2) Marine Microbiological Engineering & Research Center, Marine Biomedical Research Institute of Qingdao, Qingdao 266000, China
Rhodotorula mucilaginosa is a species of red-pigmented yeast in the phylum Basidiomycota, family Cryptococcaceae, subfamily Rhodotorulodae, genus Rhodotorula. The red-pigment is usually attributed to the carotenoids accumulated intracellularly (Hawksworth et al., 1996). With the increasing demand for natural carotenoids, the production of carotenoids by yeast fermentation has become the most interesting method. Meanwhile, attentions to red yeast such as R. mucilaginosa and other red yeasts has been on rise over the years (Frengova and Beshkova, 2009; Mata-Gómez et al., 2014).
R. mucilaginosa strains were isolated from different habitats, such as ocean, soil, animals, plants, and industrial minerals (Wirth and Goldani, 2012). In addition to the biosynthesis of carotenoids, the production of a variety of other metabolites of values, such as unsaturated fatty acids, indoleacetic acid, extracellular polysaccharides, and β-glucan, were also documented by the strains of this species, respectively (Singh et al., 2013; Ignatova et al., 2015; Ma et al., 2017). The biological functions of those metabolites on antioxidant, immune enhancement, anti-tumor activity have been well documented. Thus far R. mucilaginosa has been already used to produce carotenoids as well as applied as natural fruit preservative to inhibit plant pathogenic fungi (Akhtyamova and Sattarova, 2013; Zhang et al., 2014), and to modify mineral surface in copper biotransformation (Salvadori et al., 2014). In short, R. mucilaginosa has huge application potential to be explored in a broad market aspects covering the fields of food, medicine, energy, animal husbandry, biological control, as well as biomining industries.
Even though the application potential has been well revealed, the published genomic resource of R. mucilaginosa is still scarce. To date, merely five genome sequences of R. mucilaginosa strains, i.e., C2.5t1, RIT389, IIPL32, JGTA-S1 and ATCC 58901, have been published since 2015 (Deligios et al., 2015; Saha and Seal, 2015; Dasgupta et al., 2017; Gan et al., 2017; Landolfo et al., 2018). In order to explore the full potential of the metabolites afore-mentioned, all of the supportive knowledge pool on genomic sequencing and analysis are still in need.
Recently, our group obtained a red marine yeast isolate CYJ03 from the water sample of the northern Yellow Sea of China, and it was identified as R. mucilaginosa. This strain exhibited strong tolerances to high concentrations of NaCl, glucose as well as low temperature. A set of techniques has been established aimed at high density fermentation for the mass production of carotenoids (unpublished data). In this study, the genome sequence of R. mucilaginosa CYJ03 was reported and analyzed, and the carotenoid biosynthesis pathway and gene cluster were investigated. These data can be used to explore genes and protein functions for biochemical and biotechnological features, and to regulate metabolic pathways by genetic engineering and metabolic engineering for producing valuable metabolites.
2 Methods 2.1 Strain, Culture Condition and IdentificationR. mucilaginosa CYJ03 (CGMCC 16302) was used in the current study, the strain was grown in yeast extract peptone dextrose (YPD) medium at 28℃ for 18 h. Colonies and cell morphology were observed by spread plate method and scanning electron microscopy (Hitachi S-4800, Japan), respectively. ITS1-5.8S rRNA-ITS2 sequences were analyzed to match to the data base of strains in Rhodotorula, the phylogenetic tree was constructed with the MEGA 7 software by Neighbor-Joining method (Tamura et al., 2004).
2.2 DNA Preparation, Genome Sequencing and AssemblyThe total genomic DNA of R. mucilaginosa CYJ03 was extracted using NanoMagTM fast fungal genomic DNA preparation kit (Tianjin Charme Co., Tianjin, China) and the genome sequencing was performed using the PacBio Sequel System (Pacific Biosystems, Menlo Park, USA). Subreads were assembled and self-corrected using the Falcon Assembler (Chin et al., 2016), and the consensus sequences were obtained, which was based on the Overlap-Layout-Consensus algorithm. Then the error was corrected using the Genomic Consensus and Pilon, respectively.
