2) University of Chinese Academy of Sciences, Beijing 100049, China;
3) Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou), Guangzhou 511458, China;
4) Sansha Marine and Fisheries Bureau, Sansha 573199, China
The Pacific triton Charonia tritonis, also known as the giant triton snail, is one of the largest carnivorous gastropods in coral reef ecosystem (Zhang et al., 2013). It is widespread in tropical waters of the South China Sea (Zhang et al., 2021). It is also the primary predator of the crown of thorns starfish (CoTS) Acanthaster planci, which is the main cause of damage to many coral reefs (Poulsen, 1995; Kang and Kim, 2004; Cowan et al., 2017). With the aims of protecting the coral reef ecosystem and biodiversity using eco-friendly products, it is important to collect as much information as possible on the biology of C. tritonis, such as the predation mechanism.
Previous research has shown that many gastropods secrete acidic venom or toxin to paralyze their prey. For example, the Charonia variegate, which is also known as atlantic triton snail, utilizes its toxins to immobilize prey, and the toxins are secreted from foot or mouth. The Atlantic conch (Charonia varigat), secretes toxins from its feet or mouth to immobilize prey (Bandel, 1984). The Charonia lampas rubicunda can cause the starfish Patriella brevispina paralysis instantaneously, because its proboscis can secrete toxins (Endean, 1972). Conus produces hundreds of different short peptides termed conotoxin to access their prey including capture and digestion of prey, and defend against prey (Kaas et al., 2010). In C. tritonis, its proboscis stretches out to contact the prey, then penetrates the prey to inject toxins or acidic saliva, and quickly paralyzes the prey (Chesher, 1969; Percharde, 1972; Bose et al., 2017b). It has been reported that the acidic material can promote penetration of the calcareous body wall of the prey or traumatize their prey to avoid predation (Garcia Ortega et al., 2011). However, there are few reports on whether the acidic material is a C. tritonis toxin. In addition, no studies have reported toxin classification, identification, diversity and conotoxin homologous genes of C. tritonis.
In the present study, three-dimensional structure of C. tritonis CTXs were constructed and the disulfide bridge were predicted by using the amino acid sequence of conotoxin as a model. The phylogenetic tree showed that CTXs Clustered with the C, V, B1 conotoxin superfamily. To investigate the possible biological roles of CTXs, the predation control and tissue distribution were detected, the results reveal the CTXs may take part in the predation process of C. tritonis and will provide further insight and a theoretical basis for revealing the conotoxin action and secretion mechanism of C. tritonis.
2 Materials and Methods 2.1 Sample CollectionC. tritonis (n = 9) were collected from the South China Sea, which were kept active and healthy in a cement pool with seawater. Only three were in a resting stage and their mantle, foot muscle, proboscides, tentacles and salivary gland tissues were extracted. The mantle, foot muscle, proboscides, tentacles, salivary gland, digestive gland, gonad and liver tissues were from the other six C. tritonis in capture control and treatment groups. All tissues were stored at −80℃ after quickly frozen in liquid nitrogen.
2.2 Nucleotide Sequence and Bioinformatic AnalysesNucleotide sequence similarities were examined by BLAST software (http://smart.embl-heidelberg.de/). Using ORF Finder (https://www.ncbi.nlm.nih.gov/orffinder/) to analyze the four CTX genes open reading frame (ORF). The SMART online tool (http://smart.embl-heidelberg.de/) was used to analyzed the amino acid sequence and structural domain of CTXs. The SWISS-MODEL online tool (https://www.swissmodel.expasy.org/interactive) and PyMOL were used for predicting and polishling the three-dimensional structure of CTXs amino acids. Multiple sequence alignment of CTXs amino acid sequences was detected using ClustalX and CLC Main Workbench 6.0 software to highlight regions of conservation. With Molecular Evolutionary Genetics Analysis (MEGA) 6.0 software (Tamura, 2013) and Neighbor joining method of 1000 bootstrap cycles, a phylogenetic tree was constructed.
2.3 RNA Extraction and Reverse Transcription from All TissuesTo analyze the expression of CTXs in different tissues, Chanoria tritonis mantle, foot muscle, proboscides, tentacles, salivary gland, digestive gland and liver tissues were collected. RNA was extracted from all tissues by using the universal RNA extraction kit (Magen). Then, using the reverse transcription kit (Toyobo) with gDNA Remover to perform cDNA first-strand synthesis.
