2) Frontiers Science Center for Deep Ocean Multispheres and Earth System (FDOMES), Ocean University of China, Qingdao 266100, China;
3) Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China;
4) Fisheries and Oceans Canada, Pacific Biological Station, Nanaimo, British Columbia V9T 6N7, Canada;
5) College of Marine Sciences, Shanghai Ocean University, Shanghai 201306, China
While many fish species have been heavily exploited and thus depleted worldwide, cephalopods have taken ad-vantage of changing environmental conditions and expanded their populations over the last two decades (Doubleday et al., 2016). The coastal cephalopod catch in the China Seas (Bohai Sea, Yellow Sea, East China Sea, and South China Sea) has generally increased along with a warming environment since 2000, although rates of change are species-dependent (Pang et al., 2018). Octopus in the China Seas play an increasingly important role in the Chi-nese coastal cephalopod fisheries. Their contribution to catch has increased in recent years (Fig. 1) at a rate that is similar to global octopus catch over the last two decades (FAO, 2012).
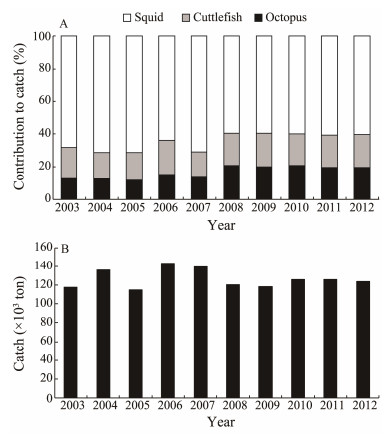
|
Fig. 1 (A) Catch ratio of squid, cuttlefish, and octopus in the Chinese coastal fishery; (B) Catch of octopus in the Chinese coastal fishery. |
The most common octopus species in Chinese coastal areas is Amphioctopus fangsiao, an important commercial species in the Chinese cephalopod fishery that has overtaken golden cuttlefish (Sepia esculenta) and common Chi-nese cuttlefish (Sepiella maindroni de Rochebrune) over the last century. A. fangsiao is mainly distributed along coastal areas of the Western Pacific and is commonly seen from Japanese waters to the China Seas. Coastal octopus is cultured using the cage and otter trawl methods. In the 1970s, A. fangsiao catch during early spring comprised 85% of the annual cephalopod yield in the China Seas (Dong, 2014). However, due to poor species identification in octopus fisheries, the exact number of A. fangsiao caught from year to year is unavailable.
Previous research on A. fangsiao is rather limited and has focused on population genetics analysis and embryonic development for aquaculture purposes (Lu et al., 2010; Dong, 2014). Tank culturing experiments indicate that ma-turation is affected by temperature, food availability and photoperiod (Dong, 2014). Geographical isolation distinguishes multiple stocks due to limited mobility (Lu et al., 2010). It is unclear how many stocks exist in the China Seas. Despite the increasing proportion of octopus catch in recent years, studies on natural populations of A. fangsiao are generally lacking and the mechanism of their do-minance in some coastal areas is unclear.
Haizhou Bay is a shallow open coastal area in the west southern Yellow Sea (Fig. 2). This area is an important spawning and nursery ground for many fish species (Tang et al., 2011; Pang et al., 2015), and one of the most productive fishery grounds in the China Seas (Tang et al., 2011; Zhang, 2013). However, Haizhou Bay has become degraded in recent decades as a result of long-term high fishing pressure (Su et al., 2015). This study was initiated to present a general view on the biology of the A. fangsiao population in Chinese coastal areas, and specifically to characterize the subpopulation in Haizhou Bay. This is the first study to describe the A. fangsiao population growth and distribution pattern in an over-exploited ecosystem.