2.3 Gene Prediction and AnnotationGene prediction was performed using GeneMark-ES (v4.33) (Lomsadze et al., 2005). For gene prediction, assembly scaffolds were masked using RepeatMasker (v4.0.7). The genome assembly was annotated using the software Diamond (at an E value of 10-5) (Akhter et al., 2012) with BLASTX algorithm against NR, Swissprot, and GO term databases, KEGG for metabolic pathways, KOG for eukaryotic clusters of orthologs, eggNOG for orthologous groups of proteins (Koonin et al., 2004; Minoru et al., 2004; Huerta-Cepas et al., 2016).
2.4 Carotenoid Biosynthesis Pathway and Gene Cluster AnalysisProteins predicted to be involved in different steps of the carotenoid biosynthetic pathway were annotated by R. mucilaginosa CYJ03 genomic sequences against KEGG database. And the carotenoid biosynthesis pathway was mapped based on the data obtained. The secondary metabolite biosynthesis gene clusters were identified, annotated, and compared in fungi genomes using the online software antiSMASH (Medema et al., 2011).
3 Results and Discussion 3.1 Identification of R. mucilaginosa CYJ03 and Phylogenetic AnalysisCYJ03 could grow aerobically on YPD agar characterized by forming orange and smooth colonies with moist appearance (Fig. 1A). Under scanning electron microscopy the cells of CYJ03 were 30-35 μm in length and 20-22 μm in width, ellipsoid in shape with budding observed (Fig. 1B).
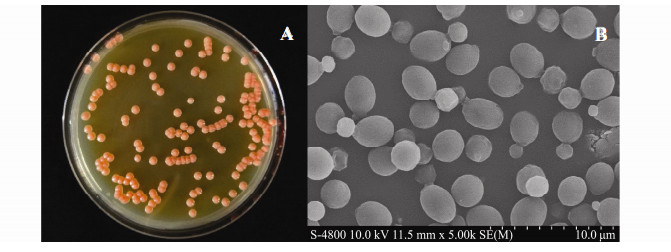
|
Fig. 1 (A) Colony morphology of R. mucilaginosa CYJ03 grown on YPD agar, (B) Scanning electron microscopy of CYJ03 at 5 kX magnification. |
The phylogenetic relationship of CYJ03 to other Rhodotorula species was analyzed based on the small subunit ribosomal RNA gene analysis. It was found that CYJ03 and the type strain R. mucilaginosa CBS 316 were clustered in the same clade (Fig. 2). The identification of CYJ03 as R. mucilaginosa was subsequently confirmed using whole genome nucleotide sequencing.
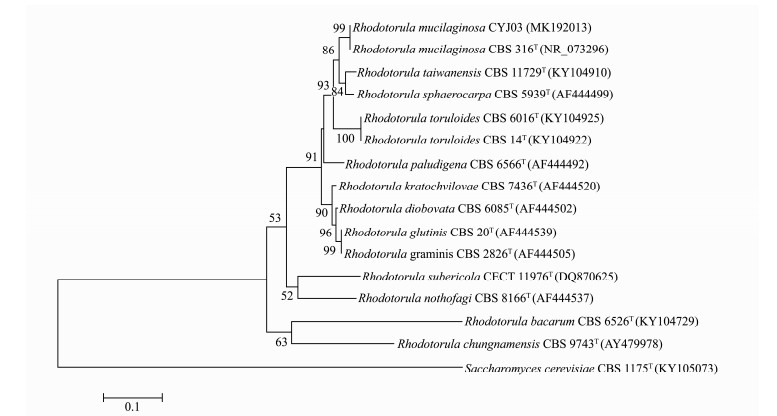
|
Fig. 2 Phylogenetic analysis of CYJ03 and related strains, Saccharomyces cerevisiae CBS 1175 was used as the root. The phylogenetic tree was constructed based on ITS1-5.8S rRNA-ITS2 sequences. Bootstrap percentages from 1000 replications are shown on the branches (values below 50% are not shown). GenBank accession number of each strain's sequence is in parentheses. T, type strain. |
Colonies of Rhodotorula species are usually distinctive by forming colors, ranging from red to colorless, mostly pink and red when grown on SDA (Sabouraud's Dextrose Agar) (Hernández-Almanza et al., 2014). These pigments produced are beneficial in protecting the producer cell structures form oxidative stress, via the blocking certain radiations with the wavelengths from 620 to 750 nm. The orange pigment of CYJ03 may be then carotenoid-like substances as to be found in other strains (Buzzini et al., 2007).