C. tritonis individuals in the predation control experiment were divided into two groups, i.e., control group without predation and treatment group during predation. In the cement pool feeding CoTS, three C. tritonis that had not eaten CoTS were captured and the remaining C. tritonis were included in the post feeding group during predation on CoTS. The mantle, foot muscle, proboscides, tentacle, salivary gland, digestive gland, gonad and liver tissues were collected from the different groups for quantitative real-time PCR (qRT-PCR) assay.
2.4 qRT-PCR Analyses of CTXs ExpressionqRT-PCR was employed to assay the distribution of CTXs in different tissues and analyze the CTXs expression difference in the predation control and treatment groups. Four pairs of primers (CTX-1-realtime-F/R; CTX-2-realtime-F/R; CTX-3-realtime-F/R; CTX-4-realtime-F/R) were designed based on the specific sequence of the four CTX genes by primer premier 5 software.
Each sample were tested in a 384 well plate and the amplifications were performed in a total volume of 10 μL, containing 5 μL SYBR Green Realtime qRT-PCR Master Mix (Toyobo), 0.3 μL forward primers and 0.3 μL reverse primers in distilled water (10 μmol L−1), 3.4 μL of distilled water and then add 1 μL template cDNA that was diluted in double distilled water. The reaction program was set 94℃ predenaturation for 3 min, 98℃ denaturation for 10 s, the annealing temperature was set 55℃/59℃/60℃ for 25 s, predenaturation was cycled one time, and 45 denaturation annealing extension cycles. 18S rRNA gene was employed as the internal reference gene. All tissues were set three biological and technical replicates. Take the 2−ΔΔCt as the calculation method. Statistical analysis was performed with Ttest by Prism 8.0. Differences were considered significant when P < 0.05. All sequences of the primers were listed in Table 1.
|
|
Table 1 Universal and specific primers |
The CTXs sequences were selected from transcriptome data of the article (Zhang et al., 2021) about C. tritonis. CTXs were identified as conotoxin homologous genes refer to the four aspects Fig.1 (Kaas et al., 2010) (Sections 1, 2, 3, 4). Evaluate the superfamily classification based on the similarity between the CTXs protein signal peptide sequence and the reported conotoxin signal peptide sequence (Fig.1, Section 2). Next, the mature sequences formation the different disulfide bonds (Fig.1, Section 3). Then CTXs amino acids fold to form a three-dimensional structure (Fig.1, Section 4 and Fig.2).

|
Fig. 1 Schematic diagram of CTXs identification and classification process. The process of evaluating the superfamily classification based on the similarity between the CTXs protein signal peptide sequence and the reported conotoxin signal peptide sequence (Section 2). The cysteine arrangement of CTXs were predicted by Conoserver (Section 3). The three-dimensional shape of disulfide bonds after folding (Section 4). The pharmacological families were defined based on the targeted receptor and the effect in Section 5. |
|
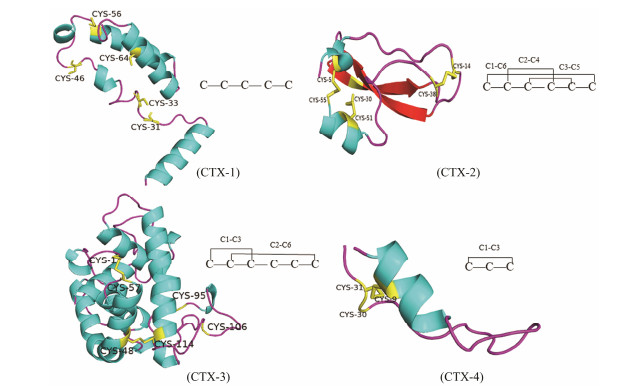
Fig. 2 3D structure and disulfide bonding pattern of C. tritonis CTX-1, CTX-2, CTX-3, CTX-4. |
The protein-protein BLAST used to match species with the highest similarity to C. tritonis CTXs, sequence annotations and amino acid sequences based on putative conserved domains and e-value. Analysis of CTX-1 revealed 43.55% identity with C. ermineus (accession no. AXL_95724.1), CTX-2 revealed 54.84% identity with C. betulinus (accession no. AMP_44780.1), CTX-3 revealed 74.50% identity with C. betulinus (accession no. ALM_87513.1), and CTX-4 revealed 80.00% identity with C. episcopatus (accession no. BAS_25475.1). The four CTXs have different cysteine residues and disulfide bonding mode. CTX-2 has the same domain with Kunitz domain inhibitor showed the disulfide bonding pattern of Cys5(C1)-Cys55(C6), Cys14(C2)-Cys38(C4), Cys30(C3)-Cys51(C5). CTX-3 revealed the disulfide bonding pattern of Cys1(C1)-Cys57(C3), Cys48(C2)-Cys114(C6). CTX-4 has three cysteine residues suggested the disulfide bonding pattern of Cys9(C1)-Cys31(C3) and CTX-1 has five cysteine residues without disulfide bonding pattern (Fig.2). The three-dimensional structure of CTXs also conform to this result (Fig.2).