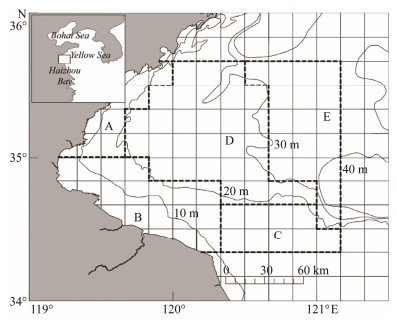
|
Fig. 2 Location and sampling design for the survey in Hai-zhou Bay, China. The five strata (encircled by bold broken lines) are divided based on depth, current, and substrate. |
Octopus samples were collected using a 300 hp survey vessel with a single bottom trawl in Haizhou Bay (Fig. 2) and its vicinity waters (119°20´E-121°10´E, 34°20´N-35°40´N) from 2011 to 2015 (except for 2012). Five seasonal bottom trawl surveys were conducted in 2011, including March and May (spring), July (summer), Septem-ber (autumn), and December (winter). Surveys were only conducted in May and October from 2013 to 2015, which represent spring and autumn. During all surveys, trawling speed was set at 2-3 knots for approximately 60 min tows. In 2011, trawl net width was 25 m, cod end mesh size was 17 mm, and net height was 6 m. From 2013 to 2015, trawl net width was 12 m, and trawl net height was 1.2 m. The survey area included 76 grids (10´ × 10´ each) divided into five strata (A-E) based on depth, current, and substrate (Fig. 2). The numbers of grids randomly selected in each stratum were 3, 5, 3, 9, and 4, respectively, during each survey.
The total number of A. fangsiao was counted and all specimens were weighed. Environmental data including sur-face and bottom water temperatures, surface and bottom water salinities, and the depth were measured by a conductivity, temperature, and depth profiler at the same time and were recorded for each tow. As A. fangsiao is a holobenthic species, we used the bottom water temperature data for further analysis.
2.2 Biological MeasurementsA total of 1389 A. fangsiao individuals were selected randomly from each cruise during the four years of surveys (915, 275, 98, and 101 for 2011, 2003, 2014, and 2015, respectively). The total length and dorsal mantle length were measured to the nearest mm, while the total weight and mantle weight were measured to the nearest gram. Additionally, the gender of 759 individuals in the survey of 2011 were identified by the structure of their reproductive system (López-Peraza et al., 2013).
2.3 Statistical AnalysisThe sex ratio of each sampling month in 2011 was calculated by dividing the number of females with the number of males. Deviation of the sex ratio from 1:1 was checked using the chi-square test with 1 degree of freedom (Microsoft Excel; Microsoft Inc., Redmond, WA, USA). P-values are presented to indicate a deviation from an equal sex ratio.
The relationship between dorsal mantle length (ML) and total weight (W) was determined using the following equation:
| $ W = aM{L^b}, $ |
where a is the coefficient of the arithmetic length-weight relationship and b is the exponent of the length-weight relationship reflecting an isometric (b = 3) or allometric (b ≠ 3) growth pattern (Froese, 2006). Length-weight relationships were determined for males and females, respectively. Length-weight relationships were also compared among the five surveys in 2011 to observe seasonal variation. ML data were grouped in 10 mm bins, and the frequency distribution was modeled using a bimodal distribution for each cruise from 2011 to 2015 (Aizawa, 1999). Peaks of the length distributions from all surveys were used to describe the growth pattern of the A. fangsiao po-pulation by each year.
To calculate catch per unit effort (CPUE), trawl duration was standardized to 60 min with a tow speed of 2 knots for each survey. To compare data from different years and stations, we defined the fishing effort as per standard net mouth area, and the CPUE was estimated as the catch weight per standard net mouth area. The units for CPUE are kilograms per area (kg km-2). The spatial distribution of CPUE in spring (May) and autumn (September/Octo-ber) from each sampling year was identified using Arc-GIS 10.2 to display the annual variation. The reported ma-ximum size of A. fangsiao is 80 mm ML (Villanueva et al., 2016); thus, we roughly divided our samples into small individuals (ML < 40 mm) and large individuals (ML > 40 mm) by half of the maximum ML. Similarly, the seasonal change in spatial distribution of A. fangsiao was compared between small and large individuals using the 2011 data to elucidate possible migration.