3.2 Genome CharacteristicsWhole-genome sequencing was performed by OE Biotech (Shanghai OE Biotech Co., Ltd., Shanghai, China) using PacBio sequencing technology, with 252156 total reads ranging from 51 bp to 59861 bp for a total of 1 billion 324 million sequenced bases (Table 1). The total size of R. mucilaginosa CYJ03 is 19037214 bp containing in 88 contigs. The largest contig measured 891449 bp, and the N50 length was 420192 bp. G+C content was 60.49%. A total of 6301 genes were predicted by applying GeneMark-ES for coding genes predicting as well as for genomes assembling (Alexandre et al., 2005). Followed functional annotation were carried out with the software Diamond to assign the NR, KOG, GO, Swissprot, eggNOG and KEGG databases to 6191 (98.25%), 3683 (58.45%), 4144 (65.77%), 4241 (67.31%), 4797 (76.13%) and 2671 (42.39%) of the predicted proteins respectively.
|
|
Table 1 Genome assembly and annotation statistics of R. mucilaginosa CYJ03 |
As indicated by the predicted genes and the corresponding protein functions, the genome of CYJ03 was facilitated with a series of genes potentially associated with some specific yet interesting biological characteristics, such as zinc binding, UV radiation resistance, lipid synthesis, carotenoid biosynthesis, and other biotransformation.
Compared to other species in the same genus, the whole genome information of R. mucilaginosa is relatively scarce. So far only five genome sequences of R. mucilaginosa isolates have been published, among them C2.5t1, RIT389, IIPL32 and JGTA-S1 were submitted to the NCBI database, whereas strain ATCC 58901 was sent to JGI Genome Portal (https://genome.jgi.doe.gov/Rhomuc1/Rhomuc1.home.html). The source, genome information and functions of these five strains and CYJ03 are detailed in Supplementary Table S1 (not showen). In this work, the whole genome sequence of R. mucilaginosa CYJ03 was submitted and published to the NCBI database.
3.3 Carotenoid Biosynthetic Pathway DepictingIn industry, red yeast is often used to produce carotenoids. The production of carotenoids is very important, they are used as natural colorants or nutritional supplements, and some carotenoids are also precursors of vitamin A, such as β-carotene, γ-carotene, torulene and torularhodin (Mata-Gómez et al., 2014). However, due to the lack of information about the carotenoid biosynthetic pathways and molecular characteristics of the Rhodotorula species may hinder these potential strains from increasing their carotenoid production through genetic or metabolic engineering. Phaffia rhodozyma has done a better job in this respect, and its carotenoid biosynthesis pathway has been relatively clear (Rodríguez-Sáiz, 2010).
Based on the genomic data obtained, the carotenoid biosynthetic pathway of R. mucilaginosa CYJ03, was mapped and depicted using the KEGG pathway analysis and annotation (Fig. 3). The biosynthesis of carotenoid begins with the formation of geranylgeranyl pyrophosphate (GGPP) followed by a series of enzymatic reactions of acetyl-CoA addition. Two molecules of GGPP were condensed into phytoene which was the first colorless carotenoid, this step was catalyzed by phytoene synthase [EC: 2.5.1.32]. Then the phytoene desaturase [EC: 1.3. 99.30] catalyzed three stepwise desaturation reactions for the formation of neurosporene. Neurosporene was then converted to lycopene, which was followed by two independent cyclization branch routes leading to the two final products β-carotene and torulene, respectively. In the reported carotenoid biosynthetic pathway, torulene is further covert to torularhodin by hydroxylation and oxidation, but torularhodin was not found in KEGG mapping (Kot et al., 2018).

