肺癌是全球第二常见、死亡率居首位的恶性肿瘤,是一种全球性健康问题,给患者个人及其家庭、社会经济发展均造成了沉重负担[1]。2020年,全球累计报告新发肺癌患者220万例,180万人死于肺癌[2]。全球因肺癌造成的疾病负担高达4090万伤残调整寿命年(DALYs)[3]。肺癌患者预后与临床分期紧密相关,IA期患者5年生存率达90%以上,而IV期患者5年生存率在10%以下[4]。因此,早期诊断对于改善肺癌患者预后、提高生存率具有重大意义[5]。
目前,肿瘤生物标记物、影像学技术、支气管镜被广泛用于肺癌早期筛查[6-10];此外,新兴液体活检[11-13]和人工智能、机器学习技术在肺癌早期筛查中均显示较高潜在应用价值[14-16]。既往临床研究显示,低剂量螺旋CT用于肺癌早期筛查、特别是用于重度吸烟者和50岁以上个体肺癌早期筛查的费用-效率显著高于其他早期筛查方法[17-20]。本研究对接受低剂量螺旋CT扫描的无症状居民资料进行回顾性分析,并比较从不吸烟者和曾经吸烟者中肺部阳性结节检出率,从而评估真实世界低剂量螺旋CT用于肺癌早期筛查的价值。
1 对象与方法 1.1 研究对象以2019年1月—2021年12月在上海市浦东新区人民医院接受低剂量螺旋CT肺癌筛查的 ≥ 18岁成人作为研究对象。所有受检者在首次接受低剂量螺旋CT扫描时均无症状且接受包括接受低剂量螺旋CT扫描在内的健康体检。所有接受低剂量螺旋CT扫描的受检者均接受问卷调查,内容包括吸烟情况(从不吸烟者、已戒烟者、现吸烟者)、吸烟量(包·年)、已戒烟者停止吸烟时间,将从未吸烟或迄今吸烟少于100根定义为从不吸烟[21]。排除基线筛查时有既往肺癌史以及吸烟史不明者。根据吸烟史资料,本研究将全部研究对象分为从不吸烟者和曾经吸烟者。本研究获得上海市浦东新区人民医院医学伦理审查委员会审批通过,患者均知情同意且同意发表研究结果。
1.2 低剂量螺旋CT扫描筛查及确诊采用西门子Somatom Definition Flash128排双源CT扫描成像仪,进行肺部非增强低剂量螺旋CT扫描,轴位面、冠状面层厚均为1 mm。全部CT扫描图像均由高年资胸部影像学家阅片,以低剂量螺旋CT扫描图像上出现最大纵径 ≥ 4 mm的非钙化肺结节定义为阳性肺结节[22]。出现阳性肺结节的受检者根据美国国立综合癌症网络(National Comprehensive Cancer Network,NCCN)指南[23]和美国放射学院(American College of Radiology,ACR)制定的肺结节诊断分级系统(Lung-RADs)[24]接受进一步随访观察和诊断学评估。
出现阳性肺结节结果的CT图像由高年资肺科或呼吸内科医师及高年资影像学家共同阅片,出现阳性肺结节的研究对象根据Lung-RADs标准进一步分类。出现多个肺结节时,选择最大单个结节进行分析。收集每个肺结节位置、大小和性质;对CT图像判断结果不一致时,经过讨论后形成最终结果。采用经皮穿刺活检或支气管镜活检进行肺结节病理检测,采用国际肺癌研究协会(International Association for the Study of Lung Cancer,IASLC)和美国癌症联合委员会(American Joint Committee on Cancer,AJCC)肺癌分期系统进行肺癌分期[25]。
1.3 统计分析采用Excel 2020软件建立数据库,应用SPSS 25.0软件进行统计学分析。计量资料以
2019年1月—2021年12月,累计有10285例 ≥ 18岁成人在上海市浦东新区人民医院接受低剂量螺旋CT肺癌筛查,其中926例受检者因既往诊断出肺癌或吸烟史不明而排除出本研究,最终有9359例符合纳入和排除标准的受检者纳入本研究,其中4492例从不吸烟者、4867例曾经吸烟者(图1)。从不吸烟者和曾经吸烟者首次CT筛查年龄、性别构成、体质指数、累计随访时间差异具有统计学意义(P< 0.05),但首次CT筛查肺结节分类、检出阳性肺结节比例差异均无统计学意义(P> 0.05),表1。
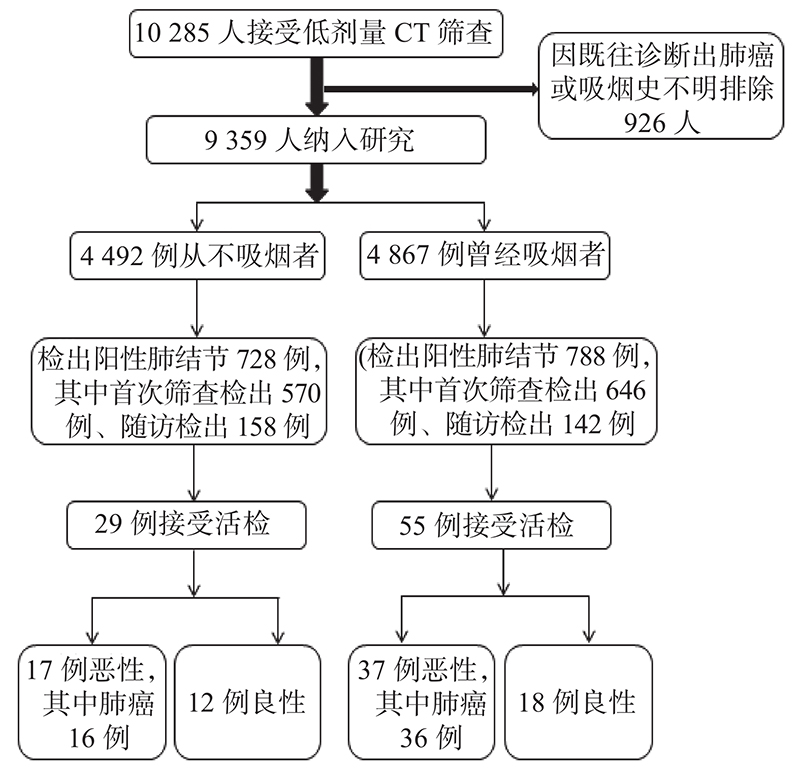
|
图 1 研究流程 Figure 1 Flowchart of the study |
|
|
表 1 研究对象人口学和临床特征 Table 1 Demographic and clinical features of study subjects |
检出肺结节阳性的从不吸烟者和曾经吸烟者首次筛查检出阳性肺结节比例、随访检出阳性肺结节比例、首次CT筛查肺结节分类、检出多发肺结节比例、首次筛查肺结节平均大小差异均无统计学意义(P > 0.05),但肺结节性质差异有统计学意义( χ2 = 47.23,P < 0.01)。此外,检出肺结节阳性的从不吸烟者肺癌发生率显著低于曾经吸烟者( χ2 = 6.42,P < 0.01),表2。
