laminarin have been proven (Yvin et al., 1999; Li et al., 2013). Yvin et al. (1999) found that laminarin and laminarin oligosaccharides had stimulating, regenerating, modulatory, and energizing effects on human dermis fibroblasts and human epidermis keratinocytes, which is useful in cosmetics. Li et al. (2013) explored the effect of laminarin on matrix metalloproteinase activity in photoaging skin. The results indicated that laminarin can regulate the metabolism of lightaged skin collagen by regulating matrix metalloproteinase activity. In addition, laminarin has a positive effect on wound healing (Choi et al., 2013).
4.6 Applications in Plant Disease Control and Growth PromotionThe fruit, leaves, and crowns of plants are vulnerable to pathogens such as Botrytis cinerea and Aspergillus flavus, causing huge economic losses. Chemical control of plant disease not only causes environmental pollution but also is harmful to human health (Hirooka and Ishii, 2013). Algal polysaccharides can increase basal metabolism and cell division as well as the level of essential oils or biomolecules in plants, which can trigger protection against pathogens (González et al., 2013). Laminarin has been applied in the control of strawberry leaf spot and powdery mildew under field conditions. The results showed that laminarin can reduce the incidences of leaf spot and powdery mildew by 50% and 70% – 80%, respectively. Its effectiveness at inhibiting B. cinerea infection was also obvious, and the inhibition rate was as high as 80% (Meszka and Bielenin, 2011). Aflatoxin contamination has been a worldwide problem. Laminarin extracted from L. digitata had inhibitory effects on growth and toxin production of A. flavus (Hu et al., 2012). Laminarin also can be used as a plant growth-promoting agent. For instance, laminarin could stimulate the germination of seeds through interfering with pathways that can lead to α-amylase formation (Yvin et al., 1998). In another study, transcriptome analysis indicated that laminarin can regulate the DEFL-mediated pathway, thus affecting abiotic stress tolerance in plants (Wu et al., 2016).
4.7 Applications in Feed AdditivesLaminarin can be used as a high-value feed additive, which can effectively improve growth performance, enhance the immune response, and regulate the gut microbiota of animals. Researchers found that the developmental competence of early-stage porcine embryos could be dramatically improved when laminarin was used as a feed additive (Jiang et al., 2018). Some important physiological parameters, such as the blastocyst formation rate, hatching rate, and total cell number in the blastocyst significantly increased when 20 mg mL−1 of laminarin was used as a food additive during the in vitro culture period of early-stage porcine embryos (Jiang et al., 2018). Walsh et al. (2013) confirmed that the supplementation of laminarin could suppress the secretion of pro-inflammatory cytokines, which could be helpful for the growth performance of pigs. After laminarin extracted from L. digitate was added to the diets of pigs, intestinal bacterial populations, volatile fatty acid concentrations, and the expression levels of cytokines and mucin genes in the ileum and colon were determined and analyzed by Smith et al. (2011). The results indicated that laminarin can improve gut health in the pig. In addition, dietary supplementation with laminarin and fucoidan could enhance pork meat quality through reducing saturated fatty acids and lowering lipid oxidation in longissimus thoracis and lumborum muscle of pig (Moroney et al., 2015).
Similarly, laminarin has been used as a feed additive in fish breeding. After growth performance, biochemical parameters, and the expression levels of immune-related genes in Epinephelus coioides fed with laminarin were measured, the results indicated that laminarin could regulate the immune response and promote the growth of fish (Yin et al., 2014).
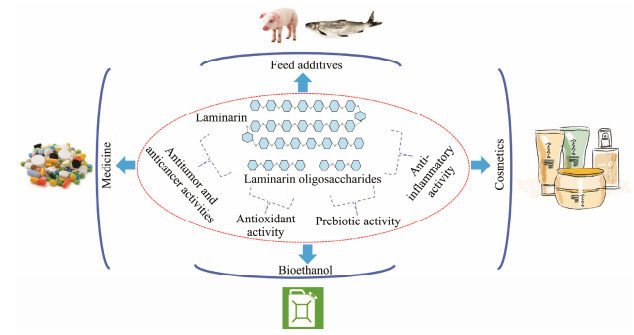
4.8 Applications in Bioethanol ProductionBioethanol, a clean fuel, is considered as an important solution to the current fossil fuel energy crisis (Amin, 2009). Although technologies for ethanol production through yeast fermentation are relatively mature, production costs remain the main barrier to bioethanol application. Due to its abundant resources, algal polysaccharide can be one of the ideal feedstocks for bioethanol production. Remarkably, laminarin is composed of glucose, which is the preferable fermentable sugar in bioethanol producers such as S. cerevisiae and Zymomonas mobilis (Al Abdallah et al., 2016). Therefore, laminarin may emerge as a potential substrate for bioethanol production. For example, an optimized combination of laminarinase and β-glucosidase-displaying yeasts could directly transform laminarin into bioethanol, and the bioethanol yield was 5.2 g L−1 (Motone et al., 2016). A two-stage bioprocess using an immobilized laminarinase and a marine-derived yeast for bioethanol production from laminarin was established by Mitsuya et al. (2017). Finally, 0.51–0.58 g L−1 bioethanol was yielded from the saccharified solution prepared via the immobilized laminarinase. Fig. 3 shows the biological activities and potential applications of laminarin and laminarin oligosaccharides.
|
Fig. 3 The biological activities and potential applications of laminarin and laminarin oligosaccharides. |
As one of the important marine algal polysaccharides derived from brown algae, laminarin has many biofunctional activities. With the development and progress of the extraction process, EAE-, MAE-, and UAE-assisted extraction methods are becoming promising extraction strategies for laminarin as they are environmentally friendly and efficient (Chen et al., 2013).