3 Results 3.1 Length-Weight RelationshipThe female to male ratio across all years was 0.97:1. The sex ratio by month did not deviate significantly from 1:1, with only a slight bias toward males in July (chi-square test, df = 1, P = 0.997). The length-weight relationships for males and females were (Fig. 3):
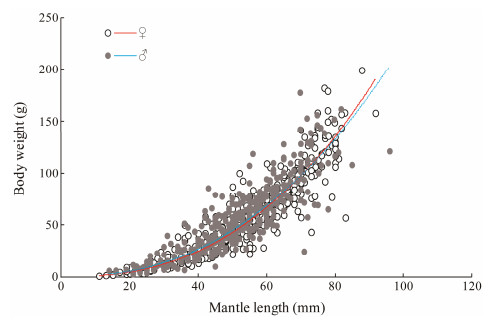
|
Fig. 3 Sex-specific relationships between mantle length and body weight in Amphioctopus fangsiao. |
| $ {W_{{\rm{Male}}}} = 0.0058M{L^{2.2885}} (n = 379, {R^2} = 0.8017) $ |
| $ {W_{{\rm{Female}}}} = 0.003M{L^{2.4451}} (n = 377, {R^2} = 0.8929) $ |
The A. fangsiao length-weight relationships also changed seasonally (Table 1). The allometric coefficient (b) ranged from 1.98 (autumn) to 2.64 (early spring), while parameter a changed from 1.3 (early spring) to 16.1 (autumn).
|
|
Table 1 Seasonal length-weight relationships of Amphioctopus fangsiao collected in 2011 |
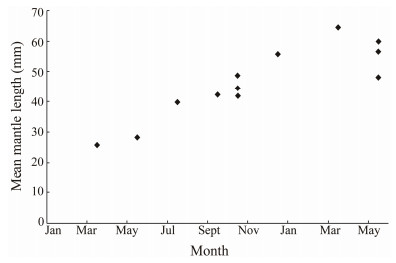
The MLs of octopus were roughly distributed in a Gau-ssian manner (Fig. 4). In 2011, two distinct length class peaks were identified in March, with one at 30 mm ML and another at 70 mm ML. The majority of individuals in May were around 60 mm ML, but another small group of individuals grew from 20 to 40 mm ML in July, to 50 mm ML in September, and 60 mm ML in December. As the largest size group of octopus appeared around March, we speculate that spring is the main spawning season for this species. Different length distribution modes were obser-ved in spring and autumn of other years, suggesting that growth varies by year. We suspect that the life span of this species is around 1 year, and in a year we observed more or less linear growth of A. fangsiao (Fig. 5).
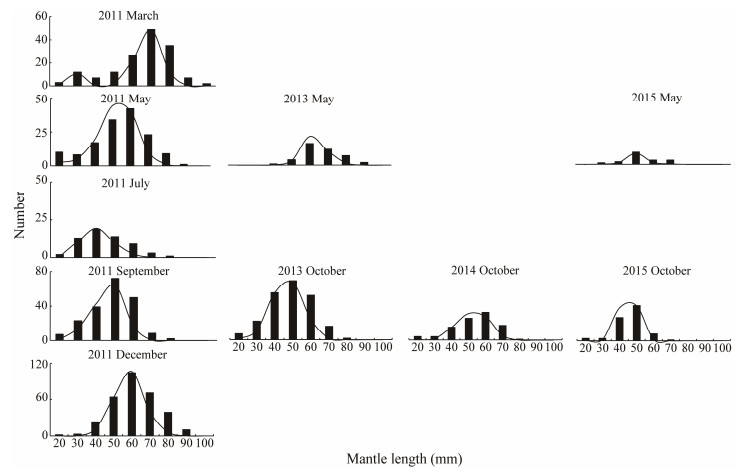
|
Fig. 4 Seasonal distribution of Amphioctopus fangsiao mantle length for all surveys in 4 years (biological data are unavailable for spring 2014). |
|
Fig. 5 Somatic growth of Amphioctopus fangsiao in a year. |
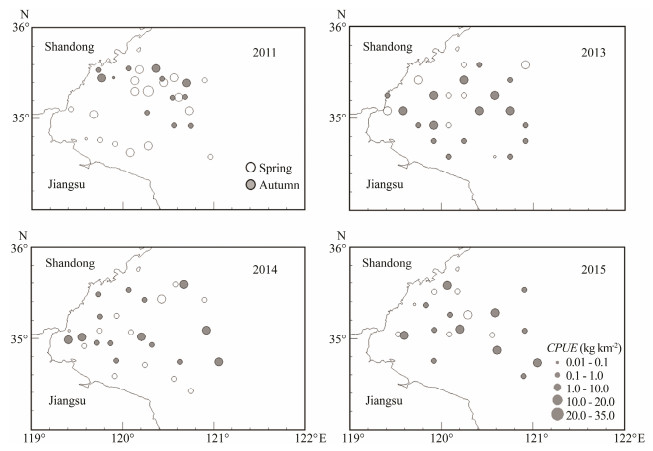
Based on the four 1-year surveys in spring (May) and autumn (Sept/Oct), the octopus population was distrib-uted around the entire bay with relatively stable popula-tion abundance (Fig. 6).