|
|
表 2 肺结节阳性者人口学和临床特征 Table 2 Demographic and clinical features of subjects with positive pulmonary nodules |
本研究旨在比较从不吸烟者和曾经吸烟者低剂量螺旋CT检出肺结节阳性者活检病理结果,结果发现检出阳性肺结节的从不吸烟者接受活检病理检查比例显著低于曾经吸烟者(29/728 vs. 55/788;χ2 = 5.82,P < 0.05),且检出肺结节阳性的从不吸烟者肺癌发生率显著低于曾经吸烟者( χ2 = 6.42,P < 0.01),但两者肺癌假阳性率差异无统计学意义者(29/728 vs. 55/788;χ2 = 0.79,P > 0.05)。从而评估真实世界低剂量螺旋CT用于肺癌早期筛查的价值。
Wisnivesky等[17]研究发现,单次低剂量螺旋CT扫描筛查肺癌每挽救1年寿命需花费2500美元,每增加230美元成本预计可延长0.1年生存期,其成本-效益比显著高于其他肺癌筛查方法。Jonas等[26]研究发现,对高危人群采用低剂量CT扫描筛查可降低肺癌死亡率,但带来的假阳性率结果可造成不必要检测、侵入性诊断、过度诊断、忧虑情绪增加以及放射性肿瘤。唐威等[27]对4 690例 ≥ 40岁的无症状者采用低剂量CT检查,并根据性别、吸烟和被动吸烟史将受检人群分为高危组、中危组和低危组,发现低剂量CT扫描用于肺癌早诊率达76%,且高危组、中危组和低危组肺癌检出率差异无统计学意义(P = 0.054)。对2011年6月—2012年12月在上海中山医院行胸部低剂量CT检查的2251例无症状人群筛查结果分析发现,与吸烟相关的肺癌高、中、低危组中肺结节检出率分别为8.8%、9.5%和10.1%(P = 0.864),且9.9%受检者至少可以检出1个阳性肺结节,提示低剂量螺旋CT在肺癌筛查中具有较高应用价值[28]。对上海市闵行区7496例年龄 ≥ 40岁、肺癌家族史、职业接触史、吸烟 > 20包/年的吸烟史等社区肺癌高危人群进行胸部低剂量CT检查,发现14.1%居民有肺部非钙化结节( ≥ 4 mm),且年龄 ≥ 55岁居民阳性肺结节检出率显著高于年龄 < 55岁者(14.9% vs. 9.7%;χ2 = 22.27,P < 0.001),表明胸部低剂量CT筛查有助于提高肺癌早期诊断率和非钙化结节检出率,特别值得在 ≥ 55岁社区居民中推广应用 [29]。近期一项纳入11个随机对照临床研究、涵盖94445例研究对象的meta分析结果显示,采用低剂量CT筛查肺癌可显著降低年龄 ≥ 40岁且偶遇显著吸烟暴露史的高危人群肺癌相关死亡率[30]。
本研究结果表明,从不吸烟者和曾经吸烟者首次CT筛查肺结节分类、检出阳性肺结节比例差异均无统计学意义(P > 0.05),与既往研究结果一致 [27-28]。但肺结节阳性的从不吸烟者和曾经吸烟者肺结节性质及肺癌发生率差异均有统计学意义(P < 0.01),提示曾经吸烟者检出肺癌比例更高,应尽早对这些高危人群开展肺癌筛查,以提高检出率、降低死亡率。
本研究存在如下不足:①本研究是单中心回顾性研究,低剂量螺旋CT筛查和随访策略未予严格控制。② 本研究未对从不吸烟者接受低剂量螺旋CT肺癌筛查和未接受筛查效果进行比较,因而不能提供从不吸烟者接受低剂量螺旋CT肺癌筛查的直接证据。③ 鉴于未能获得二手烟暴露、大气污染等肺癌可能危险因素数据,这可能会成为从不吸烟者肺癌筛查的影响因素。④ 本研究系基于医院的调查,故可能存在选择性偏倚。
本研究结果表明,胸部低剂量螺旋CT筛查可显著提升从不吸烟者肺结节检出率,虽然其肺癌检出率低于曾经吸烟者,但胸部低剂量螺旋CT筛查用于筛查肺癌的假阳性率与曾经吸烟者差异无统计学意义。鉴于胸部低剂量螺旋CT的肺癌筛查效能,其值得在无症状成人中用于肺癌筛查。
| [1] |
Mohindra NA, Patel JD. Top advances in lung cancer, 2021[J]. Cancer, 2022, 128(19): 3434-3437. DOI:10.1002/cncr.34406 |
| [2] |
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. DOI:10.3322/caac.21660 |
| [3] |
Zhang JR, Li JF, Xiong S, et al. Global burden of lung cancer: implications from current evidence[J]. Ann Cancer Epidemiol, 2021, 5: 4. DOI:10.21037/ace-20-31 |
| [4] |