Sequence alignment of CTXs showed poor sequence conservation and different cysteine residue positions (Fig.3a). The phylogenetic tree showed that the sequence identified in Gigantopelta aegis (accession no. XP_041354957.1) was orthologous to the identified CTX-2 in C. tritonis. The sequence identified in Conus ermineus (accession no. AXL 95723.1) was orthologous to the identified CTX-1. The sequence identified in Conus betulinus (accession no. ALM 87513.1) was orthologous to the identified CTX-3. The sequence identified in Conus episcopatus (accession no. BAS 25475.1) and CTX-4 were clustered into one branch (Fig.3b). The tree shows that the sequence identification of CTXs and vertebrates were relatively low, but were closely related to gastropod mollusks. This phylogenetic tree analysis indicated that CTXs were conserved during evolution.

|
Fig. 3 (a) Amino acid sequence alignment result of CTXs. The height and letter size of the pink bar graph were positively correlated with the degree of conservation of the corresponding amino acids. (b) Phylogenetic tree of CTXs and other species. (c) Phylogenetic tree of C. tritonis CTXs and Conus superfamily genes. |
Signal peptide sequence of CTXs in Table 2 could be assigned to the gene superfamily present in the ConoServer database (Biggs et al., 2010; Luo et al., 2013). The phylogenetic tree of CTXs and superfamilies (D, L, C, A, S, E, M, V, B1, I2) were analyzed. This tree demonstrated that the CTX-1 and CTX-4 sequences of C. tritonis and the C superfamily were clustered into one branch. CTX-2 sequence and the V superfamily were clustered into one branch. CTX-3 sequence and the B1 superfamily were clustered into one branch. The phylogenetic tree of superfamily supported the ConoServer prediction results (Fig.3c).
|
|
Table 2 Results of CTXs cysteine framework and BLAST |
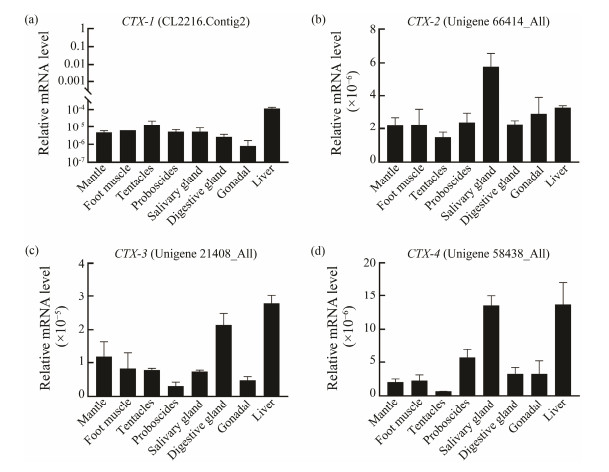
The tissues distribution results showed that CTXs were highly expressed in liver, salivary gland and digestive gland (Figs.4a, 4b, 4c, 4d). CTX-1, CTX-3 and CTX-4 were highly expressed in liver (Figs.4a, 4c, 4d). CTX-2 expression in salivary gland was two times higher than that in liver (Fig.4b). CTX-3 was highly expressed in digestive gland and liver, but low in salivary gland (Fig.4c). Moreover, CTX-4 expression in salivary gland and liver was three times higher than in digestive gland (Fig.4d).