|
Fig. 3 Diagram of the carotenoid biosynthetic pathway of CYJ03. crtB, phytoene synthase; AL1, phytoene desaturase; AL2, phytoene synthase/lycopene cyclase. |
Combined with the results of gene annotation, it was found that in the carotenoid biosynthetic pathway by CYJ03 three genes, crtB, AL1 and AL2, were involved and encoded phytoene synthase, phytoene desaturase, and phytoene synthase/lycopene beta-cyclase, respectively. It is worth mentioning that AL2 gene encoded a bifunctional protein which exerts both phytoene synthase and lycopene cyclase activities. This finding is similar to what has been observed in several other fungal species (Arrach et al., 2001; Sanz et al., 2011).
3.4 Carotenoid Biosynthetic Gene Cluster of R. mucilaginosa CYJ03In this study the scanning of CYJ03 genome ended with the finding of twenty-five gene clusters corresponding to secondary metabolite biosynthesis by using antiSMASH software. Three out of 25 of the clusters were found to be involved with terpenes biosynthesis, with 13 clusters being identified as the one in charge of carotenoid biosynthesis. The important genes of this cluster have been annotated, and the results were all shown in Table 2.
|
|
Table 2 Genes of the carotenoid biosynthetic gene cluster in R. mucilaginosa CYJ03 |
It is found that multiple genes of cluster 13 have high homology with what have been identified in other fungi strains. In different fungi the carotenoid gene clusters arrangement varied in layout (Fig. 4). Compared to the observations in other fungi, the six main carotenoid biosynthetic genes of CYJ03 were closely located in a compacted array. The six genes encoded a series enzymes for carotenoid biosynthesis namely phytoene desaturase (AL1), putative oligopeptide transporter, putative carotenoid oxygenase, phytoene synthase/lycopene cyclase (AL2), SGS-domain-containing protein and DUF1212 family protein, respectively. In general the carotenoid biosynthetic gene cluster of CYJ03 shares low similarity to that of other fungi, e.g., cluster 13 of CYJ03 only share merely 27% similarity compared to Rhodosporidium toruloides strain CECT1137.

|
Fig. 4 Alignments of homologous carotenoid biosynthetic gene clusters between R. mucilaginosa CYJ03 and other strains. This map was drawn by using the antiSMASH online software, the ClusterBlast was performed. The top10 strains matching with CYJ03 gene cluster are displayed. Homologous genes are marked with the same colour, and numbers in parentheses represent similarity. Numbers indicate the individual genes/enzymes. Names and gene numbers from the R. mucilaginosa CYJ03 genome are listed in Table 2. |
The use of -omics and genetic engineering tools to study carotenoid metabolism mechanisms and improve synthesis efficiency is emerging with a panel of key biosynthetic steps elucidated. Landolfo et al. (2018) studied the expression of CAR2 and CAR1 genes from R. mucilaginosa C2.5t1, which encode phytoene dehydrogenase and phytoene synthase/lycopene cyclase at the late steps of carotenogenic pathway, respectively. The results showed that there was no clear induction trend of these two genes, and no significant variations were observed during growth. Therefore, the amount of carotenoids produced by R. mucilaginosa is more likely determined by the regulation of enzyme activity rather than the regulation at transcript levels, e.g., induction of the biosynthesis of relevent genes (Wozniak et al., 2011). Prado-Cabrero et al. (2007) targeted deleted the carX gene from Fusarium fujikuroi, which encodes a putative carotenoid oxygenase involved in the formation of neurosporaxanthin (a major carotenoid). The deletion of carX gene did not impede neurosporaxanthin biosynthesis, but did significantly evaluate the total carotenoid production. It was concluded that carotenoid oxygenase participates in the regulation of carotenoid synthesis through a negative feedback mechanism. In Sporidiobolus pararoseus NGR, transcriptional levels analysis of genes encoding phytoene desaturase and lycopene cyclase/phytoene synthase were investigated by applying salt stress conditions. The results showed that their relative expressions were significantly up-regulated, which resulted in the accumulation of 3, 4-didehydrolycopene and brought about subsequent increase in the production of torulene and torularhodin (Li et al., 2017).