|
Fig. 6 Annual variation in the Amphioctopus fangsiao spatial distribution during spring and autumn; pies indicate the catch per unit effort (CPUE) values (kg km-2). |
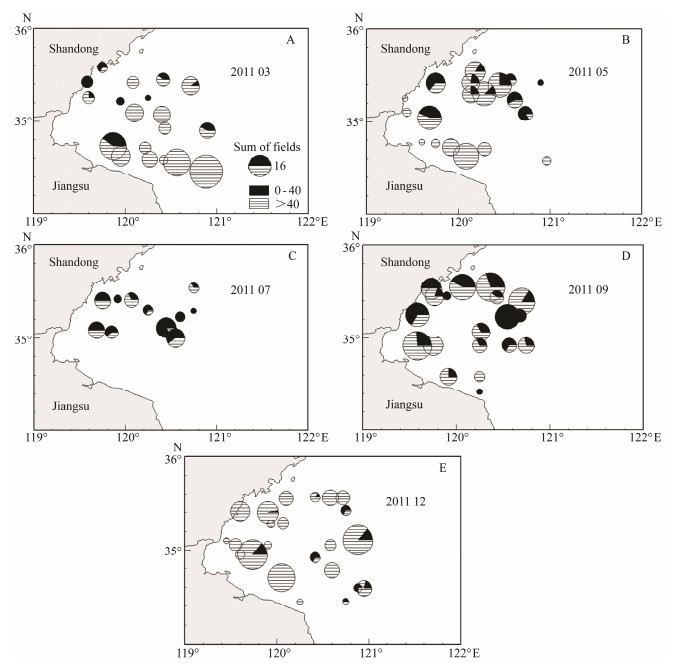
The 2011 survey suggested strong seasonal changes in the spatial distribution for small (ML < 40 mm) and large (ML > 40 mm) individuals (Fig. 7). Small octopus appear-ed in early spring near the coast, whereas the majority of large individuals remained in the outer water (A). As water temperature gradually increased, small octopuses started to occur near the shore, and were rarely found offshore (B). The number of octopus declined greatly due to death after spawning in the summer, and the proportion of small octopus increased (C). Small-size individuals kept grow-ing and spread into the entire bay in September (D). Most of the octopus developed into large individuals in Decem-ber, and only a few small octopus occurred at that time (E).
|
Fig. 7 Seasonal changes in the spatial distribution of Amphioctopus fangsiao by size (small: ML < 40 mm; large: ML > 40 mm) in 2011. Shaded areas refer to the number of small octopus, and dashed areas show the number of large octopus. |
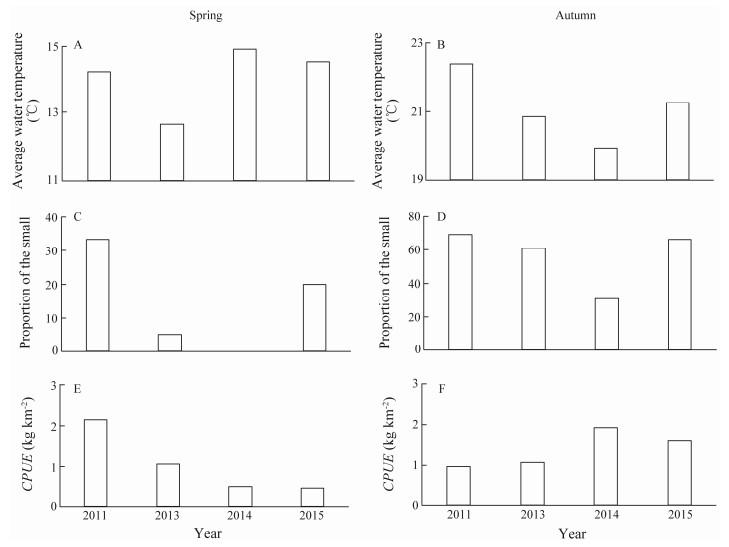
Average water temperature was positively related with the proportion of small individuals in the spring and au-tumn groups (Figs. 8A-D). The autumn groups had a high-er proportion of small individuals than the spring groups during all 4 years (Figs. 8C, D). In contrast, a possible ne-gative relationship was found between autumn CPUE and ambient water temperature and the proportion of the small catch (Figs. 8B, D, F). The spring CPUE was high in 2011, but low in the other years despite relatively high tempera-tures in 2014 and 2015 (Figs. 8A, E).