|
Fig. 4 CTXs expression levels in different tissues of C. tritonis. (a) Expression analysis of CTX-1; (b) Expression analysis of CTX-2; (c) Expression analysis of CTX-3; (d) Expression analysis of CTX-4. |
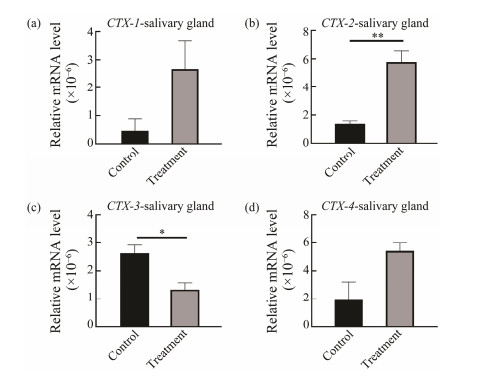
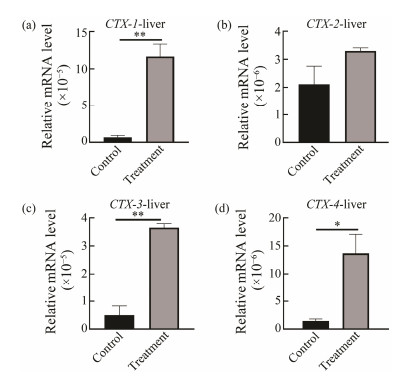
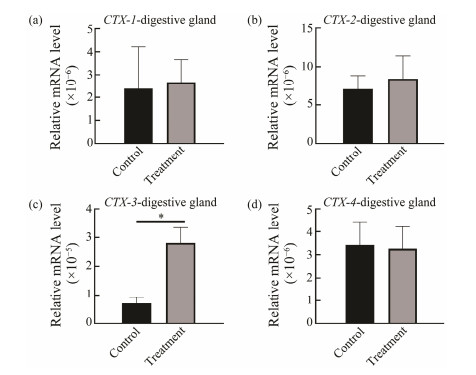
To analyze the expression of CTXs in response to predation control in the salivary gland, digestive gland and liver, qRT-PCR was performed. In the salivary gland, digestive gland and liver, expression of CTX-2 was down-regulated in the control group, but in the treatment group, CTX-2 expression was up-regulated significantly at salivary gland (P < 0.02) (Fig.5). Increased expression of CTX-2 in treatment group suggested that CTX-2 might have different response mechanisms when involved in paralysis and ingestion CoTS. However, expressions of CTX-1 (P < 0.02), CTX-4 (P < 0.05) and CTX-3 (P < 0.02) were significantly up-regulated in liver but down-regulated in salivary gland compared to the control group (Fig.6). Expression of CTX-3 (P < 0.02) in digestive gland was also significantly up-regulated and CTX-1, CTX-2 and CTX-4 were also up-regulated with no significant difference (Fig.7).
|
Fig. 5 CTXs expressions in salivary gland at predation control and treatment groups. *P < 0.05, ** P < 0.02. |
|
Fig. 6 CTXs expressions in liver at predation control and treatment groups. *P < 0.05, ** P < 0.02. |
|
Fig. 7 CTXs expression in digestive gland at predation control and treatment groups. *P < 0.05. |
It has been reported that Conus can secrete venom that can paralyze the prey, such as fish, insects, and snails (Luo et al., 2007; Safavi-Hemami et al., 2011). However, the salivary gland of Charonia can produce venom, which help predation, immobilize prey and neutralize the intrinsic toxin of CoTS (Bose et al., 2017a, 2017b). Previous studies have showed that the venom secreted by C. tritonis and Conus plays the crucial role in paralyze prey, and ω-MVIIA derived from the venom of C. magus was confirmed to treat chronic pain in cancer (Miljanich, 2004; Lynch et al., 2006; McGivern, 2007; Bose et al., 2017b). C. tritonis is the natural enemy of A. planci and its predation mechanism has been the focus of our attention. Taking into account the role of CTXs in Gigantopelta aegis, C. betulinus, C. ermineus and C. episcopatus, we speculate that CTXs may play a role in different predation periods of C. tritonis.