Laminarin has many excellent health beneficial effects. The mechanisms of antitumor and anticancer activities of laminarin, such as apoptosis (ErbB signaling pathway, mitochondrial pathway, and DR-mediated pathway) and inhibition of cancer cell colony formation (SMP-30), have been summarized and discussed in this review. Laminarin oligosaccharides possess higher antioxidant and antitumor activities than laminarin (Menshova et al., 2014), while the differences of other biological activities between laminarin oligosaccharides and laminarin, such as prebiotic activity and anti-inflammatory activity, have not been studied in detail thus far. Therefore, large-scale preparation of laminarin oligosaccharides and evaluation of their more specific biological activities are a focus of research. Enzymatic degradation of laminarin by using laminarinases is one of the most promising approaches to produce laminarin oligosaccharide because of the mild reaction conditions, low energy consumption, high output, and clear laminarin oligosaccharide composition. In total, typical crystal structures of laminarinases from four GH families have been identified, which will benefit our understanding of the catalytic mechanisms and targeted production of laminarin oligosaccharides.
Moreover, modifications of laminarin such as sulfation, nanoparticle modification, and branched-chain modification can enhance some specific functional activities of laminarin (Ji et al., 2013; Cui et al., 2019). For example, specific bit sulfated modification (C2 and C6) and increase the content of side chains are effective methods to enhance the antitumor activity of laminarin. However, the detailed mechanism requires further illustration. Thus, the production of laminarin with specific structural modifications and laminarin oligosaccharides with homogeneous DP and the illumination of their structure-activity relationship will become another research focus in the near future.
AcknowledgementsThis work was supported by the National Natural Science Foundation of China (No. 31922072), the National Key Research and Development Program of China (Nos. 2019YFD0901902 and 2019YFD090 1904), the Taishan Scholar Project of Shandong Province (No. tsqn2018120 20), and the Fundamental Research Funds for the Central Universities (No. 201941002).
Adam, F., Abert-Vian, M., Peltier, G. and Chemat, F., 2012. 'Solvent-free' ultrasound-assisted extraction of lipids from fresh microalgae cells: A green, clean and scalable process. Bioresource Technology, 114: 457-465. DOI:10.1016/j.biortech.2012.02.096 (  0) 0) |
Adams, J., Ross, A., Anastasakis, K. and Hodgson, E., 2011. Seasonal variation in the chemical composition of the bioenergy feedstock Laminaria digitata for thermochemical conversion. Bioresource Technology, 102(1): 226-234. DOI:10.1016/j.biortech.2010.06.152 (  0) 0) |
Al Abdallah, Q., Nixon, B. T. and Fortwendel, J. R., 2016. The enzymatic conversion of major algal and cyanobacterial carbohydrates to bioethanol. Frontiers in Energy Research, 4: 36. (  0) 0) |
Ale, M. T., Mikkelsen, J. D. and Meyer, A. S., 2011. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Marine Drugs, 9(10): 2106-2130. DOI:10.3390/md9102106 (  0) 0) |
Amin, S., 2009. Review on biofuel oil and gas production processes from microalgae. Energy Conversion and Management, 50(7): 1834-1840. DOI:10.1016/j.enconman.2009.03.001 (  0) 0) |
Badur, A. H., Ammar, E. M., Yalamanchili, G. and Hehemann, J. H., 2020. Characterization of the GH16 and GH17 laminarinases from Vibrio breoganii 1C10. Applied Microbiology and Biotechnology, 104(1): 161-171. DOI:10.1007/s00253-019-10243-0 (  0) 0) |
Bara, M. T. F., Lima, A. L. and Ulhoa, C. J., 2003. Purification and characterization of an exo-β-1, 3-glucanase produced by Trichoderma asperellum. FEMS Microbiology Letters, 219(1): 81-85. DOI:10.1016/S0378-1097(02)01191-6 (  0) 0) |
Becker, S., Scheffel, A., Polz, M. F. and Hehemann, J. H., 2017. Accurate quantification of laminarin in marine organic matter with enzymes from marine microbes. Applied and Environmental Microbiology, 83(9): e03389-16. (  0) 0) |