|
Fig. 8 Annual changes in spring and autumn average water temperature (A, B), proportion of small (ML < 40 mm) (C, D) and catch per unit effort (CPUE) (E, F) during spring and autumn in Haizhou Bay. |
Due to rapid warming and over-exploitation in the Chi-na Seas (Pang et al., 2018), Haizhou Bay has become a degraded ecosystem with depleted fish resources (Tang et al., 2011; Pang et al., 2015). However, A. fangsiao, oc-cupying a relatively high trophic level and preying on a wide range of small fishes, bivalves, and crustaceans (Huang, 2004), has taken advantage of changing environmental conditions and maintained a stable population (Pang et al., 2018).
4.1 Growth, Body Condition, and the Spawning SeasonVariations in the cephalopod sex ratio could be explain-ed by a combination of migration and post-spawning mor-tality. We did not observe a significant sex ratio bias in A. fangsiao, which is common in many other octopus spe-cies (Mangold, 1983). A slight bias towards males in July might be caused by the death of a large number of females after spawning. The R2 value of the length-weight relation-ship for male and female octopus was relatively low, as growth is easily affected by changing environmental condi-tions (Semmens et al., 2004). This result is similar to other species, like common octopus, without a sex bias (Silva et al., 2002; Otero et al., 2007). Growth of O. ocellatus fol-lowed an allometric pattern as inferred by the b-value with-out a significant difference between males and females (Von Boletzky and Villanueva, 2014).
Previous studies indicated that the spawning season of A. fangsiao is mainly in spring (Dong, 2014). Energy con-sumption of female octopus increased greatly during the spawning season (Von Boletzky and Villanueva, 2014), which might be the reason that the majority of individuals in May were smaller than those in March of 2011 (Figs. 4 and 7). At the end of the spawning season, few individuals are left in the water, and juveniles start to appear in early summer (July 2011). In autumn, the population significantly increases with the pulse of new recruitment (Sep-tember 2011), and continues to grow in winter (December 2011). However, small individuals (ML < 40 mm) could be observed all year around in our surveys, even in winter, suggesting that octopus may have an extended spawning season or more than one spawning peak like Octopus vulgaris (Patterson, 1988; Silva et al., 2002). Three O. vulgaris reproductive strategies have been reported in different geographical areas (Katsanevakis and Verriopoulos, 2006), and countless unsynchronized spawning scenarios occur because of its phenotypic plasticity (Garofalo et al., 2010; Angeles-Gonzalez et al., 2017). Therefore, we hypo-thesized that this natural population of A. fangsiao has a comparatively protracted spawning season which is from early spring to late autumn. Although the peaks in the length distribution for the same month changed every year, growth of A. fangsiao displayed a possible linear trend from early spring to winter. Further research on age identification of this species is required to uncover the growth pattern.