Studies revealed that the composition and structure of conotoxin vary greatly among species, including C. betulinus, C. ermineus and C. episcopatus (Dutertre et al., 2013; Peng et al., 2016; Phuong et al., 2016; Li et al., 2017; Robinson et al., 2017). In the present study, CTXs were identified to have different types of cysteine framework based on conserved sequence (Abalde et al., 2018), Because the prediction by ConoServer is based on mature peptides sequence rather than specific conserved sequence (Kaas et al., 2008, 2010, 2012; Brown et al., 2016), the CTXs have different cysteine arrangement and are inconsistent with prediction. Presently there are nearly no referable reports on the pharmacological functions of CTXs in C. tritonis. Hopefully our next research will focus on such function.
The phylogenetic analysis results showed that CTXs of C. tritonis were conserved in evolutionary relationship. The mantle, foot muscle, proboscides and tentacle tissues were confirmed to participate in the hunting behavior of C. tritonis (Hall and Kingsford, 2016; Bose et al., 2017b; Hall et al., 2017). Especially, the expression difference of CTX2, CTX3, CTX4 in mantle, foot muscle, proboscides, tentacle, salivary glands, digestive glands and liver tissues of C. tritonis has been strongly proved, which means that CTXs might have different functions and response mechanisms when participating in predation.
The predation control experiment showed that significantly more CTXs were secreted during predation compared with the predation control group, which showed that CTXs possibly play a role in predation. The salivary gland, liver and digestive gland as the important digestive system exist in almost all gastropods mollusks (Cui et al., 2000), which can synthesize and secrete digestive enzymes. CTX-3 was highly expressed in salivary gland in the control group, which was consistent with the result that the salivary gland of C. tritonis can secrete venom. However, CTX-3 also expressed in liver and digestive gland compared with the control group, which means that the CTXs of C. tritonis might play an important role in preying on CoTS. The expression levels of CTXs were inconformity with the control group, which may be due to the different functions of CTXs in tissues. The expression difference in predation control revealed that CTXs might have different response mechanisms during the paralysis of CoTS. These results will provide basis for further studying the toxins' secretion mechanism of C. tritonis.
5 ConclusionsIn this study, CTXs of C. tritonis were identified and the sequence, structure, disulfide bonding bridge and phylogenetic tree were analyzed. The comprehensive results showed that the four CTXs might be different toxin genes. The expressions of CTXs in different predation periods of the giant triton snail (Charonia tritonis) revealed that the CTXs were highly expressed in salivary gland, digestive gland and liver of predation treatment group.
AcknowledgementsThis work was supported by the National Natural Science Foundation of China (No. 42176129), the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDA13020206), the Key Special Project for Introduced Talents Team of Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou) (No. GML2019ZD0402), and the Planning Project of Guangdong Province, China, Guangdong Provincial Key Laboratory of Applied Marine Biology (No. 2020B1212060058).
Abalde, S., Tenorio, M. J., Afonso, C. M. L., and Zardoya, R., 2018. Conotoxin diversity in Chelyconus ermineus (Born, 1778) and the convergent origin of piscivory in the Atlantic and Indo-Pacific cones. Genome Biology and Evolution, 10(10): 2643-2662. DOI:10.1093/gbe/evy150 (  0) 0) |
Bandel, K., 1984. The Radulae of Caribbean and Other Mesogastropoda and Neogastropoda. EJ Brill, Leiden, 1-199.