Becker, S., Tebben, J., Coffinet, S. and Wiltshire, K., 2020. Laminarin is a major molecule in the marine carbon cycle. Proceedings of the National Academy of Sciences, 117(12): 6599-6607. DOI:10.1073/pnas.1917001117 (  0) 0) |
Boons, G. J., 1996. Strategies in oligosaccharide synthesis. Tetrahedron, 52(4): 1095-1121. DOI:10.1016/0040-4020(95)00897-7 (  0) 0) |
Charoensiddhi, S., Lorbeer, A. J., Lahnstein, J. and Bulone, V., 2016. Enzyme-assisted extraction of carbohydrates from the brown alga Ecklonia radiata: Effect of enzyme type, pH and buffer on sugar yield and molecular weight profiles. Process Biochemistry, 51(10): 1503-1510. DOI:10.1016/j.procbio.2016.07.014 (  0) 0) |
Chen, H. M., Zheng, L. and Yan, X. J., 2005. The preparation and bioactivity research of agaro-oligosaccharides. Food Technology and Biotechnology, 43(1): 29-36. (  0) 0) |
Chen, S. X., Shen, J. G., Chen, B. and Wang, D. Z., 2013. The optimization of polysaccharide extraction process from Zingiber striolatum in Fanjing mountain by microwave-assisted method. Food and Fermentation Industries, 39(5): 234-237 (in Chinese with English abstract). (  0) 0) |
Choi, J. A., Oh, T. H., Choi, J. S. and Chang, D. J., 2013. Impact of β-1, 3-glucan isolated from Euglena gracilis on corneal epithelial cell migration and on wound healing in a rat alkali burn model. Current Eye Research, 38(12): 1207-1213. DOI:10.3109/02713683.2013.811262 (  0) 0) |
Choi, J. I., Kim, H. J. and Lee, J. W., 2011. Structural feature and antioxidant activity of low molecular weight laminarin degraded by gamma irradiation. Food Chemistry, 129(2): 520-523. DOI:10.1016/j.foodchem.2011.03.078 (  0) 0) |
Choi, J. I., Kim, H. J., Kim, J. H. and Lee, J. W., 2012. Enhanced biological activities of laminarin degraded by gammaray irradiation. Journal of Food Biochemistry, 36(4): 465-469. DOI:10.1111/j.1745-4514.2011.00552.x (  0) 0) |
Cong, Q., Chen, H., Liao, W. and Xiao, F., 2016. Structural characterization and effect on anti-angiogenic activity of a fucoidan from Sargassum fusiforme. Carbohydrate Polymers, 136: 899-907. DOI:10.1016/j.carbpol.2015.09.087 (  0) 0) |
Cui, D., Ma, J., Liang, T. and Sun, L., 2019. Selenium nanoparticles fabricated in laminarin polysaccharides solutions exert their cytotoxicities in HepG2 cells by inhibiting autophagy and promoting apoptosis. International Journal of Biological Macromolecules, 137: 829-835. DOI:10.1016/j.ijbiomac.2019.07.031 (  0) 0) |
Delattre, C., Michaud, P., Courtois, B. and Courtois, J., 2005. Oligosaccharides engineering from plants and algae: Applications in biotechnology and therapeutics. Minerva Biotecnologica, 17(3): 107. (  0) 0) |
Devillé, C., Damas, J., Forget, P. and Dandrifosse, G., 2004. Laminarin in the dietary fibre concept. Journal of the Science of Food and Agriculture, 84(9): 1030-1038. DOI:10.1002/jsfa.1754 (  0) 0) |
Devillé, C., Gharbi, M., Dandrifosse, G. and Peulen, O., 2007. Study on the effects of laminarin, a polysaccharide from seaweed, on gut characteristics. Journal of the Science of Food and Agriculture, 87(9): 1717-1725. DOI:10.1002/jsfa.2901 (  0) 0) |
Dong, W., Huang, J., Li, Y. and Tan, Y., 2015. Crystal structural basis for Rv0315, an immunostimulatory antigen and inactive beta-1, 3-glucanase of Mycobacterium tuberculosis. Scientific Reports, 5: 15073. DOI:10.1038/srep15073 (  0) 0) |
Dore, C. M. P. G., Alves, M. G. D. C. F., Will, L. S. E. P. and Costa, T. G., 2013. A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydrate Polymers, 91(1): 467-475. DOI:10.1016/j.carbpol.2012.07.075 (  0) 0) |
Ermakova, S., Men'shova, R., Vishchuk, O. and Kim, S. M., 2013. Water-soluble polysaccharides from the brown alga Eisenia bicyclis: Structural characteristics and antitumor activity. Algal Research, 2(1): 51-58. DOI:10.1016/j.algal.2012.10.002 (  0) 0) |
Ezzine, A., Chahed, H., Hannachi, M. and Hardouin, J., 2016. Biochemical and molecular characterization of a new glycoside hydrolase family 17 from Sclerotinia sclerotiorum. Journal of New Sciences, 28(8): 1610-1621. (  0) 0) |