Physical condition of cephalopod species varies largely due to different development stages, food availability, go-nad development, and environmental changes (Forsythe et al., 1987, 1988; Semmens et al., 2004; Pecl and Jackson, 2008). In the present study, the length-weight relationship changed with life stage during different seasons, which was reflected by the a and b values (Table 1). Generally, parameter a represents the nutritional condition of an individual (Anderson and Gutreuter, 1983), and b describes the growth pattern (Ricker, 1975). Spawning is a high energy-cost activity and, as expected, the physical con-dition of octopus is the poorest in spring (from March 2011), following nearly isometric growth. The physical con-dition was high due to the recruitment of juvenile octopus in summer (July 2011) and arrived the peak in autumn with extreme allometric growth (September 2011). The phy-sical condition declined and the growth slowed down when migration began in winter (December 2011) and maturation started. The growth pattern and physical condition varied greatly throughout the year. These observations indicate that this octopus species should spawn mainly in spring.
4.2 Seasonal Changes in the Distribution of Small OctopusThe migration pattern of octopus species could be different from that of other fish species. As octopus crawl on the bottom, migration is merely a short-distance movement. There are no accurate records on migration of this species, while similar species, such as common octopus, has been postulated to conduct a spawning migration from offshore to inshore waters in some areas (Guerra, 1981).
As A. fangsiao is a holobenthic species, it might also migrate from offshore water to inshore water through its life based on our results. Mature individuals may migrate to inshore areas for spawning during spring. Then the lar-vae and juveniles grow and develop during the summer and autumn, migrating from inshore to offshore. In winter, the majority of octopus move to offshore water for overwintering along the cold Yellow Sea Coastal Current. How-ever, further studies on recruitment and spatial distribution of spawners are needed to identify nursery and spawn-ing grounds of this species in the Yellow Sea.
4.3 Potential Effects of Water Temperature on Population Growth and BiomassAverage water temperature can lead to the annual vari-ation in growth and biomass of fishes, particularly for fast-growing species with high adaptability. High water temperature can accelerate all aspects of the life cycle of octopus in nature if food is not limited, including hatching rate, growth, maturation, and embryonic development (Forsythe and Hanlon, 1988; Segawa and Nomoto, 2002; Semmens et al., 2004). Although no statistical analysis was conducted in this study, potential association between the water temperature and the population growth and bio-mass could be observed, which can serve as a reference for future studies.
In our study, temperatures in 2011 and 2015 were high-er than in 2013 and 2014. Catches in autumn may have been negatively related to water temperature. Population size in autumn was mainly determined by new hatchlings in spring. The low frequency of small individuals but high CPUE in autumn 2014 suggests that there were more large individuals, which may be due to the extended spawning season in a cold environment. However, the CPUE data were not associated with water temperature in spring, when the majority of spawning occurs. The spawning migration began early and mature individuals came inshore in the warm spring of 2011, while spawning activity may have started late in the cold springs of 2013 and 2014. The po-pulation had not recovered in spring 2015, which may have been caused by uncharacteristically late spawning during the cold 2014.
As seasonal population size and frequency of small A. fangsiao individuals might be affected by relevant water temperature, we suggest that temperature is the main factor affecting the natural population dynamics of octopus (Forsythe and Hanlon, 1988; Angeles-Gonzalez et al., 2017). Additionally, other factors, such as light, food quality, and water currents (Forsythe and Van Heukelem, 1987; Ro-berts and van der Berg, 2002), as well as initial hatchling size and sex ratio are also crucial to the natural dynamics of octopus (Leporati et al, 2007). Therefore, life history traits, such as age, growth, and maturation, should be further studied to better understand the natural population dy-namics. In addition, the influence of fishing activity should be included as a key factor in future work. This will ensure the accurate fishery data and the executive fishery management measures will be implemented in Haizhou Bay, Yellow Sea.
AcknowledgementsThis research was partially supported by the National Key R & D Program of China (Nos. 2018YFD0900902, 2018YFD0900903), the National Natural Science Foundation of China (Nos. 41861134037, 41930534), and the 'Fundamental Research Funds for the Central Universities' to Ocean University of China (Nos. 201762015, 2018 2201). We thank Dr. Robert Boenish (University of Maine) for proofreading this manuscript and giving many valuable suggestions.
Aizawa, Y., 1999. Consideration of the methods for estimating the age-composition from the length frequency data with MS-Excel. Bulletin of the Japanese Society of Fisheries Oceanography, 63: 205-214. (  0) 0) |
Anderson, R. O. and Gutreuter, S. J., 1983. Length, weight, and associated structural indices. Fisheries techniques, American Fisheries Society, Bethesda, EE, UU, 283-300.