(  0) 0) |
Biggs, J. S., Watkins, M., Puillandre, N., Ownby, J. P., Lopez-Vera, E., Christensen, S., et al., 2010. Evolution of Conus peptide toxins: Analysis of Conus californicus Reeve, 1844. Molecular Phylogenetics and Evolution, 56(1): 1-12. DOI:10.1016/j.ympev.2010.03.029 (  0) 0) |
Bose, U., Suwansa-Ard, S., Maikaeo, L., Motti, C. A., Hall, M. R., and Cummins, S. F., 2017a. Neuropeptides encoded within a neural transcriptome of the giant triton snail Charonia tritonis, a Crown-of-Thorns Starfish predator. Peptides, 98: 3-14. DOI:10.1016/j.peptides.2017.01.004 (  0) 0) |
Bose, U., Wang, T., Zhao, M., Motti, C. A., Hall, M. R., and Cummins, S. F., 2017b. Multiomics analysis of the giant triton snail salivary gland, a crown-of-thorns starfish predator. Scientific Reports, 7: 6000. DOI:10.1038/s41598-017-05974-x (  0) 0) |
Brown, E., Masinde, E. L. K., and Woodcock, B. G., 2016. Effects of conopeptide-containing venom from seven species of Conidae gastropoda on the chick biventer-cervicis nerve-muscle assessed using the ConoServer database. International Journal of Clinical Pharmacology and Therapeutics, 54(7): 544-554. DOI:10.5414/cp202667 (  0) 0) |
Chesher, R. H., 1969. Destruction of Pacific corals by sea star Acanthaster planci. Science, 165(3890): 280-283. DOI:10.1126/science.165.3890.280 (  0) 0) |
Cowan, Z. L., Pratchett, M., Messmer, V., and Ling, S., 2017. Known predators of crown-of-thorns starfish (Acanthaster spp.) and their role in mitigating, if not preventing, population outbreaks. Diversity Basel, 9(1): 7. DOI:10.3390/d9010007 (  0) 0) |
Cui, L. B., Lu, Y. H., Liu, C. L., and Tang, H., 2000. Light and electron microscopic studies on the salivary glands of Haliotis discus hannai Ino. Acta Oceanologica Sinica, 22(1): 141-144 (in Chinese with English abstract). (  0) 0) |
Dutertre, S., Jin, A. H., Kaas, Q., Jones, A., Alewood, P. F., and Lewis, R. J., 2013. Deep venomics reveals the mechanism for expanded peptide diversity in cone snail venom. Molecular & Cellular Proteomics, 12(2): 312-329. DOI:10.1074/mcp.M112.021469 (  0) 0) |
Endean, R., 1972. Aspects of molluscan pharmacology. Chemical Zoology, 7: 421-466. (  0) 0) |
Garcia Ortega, L., Alegre Cebollada, J., Garcia Linares, S., Bruix, M., Martinez del Pozo, A., and Gavilanes, J. G., 2011. The behavior of sea anemone actinoporins at the water-membrane interface. Biochimica et Biophysica Acta Biomembranes, 1808(9): 2275-2288. DOI:10.1016/j.bbamem.2011.05.012 (  0) 0) |
Hall, A. E., and Kingsford, M. J., 2016. Variation in the population demographics of Scolopsis bilineatus in response to predators. Coral Reefs, 35(4): 1173-1185. DOI:10.1007/s00338-016-1486-0 (  0) 0) |
Hall, M. R., Kocot, K. M., Baughman, K. W., Fernandez-Valverde, S. L., Gauthier, M. E. A., Hatleberg, W. L., et al., 2017. The crown-of-thorns starfish genome as a guide for biocontrol of this coral reef pest. Nature, 544(7649): 231-234. DOI:10.1038/nature22033 (  0) 0) |
Kaas, Q., Westermann, J. C., and Craik, D. J., 2010. Conopeptide characterization and classifications: An analysis using ConoServer. Toxicon, 55(8): 1491-1509. DOI:10.1016/j.toxicon.2010.03.002 (  0) 0) |
Kaas, Q., Westermann, J. C., Halai, R., Wang, C. K. L., and Craik, D. J., 2008. ConoServer, a database for conopeptide sequences and structures. Bioinformatics, 24(3): 445-446. DOI:10.1093/bioinformatics/btm596 (  0) 0) |
Kaas, Q., Yu, R., Jin, A. H., Dutertre, S., and Craik, D. J., 2012. ConoServer: Updated content, knowledge, and discovery tools in the conopeptide database. Nucleic Acids Research, 40(D1): D325-D330. DOI:10.1093/nar/gkr886 (  0) 0) |