With the established CYJ03 genome data in the future the role of key genes for carotenoid biosynthesis of CYJ03 will be further studied and hopefully the techniques for the overproduction of carotenoids will be available through the sophisticated bioengineering manipulations.
4 ConclusionsA carotenoid-producing red yeast strain CYJ03 was isolated from ocean samples and it was subsequently identified as R. mucilaginosa. Whole-genome sequencing was completed, and in-depth analysis of the carotenoid biosynthesis pathway and the relevant gene clusters were conducted, respectively. The elucidation of carotenoid biosynthesis pathway and the relevant key genes have extended the knowledge of carotenoids biosynthesis by Rhodotorula. In addition, the release of the whole genome sequence of R. mucilaginosa CYJ03 enables the exploration of application potential of the metabolites of interest. In the future it would be of great values to develop genetic manipulation tools to facilitate the over production of carotenoids and other valuable metabolites.
Nucleotide Sequence Accession NumbersRhodotorula mucilaginosa CYJ03 genome sequencing project has been deposited in NCBI under BioProject (No. PRJNA506114) and BioSample SAMN10448795. This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accession RZHN00000 000. The version described in this paper is version RZHN 01000000.
AcknowledgementsThe study is supported by the Postdoctoral Applied Research Project of Qingdao.
Akhter, S., Aziz, R. K. and Edwards, R. A., 2012. Phispy: A novel algorithm for finding prophages in bacterial genomes that combines similarityand composition-based strategies. Nucleic Acids Research, 40(16): 329-334. (  0) 0) |
Arrach, N., Fernández-Martín,, R, ., Cerdá-Olmedo, E. and Avalos, J., 2001. A single gene for lycopene cyclase, phytoene synthase, and regulation of carotene biosynthesis in Phycomyces. Proceedings of the National Academy of Sciences of the United States of America, 98(4): 1687-1692. DOI:10.1073/pnas.98.4.1687 (  0) 0) |
Buzzini, P., Innocenti, M., Turchetti, B., Libkind, D., van Broock, M. and Mulinacci, N., 2007. Carotenoid profiles of yeasts belonging to the genera Rhodotorula, Rhodosporidium, Sporobolomyces, and Sporidiobolus. Canadian Journal of Microbiology, 53(8): 1024-1031. DOI:10.1139/W07-068 (  0) 0) |
Chin, C. S., Peluso, P., Sedlazeck, F. J., Nattestad, M., Concepcion, G. T., Clum, A., Dunn, C., O'Malley, R., FigueroaBalderas, R. and Morales, -Cruz A., 2016. Phased diploid genome assembly with single-molecule real-time sequencing. Nature Methods, 13(12): 1050-1054. DOI:10.1038/nmeth.4035 (  0) 0) |
Dasgupta, D., Sharma, T., Bhatt, A., Bandhu, S. and Ghosh, D., 2017. Cultivation of oleaginous yeast Rhodotorula mucilaginosa IIPL32 in split column airlift reactor and its influence on fuel properties. Biocatalysis & Agricultural Biotechnology, 10: 308-316. (  0) 0) |
Deligios, M., Fraumene, C., Abbondio, M., Mannazzu, I., Tanca, A., Addis, M. F. and Uzzau, S., 2015. Draft genome sequence of Rhodotorula mucilaginosa, an emergent opportunistic pathogen. Genome Announcements, 3(2): 1-2. (  0) 0) |
Frengova, G. I. and Beshkova, D. M., 2009. Carotenoids from Rhodotorula and Phaffia: Yeasts of biotechnological importance. Journal of Industrial Microbiology & Biotechnology, 36(2): 163-180. (  0) 0) |