Gaborieau, M. and Castignolles, P., 2011. Size-exclusion chromatography (SEC) of branched polymers and polysaccharides. Analytical and Bioanalytical Chemistry, 399(4): 1413-1423. DOI:10.1007/s00216-010-4221-7 (  0) 0) |
Gao, M. X., Liu, H. W. and Zong, M. Y., 2006. Extracting polysaccharides of Laminarin japonica by microwave. Food Research and Development, 8: 69-72 (in Chinese with English abstract). (  0) 0) |
Garcia-Vaquero, M., Rajauria, G., O'doherty, J. and Sweeney, T., 2017. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Research International, 99: 1011-1020. DOI:10.1016/j.foodres.2016.11.016 (  0) 0) |
González, A., Castro, J., Vera, J. and Moenne, A., 2013. Seaweed oligosaccharides stimulate plant growth by enhancing carbon and nitrogen assimilation, basal metabolism, and cell division. Journal of Plant Growth Regulation, 32(2): 443-448. DOI:10.1007/s00344-012-9309-1 (  0) 0) |
Graiff, A., Ruth, W., Kragl, U. and Karsten, U., 2016. Chemical characterization and quantification of the brown algal storage compound laminarin - A new methodological approach. Journal of Applied Phycology, 28(1): 533-543. DOI:10.1007/s10811-015-0563-z (  0) 0) |
Hahn, T., Zayed, A., Kovacheva, M. and Stadtmüller, R., 2016. Dye affinity chromatography for fast and simple purification of fucoidan from marine brown algae. Engineering in Life Sciences, 16(1): 78-87. DOI:10.1002/elsc.201500044 (  0) 0) |
He, H., Gu, G. and Du, Y., 2003. Synthesis of laminarin oligosaccharide derivatives having D-arabinofuranosyl side-chains. Journal of Carbohydrate Chemistry, 22(5): 275-283. DOI:10.1081/CAR-120023470 (  0) 0) |
Hirabayashi, J., Hashidate, T., Arata, Y. and Nishi, N., 2002. Oligosaccharide specificity of galectins: A search by frontal affinity chromatography. Biochimica et Biophysica Acta (BBA) - General Subjects, 1572(2-3): 232-254. DOI:10.1016/S0304-4165(02)00311-2 (  0) 0) |
Hirooka, T. and Ishii, H., 2013. Chemical control of plant diseases. Journal of General Plant Pathology, 79(6): 390-401. DOI:10.1007/s10327-013-0470-6 (  0) 0) |
Hu, L. B., Li, H. B., Sun, J. L. and Zeng, J., 2012. Effect of laminarin on Aspergillus Flavus growth and aflatoxin production. Advanced Materials Research, 343-344: 1168-1171. (  0) 0) |
Iji, P. and Tivey, D., 1998. Natural and synthetic oligosaccharides in broiler chicken diets. World's Poultry Science Journal, 54(2): 129-143. DOI:10.1079/WPS19980010 (  0) 0) |
Imbs, T. I., Ermakova, S. P., Malyarenko, O. S. and Isakov, V. V., 2016. Structural elucidation of polysaccharide fractions from the brown alga Coccophora langsdorfii and in vitro investigation of their anticancer activity. Carbohydrate Polymers, 135: 162-168. DOI:10.1016/j.carbpol.2015.08.062 (  0) 0) |
Ji, C. F. and Ji, Y. B., 2014. Laminarin-induced apoptosis in human colon cancer LOVO cells. Oncology Letters, 7(5): 1728-1732. DOI:10.3892/ol.2014.1952 (  0) 0) |
Ji, C. F., Ji, Y. B. and Meng, D. Y., 2013. Sulfated modification and anti-tumor activity of laminarin. Experimental and Therapeutic Medicine, 6(5): 1259-1264. DOI:10.3892/etm.2013.1277 (  0) 0) |
Ji, Y. B., Ji, C. F. and Zhang, H., 2012. Laminarin induces apoptosis of human colon cancer LOVO cells through a mitochondrial pathway. Molecules, 17(8): 9947-9960. DOI:10.3390/molecules17089947 (  0) 0) |
Jia, Y. and Min, W., 2010. Laminarin purification and extraorgan antioxidation. Academic Periodical of Farm Products Processing, 8: 26-29 (in Chinese with English abstract). (  0) 0) |
Jiang, H., Liang, S., Yao, X. R. and Jin, Y. X., 2018. Laminarin improves developmental competence of porcine early stage embryos by inhibiting oxidative stress. Theriogenology, 115: 38-44. DOI:10.1016/j.theriogenology.2018.04.019 (  0) 0) |
Kadam, S. U., O'Donnell, C. P., Rai, D. K. and Hossain, M. B., 2015. Laminarin from Irish brown seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound assisted extraction, characterization and bioactivity. Marine Drugs, 13(7): 4270-4280. DOI:10.3390/md13074270 (  0) 0) |
Kadam, S. U., Tiwari, B. K. and O'Donnell, C. P., 2015. Extraction, structure and biofunctional activities of laminarin from brown algae. International Journal of Food Science & Technology, 50(1): 24-31. (  0) 0) |