Kang, K. H., and Kim, J. M., 2004. The predation of trumpet shell, Charonia sp., on eight different marine invertebrate species. Aquaculture Research, 35(13): 1202-1206. DOI:10.1111/j.1365-2109.2004.01124.x (  0) 0) |
Li, Q., Barghi, N., Lu, A., Fedosov, A. E., Bandyopadhyay, P. K., Lluisma, A. O., et al., 2017. Divergence of the venom exogene repertoire in two sister species of turriconus. Genome Biology and Evolution, 9(9): 2211-2225. DOI:10.1093/gbe/evx157 (  0) 0) |
Luo, S. L., Christensen, S., Zhangsun, D. T., Wu, Y., Hu, Y., Zhu, X. P., et al., 2013. A novel inhibitor of α9α10 nicotinic acetylcholine receptors from Conus vexillum delineates a new conotoxin superfamily. PLoS One, 8(1): e54648. DOI:10.1371/jour-nal.pone.0054648 (  0) 0) |
Luo, S. L., Wu, Y., Zhu, X. P., and Feng, J. C., 2007. Cloning of novel O-superfamily conotoxin of Conus capitaneus collected from Hainan. Chinese Journal of Marine Drugs, 26(1): 1-7 (in Chinese with English abstract). (  0) 0) |
Lynch, S. S., Cheng, C. M., and Yee, J. L., 2006. Intrathecal ziconotide for refractory chronic pain. Annals of Pharmacotherapy, 40(7-8): 1293-1300. DOI:10.1345/aph.1G584 (  0) 0) |
McGivern, J. G., 2007. Ziconotide: A review of its pharmacology and use in the treatment of pain. Neuropsychiatric Disease and Treatment, 3(1): 69-85. DOI:10.2147/nedt.2007.3.1.69 (  0) 0) |
Miljanich, G. P., 2004. Ziconotide: Neuronal calcium channel blocker for treating severe chronic pain. Current Medicinal Chemistry, 11(23): 3029-3040. DOI:10.2174/0929867043363884 (  0) 0) |
Peng, C., Yao, G., Gao, B. M., Fan, C. X., Bian, C., Wang, J. T., et al., 2016. High-throughput identification of novel conotoxins from the Chinese tubular cone snail (Conus betulinus) by multi-transcriptome sequencing. Gigascience, 5: 17. DOI:10.1186/s13742-016-0122-9 (  0) 0) |
Percharde, P. L., 1972. Observations on the gastropod, Charonia variegata, in Trinidad and Tobago. Nautilus, 85: 84-92. (  0) 0) |
Phuong, M. A., Mahardika, G. N., and Alfaro, M. E., 2016. Dietary breadth is positively correlated with venom complexity in cone snails. BMC Genomics, 17: 401. DOI:10.1186/s12864-016-2755-6 (  0) 0) |
Poulsen, A. L., 1995. Coral reef gastropods – A sustainable resource?. Pacific Conservation Biology, 2: 142-145. DOI:10.1071/PC960142 (  0) 0) |
Robinson, S. D., Li, Q., Lu, A., Bandyopadhyay, P. K., Yandell, M., Olivera, B. M., et al., 2017. The venom repertoire of Conus gloriamaris (Chemnitz, 1777), the glory of the sea. Marine Drugs, 15(5): 145. DOI:10.3390/md15050145 (  0) 0) |
Safavi-Hemami, H., Siero, W. A., Gorasia, D. G., Young, N. D., Macmillan, D., Williamson, N. A., et al., 2011. Specialisation of the venom gland proteome in predatory cone snails reveals functional diversification of the conotoxin biosynthetic pathway. Journal of Proteome Research, 10(9): 3904-3919. DOI:10.1021/pr1012976 (  0) 0) |
Tamura, K., Stecher, G., Peterson, D., Filipski, A., and Kumar, S., 2013. MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology & Evolution, 30(12): 2725-2729. (  0) 0) |
Zhang, G. G., Xu, M., Zhang, C. L., Jia, H. X., Zhang, H., He, M. X., et al., 2021. Comparative transcriptomic and expression profiles between the foot muscle and mantletissues in the giant triton snail Charonia tritonis. Frontiers in Physiology, 12(12): 632 518. DOI:10.3389/fphys.2021.632518 (  0) 0) |
Zhang, L. P., Xia, J., Peng, P. F., Li, P., Luo, P., and Hu, C. Q., 2013. Characterization of embryogenesis and early larval development in the Pacific triton, Charonia tritonis (Gastropoda: Caenogastropoda). Invertebrate Reproduction & Development, 57(3): 237-246. DOI:10.1080/07924259.2012.753472 (  0) 0) |
 2023, Vol. 22
2023, Vol. 22