(  0) 0) |
Angeles-Gonzalez, L. E., Calva, R., Santos-Valencia, J., Avila-Poveda, O. H., Olivares, A., Diaz, F. and Rosas, C., 2017. Tem-perature modulates spatio-temporal variability of the functional reproductive maturation of Octopus maya (Cephalopo-da) on the shelf of the Yucatan Peninsula, Mexico. Journal of Molluscan Studies, 83(3): 280-288. DOI:10.1093/mollus/eyx013 (  0) 0) |
Dong, G., 2014. The basic biological studies on the artificial re-production of Octopus ocellatus. PhD thesis, Ocean University of China.
(  0) 0) |
Doubleday, Z. A., Prowse, T. A., Arkhipkin, A., Pierce, G. J., Sem-mens, J., Steer, M., Leporati, S. C., Lourenço, S., Quetglas, A., Sauer, W. and Gillanders, B. M., 2016. Global proliferation of cephalopods. Current Biology, 26(10): 406-407. DOI:10.1016/j.cub.2016.04.002 (  0) 0) |
FAO,, 2012. The state of world fisheries and aquaculture. Oppor-tunities and challenges.. Food and Agriculture Organization of the United Nations, Rome, 230pp.
(  0) 0) |
Forsythe, J. W. and Hanlon, R. T., 1988. Effect of temperature on laboratory growth, reproduction and life span of Octopus bi-maculoides. Marine Biology, 98(3): 369-379. DOI:10.1007/BF00391113 (  0) 0) |
Forsythe, J. W., and Van Heukelem, W. F., 1987. Growth. In: Ce-phalopod Life Cycles, Vol. Ⅱ. Comparative Reviews. Boyle, P. R., ed., Academic Press, London, 135-156.
(  0) 0) |
Froese, R., 2006. Cube law, condition factor and weight-length relationships: History, meta-analysis and recommendations. Journal of Applied Ichthyology, 22(4): 241-253. DOI:10.1111/j.1439-0426.2006.00805.x (  0) 0) |
Garofalo, G., Ceriola, L., Gristina, M., Fiorentino, F. and Pace, R., 2010. Nurseries, spawning grounds and recruitment of Oc-topus vulgaris in the Strait of Sicily, central Mediterranean Sea. ICES Journal of Marine Science, 67(7): 1363-1371. (  0) 0) |
Guerra, F., 1981. Structural aspects of stochastic mechanics and stochastic field theory. Physics Reports, 77(3): 263-312. DOI:10.1016/0370-1573(81)90078-8 (  0) 0) |
Huang, M. Z., 2004. Study on feeding habits and nutrient level of four cephalopod species from Taiwan Strait and its adja-cent areas. Journal of Oceanography in Taiwan Strait, 23(3): 331-340 (in Chinese with English abstract). (  0) 0) |
Katsanevakis, S. and Verriopoulos, G., 2006. Modelling the effect of temperature on hatching and settlement patterns of meroplanktonic organisms: The case of octopus. Scientia Marina, 70(4): 699-708. DOI:10.3989/scimar.2006.70n4699 (  0) 0) |
Leporati, S. C., Pecl, G. T. and Semmens, J. M., 2007. Cephalopod hatchling growth: The effects of initial size and seasonal temperatures. Marine Biology, 151(4): 1375-1383. DOI:10.1007/s00227-006-0575-y (  0) 0) |
López-Peraza, D. J., Hernández-Rodríguez, M., Barón-Sevilla, B. and Bückle-Ramirez, L. F., 2013. Histological analysis of the reproductive system and gonad maturity of Octopus ru-bescens. International Journal of Morphology, 31(4): 1459-1469. DOI:10.4067/S0717-95022013000400050 (  0) 0) |
Lü, Z. M., Li, H., Wu, C. W., Fan, Z. J. and Zhang, J. S., 2010. Genetic variation of Octopus ocellatus populations in China's coastal waters based on the COI gene analysis. Acta Ocea-nologica Sinica, 32(1): 130-138. (  0) 0) |
Mangold, K., 1983. Octopus vulgaris. Cephalopod Life Cycles, 1: 335-364. (  0) 0) |