Karsten, C. M., Pandey, M. K., Figge, J. and Kilchenstein, R., 2012. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcǃRòB and dectin-1. Nature Medicine, 18(9): 1401. DOI:10.1038/nm.2862 (  0) 0) |
Kim, K. H., Kim, Y. W., Kim, H. B. and Lee, B. J., 2006. Antiapoptotic activity of laminarin polysaccharides and their enzymatically hydrolyzed oligosaccharides from Laminaria japonica. Biotechnology Letters, 28(6): 439-446. DOI:10.1007/s10529-005-6177-9 (  0) 0) |
Kim, M. J., Nam, S. W., Tamano, K. and Machida, M., 2011. Optimization for production of exo-β-1, 3-glucanase (laminarinase) from Aspergillus oryzae in Saccharomyces cerevisiae. Korean Society for Biotechnology and Bioengineering Journal, 26(5): 427-432. (  0) 0) |
Kumar, K., Correia, M. A., Pires, V. M. and Dhillon, A., 2018. Novel insights into the degradation of β-1, 3-glucans by the cellulosome of Clostridium thermocellum revealed by structure and function studies of a family 81 glycoside hydrolase. International Journal of Biological Macromolecules, 117: 890-901. DOI:10.1016/j.ijbiomac.2018.06.003 (  0) 0) |
Kusaykin, M. I., Belik, A. A., Kovalchuk, S. N. and Dmitrenok, P. S., 2017. A new recombinant endo-1, 3-β-D-glucanase from the marine bacterium Formosa algae KMM 3553: Enzyme characteristics and transglycosylation products analysis. World Journal of Microbiology and Biotechnology, 33(2): 40. DOI:10.1007/s11274-017-2213-x (  0) 0) |
Labourel, A., Jam, M., Legentil, L. and Sylla, B., 2015. Structural and biochemical characterization of the laminarinase ZgLamCGH16 from Zobellia galactanivorans suggests preferred recognition of branched laminarin. Acta Crystallographica Section D: Biological Crystallography, 71(2): 173-184. DOI:10.1107/S139900471402450X (  0) 0) |
Leal, B. E. S., Prado, M. R., Grzybowski, A. and Tiboni, M., 2017. Potential prebiotic oligosaccharides from aqueous thermopressurized phosphoric acid hydrolysates of microalgae used in treatment of gaseous steakhouse waste. Algal Research, 24: 138-147. DOI:10.1016/j.algal.2017.03.020 (  0) 0) |
Lee, K. C., Arai, T., Ibrahim, D. and Kosugi, A., 2014. Purification and characterization of a thermostable laminarinase from Penicillium rolfsii c3-2 (1) IBRL. BioResources, 9(1): 1072-1084. (  0) 0) |
Lee, O. K. and Lee, E. Y., 2016. Sustainable production of bioethanol from renewable brown algae biomass. Biomass and Bioenergy, 92: 70-75. DOI:10.1016/j.biombioe.2016.03.038 (  0) 0) |
Li, J., Xie, L., Qin, Y. and Liang, W., 2013. Effect of laminarin polysaccharide on activity of matrix metalloproteinase in photoaging skin. China Journal of Chinese Materia Medica, 38(14): 2370-2373. (  0) 0) |
Li, W., Huan, X., Zhou, Y. and Ma, Q., 2009. Simultaneous cloning and expression of two cellulase genes from Bacillus subtilis newly isolated from Golden Takin (Budorcas taxicolor Bedfordi). Biochemical and Biophysical Research Communications, 383(4): 397-400. DOI:10.1016/j.bbrc.2009.04.027 (  0) 0) |
Mak, W., Hamid, N., Liu, T. and Lu, J., 2013. Fucoidan from New Zealand Undaria pinnatifida: Monthly variations and determination of antioxidant activities. Carbohydrate Polymers, 95(1): 606-614. DOI:10.1016/j.carbpol.2013.02.047 (  0) 0) |
Menshova, R. V., Ermakova, S. P., Anastyuk, S. D. and Isakov, V. V., 2014. Structure, enzymatic transformation and anticancer activity of branched high molecular weight laminaran from brown alga Eisenia bicyclis. Carbohydrate Polymers, 99: 101-109. DOI:10.1016/j.carbpol.2013.08.037 (  0) 0) |
Menu-Bouaouiche, L., Vriet, C., Peumans, W. J. and Barre, A., 2003. A molecular basis for the endo-β1, 3-glucanase activity of the thaumatin-like proteins from edible fruits. Biochimie, 85(1-2): 123-131. DOI:10.1016/S0300-9084(03)00058-0 |
Meszka, B. and Bielenin, A., 2011. Activity of laminarin in control of strawberry diseases. Phytopathologia, 62: 15-23. (  0) 0) |
Mitsuya, D., Sugiyama, T., Zhang, S. and Takeuchi, Y., 2018. Enzymatic properties and the gene structure of a cold-adapted laminarinase from Pseudoalteromonas species LA. Journal of Bioscience and Bioengineering, 126(2): 169-175. DOI:10.1016/j.jbiosc.2018.02.018 (  0) 0) |