Gan, H. M., Thomas, B. N., Cavanaugh, N. T., Morales, G. H., Mayers, A. N., Savka, M. A. and Hudson, A. O., 2017. Whole genome sequencing of Rhodotorula mucilaginosa isolated from the chewing stick (Distemonanthus benthamianus): Insights into Rhodotorula phylogeny, mitogenome dynamics and carotenoid biosynthesis. PeerJ, 5(1): e4030. (  0) 0) |
Hawksworth, D. L., Kirk, P. M., Sutton, B. C. and Pegler, D. N., 1996. Ainsworth & Bisby's dictionary of the fungi. Revista do Instituto de Medicina Tropical de São Paulo, 38(4): 17-19. (  0) 0) |
Hernández-Almanza, A., Montanez, J. C., Aguilar-González,, M., A., Martínez-Ávila, C., Rodríguez-Herrera, R. and Aguilar, C. N., 2014. Rhodotorula glutinis as source of pigments and metabolites for food industry. Food Bioscience, 5(Complete): 64-72. (  0) 0) |
Huerta-Cepas, J., Szklarczyk, D., Forslund, K., Cook, H., Heller, D., Walter, M. C., Rattei, T., Mende, D. R., Sunagawa, S. and Kuhn, M., 2016. eggNOG 4.5: A hierarchical orthology framework with improved functional annotations for eukaryotic, prokaryotic and viral sequences. Nucleic Acids Research, 44: D286-D293. DOI:10.1093/nar/gkv1248 (  0) 0) |
Ignatova, L. V., Brazhnikova, Y. V., Berzhanova, R. Z. and Mukasheva, T. D., 2015. Plant growth-promoting and antifungal activity of yeasts from dark chestnut soil. Microbiological Research, 175: 78-83. DOI:10.1016/j.micres.2015.03.008 (  0) 0) |
Koonin, E. V., Fedorova, N. D., Jackson, J. D., Jacobs, A. R., Krylov, D. M., Makarova, K. S., Mazumder, R., Mekhedov, S. L., Nikolskaya, A. N. and Rao, B. S., 2004. A comprehensive evolutionary classification of proteins encoded in complete eukaryotic genomes. Genome Biology, 5(2): R7-R7. DOI:10.1186/gb-2004-5-2-r7 (  0) 0) |
Kot, A. M., Błażejak, S., Gientka, I., Kieliszek, M. and Bryś, J., 2018. Torulene and torularhodin: 'New' fungal carotenoids for industry?. Microbial Cell Factories, 17(1): 49. DOI:10.1186/s12934-018-0893-z (  0) 0) |
Landolfo, S., Ianiri, G., Camiolo, S., Porceddu, A., Mulas, G., Chessa, R., Zara, G. and Mannazzu, I., 2018. CAR gene cluster and transcript levels of carotenogenic genes in Rhodotorula mucilaginosa. Microbiology, 164(1): 78-87. DOI:10.1099/mic.0.000588 (  0) 0) |
Li, C., Zhang, N., Li, B., Xu, Q., Song, J., Wei, N., Wang, W. and Zou, H., 2017. Increased torulene accumulation in red yeast Sporidiobolus pararoseus NGR as stress response to high salt conditions. Food Chemistry, 237: 1041-1047. DOI:10.1016/j.foodchem.2017.06.033 (  0) 0) |
Lomsadze, A., Terhovhannisyan, V., Chernoff, Y. O. and Borodovsky, M., 2005. Gene identification in novel eukaryotic genomes by self-training algorithm. Nucleic Acids Research, 33(20): 6494-6506. DOI:10.1093/nar/gki937 (  0) 0) |
Ma, W., Chen, X., Wang, B., Lou, W., Chen, X., Hua, J., Sun, Y. J., Zhao, Y. and Peng, T., 2017. Characterization, antioxidativity, and anti-carcinoma activity of exopolysaccharide extract from Rhodotorula mucilaginosa CICC 33013. Carbohydrate Polymers, 181: 768. (  0) 0) |
Mata-Gómez, L.C., Montañez, J. C., Méndez-Zavala, A. and Aguilar, C. N., 2014. Biotechnological production of carotenoids by yeasts: An overview. Microbial Cell Factories, 13(1): 12p. DOI:10.1186/1475-2859-13-12 (  0) 0) |