Otero, J., González, Á. F., Sieiro, M. P. and Guerra, Á., 2007. Re-productive cycle and energy allocation of Octopus vulgaris in Galician waters, NE Atlantic. Fisheries Research, 85(1): 122-129. DOI:10.1016/j.fishres.2007.01.007 (  0) 0) |
Pang, Y., Tian, Y., Fu, C., Wang, B., Li, J., Ren, Y. and Wan, R., 2018. Variability of coastal cephalopods in overexploited Chi-na Seas under climate change with implications on fisheries management. Fisheries Research, 208: 22-33. DOI:10.1016/j.fishres.2018.07.004 (  0) 0) |
Pang, Z., Xu, B., Zan, X. and Ren, Y., 2015. Shrimp community structure and its relationships with environmental factors in Haizhou Bay and adjacent waters in spring. Acta Ecologica Sinica, 35(6): 191-195. DOI:10.1016/j.chnaes.2015.09.005 (  0) 0) |
Patterson, K. R., 1988. Life history of Patagonian squid Loligo gahi and growth parameter estimates using least-squares fits to linear and von Bertalanffy models. Marine Ecology Pro-gress Series, 47(1): 65-74. DOI:10.3354/meps047065 (  0) 0) |
Pecl, G. T. and Jackson, G. D., 2008. The potential impacts of climate change on inshore squid: Biology, ecology and fisheries. Reviews in Fish Biology and Fisheries, 18(4): 373-385. DOI:10.1007/s11160-007-9077-3 (  0) 0) |
Ricker, W. E., 1975. Computation and Interpretation of Biological Statistics of Fish Populations. Bulletin of the Fisheries Re-search Board of Canada, Bulletin 191, Ottawa, 382pp.
(  0) 0) |
Roberts, M. J. and van der Berg, M., 2002. Recruitment variability of chokka squid -Role of currents on the Agulhas Bank (South Africa) in paralarval distribution and food abundance. Bulletin of Marine Science, 71(2): 691-710. (  0) 0) |
Segawa, S. and Nomoto, A., 2002. Laboratory growth, feeding, oxygen consumption and ammonia excretion of Octopus ocel-latus. Bulletin of Marine Science, 71(2): 801-813. (  0) 0) |
Semmens, J. M., Pecl, G. T., Villanueva, R., Jouffre, D., Sobrino, I., Wood, J. B. and Rigby, P. R., 2004. Understanding octopus growth: Patterns, variability and physiology. Marine and Fresh-water Research, 55(4): 367-377. DOI:10.1071/MF03155 (  0) 0) |
Silva, L., Sobrino, I. and Ramos, F., 2002. Reproductive biology of the common octopus, Octopus vulgaris Cuvier, 1797 (Cephalopoda: Octopodidae) in the Gulf of Cádiz (SW Spain). Bulletin of Marine Science, 71(2): 837-850. (  0) 0) |
Su, W., Xue, Y., Zhang, C. and Ren, Y., 2015. Spatio-seasonal patterns of fish diversity, Haizhou Bay, China. Chinese Journal of Oceanology and Limnology, 33(1): 121-134. DOI:10.1007/s00343-015-3311-y (  0) 0) |
Tang, F. H., Shen, X. Q. and Wang, Y. L., 2011. Dynamics of fisheries resources near Haizhou Bay waters. Fisheries Science, 30(6): 335-341. (  0) 0) |
Villanueva, R., Vidal, E. A., Fernández-Álvarez, F. Á. and Nabhitabhata, J., 2016. Early mode of life and hatchling size in cephalopod molluscs: Influence on the species distributional ranges. PLoS One, 11(11): e0165334. DOI:10.1371/journal.pone.0165334 (  0) 0) |
Von Boletzky, S., and Villanueva, R., 2014. Cephalopod biology. In: Cephalopod Culture. Iglesias, J., et al., eds., Springer, Dord-recht, 3-16.
(  0) 0) |
Zhang, Y. J., 2013. Spatial and temporal variations of macro-invertebrate community structure and diversity in Haizhou Bay and adjacent waters. Master thesis. Ocean University of Chi-na.
(  0) 0) |
 2020, Vol. 19
2020, Vol. 19