Mitsuya, D., Yamamoto, M., Okai, M. and Inoue, A., 2017. Continuous saccharification of laminarin by immobilized laminarinase ulam111 followed by ethanol fermentation with a marine-derived yeast. Advances in Microbiology, 7(5): 387-403. DOI:10.4236/aim.2017.75032 (  0) 0) |
Mong, T. K. K., Lee, H. K., Durón, S. G. and Wong, C. H., 2003. Reactivity-based one-pot total synthesis of fucose GM1 oligosaccharide: A sialylated antigenic epitope of small-cell lung cancer. Proceedings of the National Academy of Sciences, 100(3): 797-802. DOI:10.1073/pnas.0337590100 (  0) 0) |
Moroney, N., O'Grady, M., Robertson, R. and Stanton, C., 2015. Influence of level and duration of feeding polysaccharide (laminarin and fucoidan) extracts from brown seaweed (Laminaria digitata) on quality indices of fresh pork. Meat Science, 99: 132-141. DOI:10.1016/j.meatsci.2014.08.016 (  0) 0) |
Motone, K., Takagi, T., Sasaki, Y. and Kuroda, K., 2016. Direct ethanol fermentation of the algal storage polysaccharide laminarin with an optimized combination of engineered yeasts. Journal of Biotechnology, 231: 129-135. DOI:10.1016/j.jbiotec.2016.06.002 (  0) 0) |
Nagasawa, N., Mitomo, H., Yoshii, F. and Kume, T., 2000. Radiation-induced degradation of sodium alginate. Polymer Degradation and Stability, 69(3): 279-285. DOI:10.1016/S0141-3910(00)00070-7 (  0) 0) |
Nakabayashi, M., Nishijima, T., Ehara, G. and Nikaidou, N., 1998. Structure of the gene encoding laminaripentaose-producing β-1, 3-glucanase (LPHase) of Streptomyces matensis DIC-108. Journal of Fermentation and Bioengineering, 85(5): 459-464. DOI:10.1016/S0922-338X(98)80062-7 (  0) 0) |
Natsuka, S., Tachibana, A., Sumiyoshi, W. and Nakakita, S. I., 2018. Preparation of a molecular library of branched β-glucan oligosaccharides derived from laminarin. Journal of Applied Glycoscience, 65(4): 45-49. DOI:10.5458/jag.jag.JAG-2018_004 (  0) 0) |
Nguyen, S. G., Kim, J., Guevarra, R. B. and Lee, J. H., 2016. Laminarin favorably modulates gut microbiota in mice fed a high-fat diet. Food & Function, 7(10): 4193-4201. (  0) 0) |
Novak, M. and Vetvicka, V., 2008. β-glucans, history, and the present: Immunomodulatory aspects and mechanisms of action. Journal of Immunotoxicology, 5(1): 47-57. DOI:10.1080/15476910802019045 (  0) 0) |
Oda, M., Tanabe, Y., Noda, M. and Inaba, S., 2016. Structural and binding properties of laminarin revealed by analytical ultracentrifugation and calorimetric analyses. Carbohydrate Research, 431: 33-38. DOI:10.1016/j.carres.2016.05.008 (  0) 0) |
O'Shea, C., McAlpine, P., Sweeney, T. and Varley, P., 2014. Effect of the interaction of seaweed extracts containing laminarin and fucoidan with zinc oxide on the growth performance, digestibility and faecal characteristics of growing piglets. British Journal of Nutrition, 111(5): 798-807. DOI:10.1017/S0007114513003280 (  0) 0) |
Pang, Z., Otaka, K., Maoka, T. and Hidaka, K., 2005. Structure of β-glucan oligomer from laminarin and its effect on human monocytes to inhibit the proliferation of U937 cells. Bioscience, Biotechnology, and Biochemistry, 69(3): 553-558. DOI:10.1271/bbb.69.553 (  0) 0) |
Pap, N., Beszédes, S., Pongrácz, E. and Myllykoski, L., 2013. Microwave-assisted extraction of anthocyanins from black currant marc. Food and Bioprocess Technology, 6(10): 2666-2674. DOI:10.1007/s11947-012-0964-9 (  0) 0) |
Park, H. K., Kim, I. H., Kim, J. and Nam, T. J., 2012. Induction of apoptosis by laminarin, regulating the insulin-like growth factor-IR signaling pathways in HT-29 human colon cells. International Journal of Molecular Medicine, 30(4): 734-738. DOI:10.3892/ijmm.2012.1084 (  0) 0) |
Park, H. K., Kim, I. H., Kim, J. and Nam, T. J., 2013. Induction of apoptosis and the regulation of ErbB signaling by laminarin in HT-29 human colon cancer cells. International Journal of Molecular Medicine, 32(2): 291-295. DOI:10.3892/ijmm.2013.1409 (  0) 0) |
Pohleven, J., Štrukelj, B., and Kos, J., 2012. Affinity Chromatography. InTech, Janeza Trdine 9, 51000 Rijeka, Croatia, 49-74.