Medema, M. H., Blin, K., Cimermancic, P., De Jager, V., Zakrzewski, P., Fischbach, M. A., Weber, T., Takano, E. and Breitling, R., 2011. AntiSMASH: Rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Research, 39(web server issue): W339. (  0) 0) |
Minoru, K., Goto, S., Kawashima, S., Okuno, Y. and Hattori, M., 2004. The KEGG resource for deciphering the genome. Nucleic Acids Research, 32: 277-280. DOI:10.1093/nar/gkh063 (  0) 0) |
Akhtyamova, N. and Sattarova, R. K., 2013. Endophytic yeast Rhodotorula rubra strain TG-1: Antagonistic and plant protection activities. Biochemistry & Rhysiology, 2(1): 1000104. DOI:10.4172/2168-9652.1000104 (  0) 0) |
Prado-Cabrero, A., Scherzinger, D., Avalos, J. and Al, -Babili S., 2007. Retinal biosynthesis in fungi: Characterization of the carotenoid oxygenase CarX from Fusarium fujikuroi. Eukaryotic Cell, 6(4): 650-657. DOI:10.1128/EC.00392-06 (  0) 0) |
Rodríguez-Sáiz, M., de la Fuente, J. L. and Barredo, J. L., 2010. Xanthophyllomyces dendrorhous for the industrial production of astaxanthin. Applied Microbiology & Biotechnology, 88: 645-658. (  0) 0) |
Saha, C. and Seal, A., 2015. Early changes in shoot transcriptome of rice in response to Rhodotorula mucilaginosa JGTAS1. Genomics Data, 6: 237-240. DOI:10.1016/j.gdata.2015.09.023 (  0) 0) |
Salvadori, M. R., Ando, R. A., Oller, do Nascimento C. A., Corr, êa and B, ., 2014. Intracellular biosynthesis and removal of copper nanoparticles by dead biomass of yeast isolated from the wastewater of a mine in the Brazilian Amazonia. Plos One, 9(1): e87968. DOI:10.1371/journal.pone.0087968 (  0) 0) |
Sanz, C., Velayos, A., Álvarez, M. I., Benito, E. P. and Eslava, A. P., 2011. Functional analysis of the Phycomyces carRA gene encoding the enzymes phytoene synthase and lycopene cyclase. PloS One, 6(8): e23102. DOI:10.1371/journal.pone.0023102 (  0) 0) |
Singh, P., Tsuji, M., Singh, S. M., Roy, U. and Hoshino, T., 2013. Taxonomic characterization, adaptation strategies and biotechnological potential of cryophilic yeasts from ice cores of Midre Lovénbreen glacier, Svalbard, Arctic. Cryobiology, 66(2): 167-175. DOI:10.1016/j.cryobiol.2013.01.002 (  0) 0) |
Tamura, K., Nei, M. and Kumar, S., 2004. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proceedings of the National Academy of Sciences of the United States of America, 101(30): 11030-11035. DOI:10.1073/pnas.0404206101 (  0) 0) |
Wirth, F., and Goldani, L. Z., 2012. Epidemiology of Rhodotorula: An emerging pathogen. Interdisciplinary Perspectives on Infectious Diseases, 7pp, DOI: 10.1155/2012/465717.
(  0) 0) |
Wozniak, A., Lozano, C., Barahona, S., Niklitschek, M., Marcoleta, A., Alcaíno,, J, ., Sepulveda, D., Baeza, M. and Cifuentes, V., 2011. Differential carotenoid production and gene expression in Xanthophyllomyces dendrorhous grown in a nonfermentable carbon source. Fems Yeast Research, 11(3): 252-262. DOI:10.1111/j.1567-1364.2010.00711.x (  0) 0) |
Zhang, H., Ge, L., Chen, K., Zhao, L. N. and Zhang, X. Y., 2014. Enhanced biocontrol activity of Rhodotorula mucilaginosa, cultured in media containing chitosan against postharvest diseases in strawberries: Possible mechanisms underlying the effect. Journal of Agricultural and Food Chemistry, 62(18): 4214-4224. DOI:10.1021/jf500065n (  0) 0) |
 2020, Vol. 19
2020, Vol. 19