(  0) 0) |
Quitain, A. T., Kai, T., Sasaki, M. and Goto, M., 2013. Microwave-hydrothermal extraction and degradation of fucoidan from supercritical carbon dioxide deoiled Undaria pinnatifida. Industrial & Engineering Chemistry Research, 52(23): 7940-7946. (  0) 0) |
Ramani, R. and Ranganathaiah, C., 2000. Degradation of acry lonitrile-butadiene-styrene and polycarbonate by UV irradiation. Polymer Degradation and Stability, 69(3): 347-354. DOI:10.1016/S0141-3910(00)00081-1 (  0) 0) |
Ramnani, P., Chitarrari, R., Tuohy, K. and Grant, J., 2012. In vitro fermentation and prebiotic potential of novel low molecular weight polysaccharides derived from agar and alginate seaweeds. Anaerobe, 18(1): 1-6. DOI:10.1016/j.anaerobe.2011.08.003 (  0) 0) |
Receveur-Bréchot, V., Czjzek, M., Barre, A. and Roussel, A., Crystal structure at 1.45-Å resolution of the major allergen endo-β-1, 3-glucanase of banana as a molecular basis for the latex-fruit syndrome. Proteins: Structure, Function, and Bioinformatics, 63(1): 235-242. DOI:10.1002/prot.20876 (  0) 0) |
Renard, C. M., Lahaye, M., Mutter, M. and Voragen, F. G., 1997. Isolation and structural characterisation of rhamnogalacturonan oligomers generated by controlled acid hydrolysis of sugar-beet pulp. Carbohydrate Research, 305(2): 271-280. DOI:10.1016/S0008-6215(97)10028-3 (  0) 0) |
Rioux, L. E., Turgeon, S. L. and Beaulieu, M., 2010. Structural characterization of laminaran and galactofucan extracted from the brown seaweed Saccharina longicruris. Phytochemistry, 71(13): 1586-1595. DOI:10.1016/j.phytochem.2010.05.021 (  0) 0) |
Sakamoto, Y., Nakade, K. and Konno, N., 2011. Endo-β-1, 3-glucanase GLU1, from the fruiting body of Lentinula edodes, belongs to a new glycoside hydrolase family. Applied and Environmental Microbiology, 77(23): 8350-8354. DOI:10.1128/AEM.05581-11 (  0) 0) |
Sandini, S., La Valle, R., De Bernardis, F. and Macrì, C., 2007. The 65 kDa mannoprotein gene of Candida albicans encodes a putative β-glucanase adhesin required for hyphal morphogenesis and experimental pathogenicity. Cellular Microbiology, 9(5): 1223-1238. DOI:10.1111/j.1462-5822.2006.00862.x (  0) 0) |
Santos, T., Del Rey, F., Conde, J. and Villanueva, J., 1979. Saccharomyces cerevisiae mutant defective in exo-1, 3-beta-glucanase production. Journal of Bacteriology, 139(2): 333-338. DOI:10.1128/jb.139.2.333-338.1979 (  0) 0) |
Smith, A. J., Graves, B., Child, R. and Rice, P. J., 2018. Immunoregulatory activity of the natural product laminarin varies widely as a result of its physical properties. The Journal of Immunology, 200(2): 788-799. DOI:10.4049/jimmunol.1701258 (  0) 0) |
Smith, A., O'Doherty, J., Reilly, P. and Ryan, M., 2011. The effects of laminarin derived from Laminaria digitata on measurements of gut health: Selected bacterial populations, intestinal fermentation, mucin gene expression and cytokine gene expression in the pig. British Journal of Nutrition, 105(5): 669-677. DOI:10.1017/S0007114510004277 (  0) 0) |
Spilliaert, R., Hreggvidsson, G. O., Kristjansson, J. K. and Eggertsson, G., 1994. Cloning and sequencing of a Rhodothermus marinus gene, bglA, coding for a thermostable β-Glucanase and its expression in Escherichia coli. European Journal of Biochemistry, 224(3): 923-930. DOI:10.1111/j.1432-1033.1994.00923.x (  0) 0) |
Sun, S., Wei, X. and You, C., 2019. The construction of an in vitro synthetic enzymatic biosystem that facilitates laminaribiose biosynthesis from maltodextrin and glucose. Biotechnology Journal, 14(4): 1800493. DOI:10.1002/biot.201800493 (  0) 0) |
Takeda, T., Nakano, Y., Takahashi, M. and Konno, N., 2015. Identification and enzymatic characterization of an endo-1, 3-β-glucanase from Euglena gracilis. Phytochemistry, 116: 21-27. DOI:10.1016/j.phytochem.2015.05.010 (  0) 0) |
Thompson, T. M., Young, B. R. and Baroutian, S., 2019. Advances in the pretreatment of brown macroalgae for biogas production. Fuel Processing Technology, 195: 106151. DOI:10.1016/j.fuproc.2019.106151 (  0) 0) |
Tian, L., Li, C. M., Li, Y. F. and Huang, T. M., 2020. Laminarin from seaweed (Laminaria japonica) inhibits hepatocellular carcinoma through upregulating senescence marker protein-30. Cancer Biotherapy & Radiopharmaceuticals, 35(4): 277-283. (  0) 0) |
Tschiggerl, H., Breitwieser, A., de Roo, G. and Verwoerd, T., 2008. Exploitation of the S-layer self-assembly system for site directed immobilization of enzymes demonstrated for an extremophilic laminarinase from Pyrococcus furiosus. Journal of Biotechnology, 133(3): 403-411. DOI:10.1016/j.jbiotec.2007.09.018 (  0) 0) |
Usoltseva, R. V., Anastyuk, S. D., Shevchenko, N. M. and Zvyagintseva, T. N., 2016. The comparison of structure and anticancer activity in vitro of polysaccharides from brown algae Alaria marginata and A. angusta. Carbohydrate Polymers, 153: 258-265. DOI:10.1016/j.carbpol.2016.07.103 (  0) 0) |
Walsh, A., Sweeney, T., O'Shea, C. and Doyle, D., 2013. Effect of dietary laminarin and fucoidan on selected microbiota, intestinal morphology and immune status of the newly weaned pig. British Journal of Nutrition, 110(9): 1630-1638. DOI:10.1017/S0007114513000834 (  0) 0) |
Walsh, A., Sweeney, T., O'Shea, C. and Doyle, D., 2013. Effect of supplementing varying inclusion levels of laminarin and fucoidan on growth performance, digestibility of diet components, selected faecal microbial populations and volatile fatty acid concentrations in weaned pigs. Animal Feed Science and Technology, 183(3-4): 151-159. DOI:10.1016/j.anifeedsci.2013.04.013 (  0) 0) |
Wang, D., Kim, D. H., Seo, N. and Yun, E. J., 2016. A novel glycoside hydrolase family 5 β-1, 3-1, 6-endoglucanase from Saccharophagus degradans 2-40T and its transglycosylase activity. Applied and Environmental Microbiology, 82(14): 4340-4349. DOI:10.1128/AEM.00635-16 (  0) 0) |
Wijesekara, I., Pangestuti, R. and Kim, S. K., 2011. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydrate Polymers, 84(1): 14-21. DOI:10.1016/j.carbpol.2010.10.062 (  0) 0) |
Wu, H. M., Liu, S. W., Hsu, M. T. and Hung, C. L., 2009. Structure, mechanistic action, and essential residues of a GH-64 enzyme, laminaripentaose-producing β-1, 3-glucanase. Journal of Biological Chemistry, 284(39): 26708-26715. DOI:10.1074/jbc.M109.010983 (  0) 0) |
Wu, Y. R., Lin, Y. C. and Chuang, H., 2016. Laminarin modulates the chloroplast antioxidant system to enhance abiotic stress tolerance partially through the regulation of the defensin-like gene expression. Plant Science, 247: 83-92. DOI:10.1016/j.plantsci.2016.03.008 (  0) 0) |
Yin, G., Li, W., Lin, Q. and Lin, X., 2014. Dietary administration of laminarin improves the growth performance and immune responses in Epinephelus coioides. Fish & Shellfish Immunology, 41(2): 402-406. (  0) 0) |
Yvin, J. C., Alban, S., and Franz, G., 2006. Anti-inflammatory and healing medicine based on laminarin sulphate. United States, 7 March 2006, US7008931B2.
(  0) 0) |
Yvin, J. C., Levasseur, F., Amin-Gendy, C., and Tran Thanh, K. N., 1998. Laminarin as a seed germination and plant growth accelerator. United States, 12 May 1998, US5750472A.
(  0) 0) |
Yvin, J. C., Levasseur, F., and Hud'Homme, F., 1999. Use of laminarin and oligosaccharides derived therefrom in cosmetics and for preparing a skin treatment drug. United States, 9 November 1999, US5980916A.
(  0) 0) |
Zaporozhets, T., Besednova, N., Kuznetsova, T. and Zvyagintseva, T., 2014. The prebiotic potential of polysaccharides and extracts of seaweeds. Russian Journal of Marine Biology, 40(1): 1-9. DOI:10.1134/S1063074014010106 (  0) 0) |
Zargarzadeh, M., Amaral, A. J., Custódio, C. A. and Mano, J. F., 2020. Biomedical applications of laminarin. Carbohydrate Polymers, 232: 115774. DOI:10.1016/j.carbpol.2019.115774 (  0) 0) |
Zha, X. Q., Xiao, J. J., Zhang, H. N. and Wang, J. H., 2012. Polysaccharides in Laminaria japonica (LP): Extraction, physicochemical properties and their hypolipidemic activities in diet-induced mouse model of atherosclerosis. Food Chemistry, 134(1): 244-252. DOI:10.1016/j.foodchem.2012.02.129 (  0) 0) |
Zhang, H. and Row, K. H., 2015. Extraction and separation of polysaccharides from Laminaria japonica by size-exclusion chromatography. Journal of Chromatographic Science, 53(4): 498-502. DOI:10.1093/chromsci/bmu073 (  0) 0) |
Zhou, J., Wu, H., Liu, Q. and Jie, M., 2009. Study on antioxidative activity of laminarin in vitro. Journal of Guangdong Pharmaceutical College, 25(4): 397-400 (in Chinese with English abstract). (  0) 0) |
Zhou, P., Chen, Z., Yan, Q. and Yang, S., 2013. The structure of a glycoside hydrolase family 81 endo-β-1, 3-glucanase. Acta Crystallographica Section D: Biological Crystallography, 69(10): 2027-2038. DOI:10.1107/S090744491301799X (  0) 0) |
Zhu, Y., Li, Q., Mao, G. and Zou, Y., 2014. Optimization of enzyme-assisted extraction and characterization of polysaccharides from Hericium erinaceus. Carbohydrate Polymers, 101: 606-613. DOI:10.1016/j.carbpol.2013.09.099 (  0) 0) |
 2021, Vol. 20
2021, Vol. 20