Ning J, Ge T, Jiang ML, et al. Early diagnosis of lung cancer: which is the optimal choice?[J]. Aging, 2021, 13(4): 6214-6227. DOI:10.18632/aging.202504 |
| [5] |
Cainap C, Pop LA, Balacescu O, et al. Early diagnosis and screening in lung cancer[J]. Am J Cancer Res, 2020, 10(7): 1993-2009. |
| [6] |
Seijo LM, Peled N, Ajona D, et al. Biomarkers in lung cancer screening: Achievements, promises, and challenges[J]. J Thorac Oncol, 2019, 14(3): 343-357. DOI:10.1016/j.jtho.2018.11.023 |
| [7] |
Tammemagi MC, Lam S. Screening for lung cancer using low dose computed tomography[J]. BMJ, 2014, 348: g2253. DOI:10.1136/bmj.g2253 |
| [8] |
Xiang D, Zhang BC, Doll D, et al. Lung cancer screening: from imaging to biomarker[J]. Biomark Res, 2013, 1(1): 4. DOI:10.1186/2050-7771-1-4 |
| [9] |
El-Bayoumi E, Silvestri GA. Bronchoscopy for the diagnosis and staging of lung cancer[J]. Semin Respir Crit Care Med, 2008, 29(3): 261-270. DOI:10.1055/s-2008-1076746 |
| [10] |
Mascaux C, Peled N, Garg K, et al. Early detection and screening of lung cancer[J]. Expert Rev Mol Diagn, 2010, 10(6): 799-815. DOI:10.1586/erm.10.60 |
| [11] |
Li W, Liu JB, Hou LK, et al. Liquid biopsy in lung cancer: significance in diagnostics, prediction, and treatment monitoring[J]. Mol Cancer, 2022, 21(1): 25. DOI:10.1186/s12943-022-01505-z |
| [12] |
Pisapia P, Malapelle U, Troncone G. Liquid biopsy and lung cancer[J]. Acta Cytol, 2019, 63(6): 489-496. DOI:10.1159/000492710 |
| [13] |
Guibert N, Pradines A, Favre G, et al. Current and future applications of liquid biopsy in nonsmall cell lung cancer from early to advanced stages[J]. Eur Respir Rev, 2020, 29(155): 190052. DOI:10.1183/16000617.0052-2019 |
| [14] |
Zhang K, Chen KZ. Artificial intelligence: opportunities in lung cancer[J]. Curr Opin Oncol, 2022, 34(1): 44-53. DOI:10.1097/CCO.0000000000000796 |
| [15] |
Ardila D, Kiraly AP, Bharadwaj S, et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography[J]. Nat Med, 2019, 25(6): 954-961. DOI:10.1038/s41591-019-0447-x |
| [16] |
Avanzo M, Stancanello J, Pirrone G, et al. Radiomics and deep learning in lung cancer[J]. Strahlenther Onkol, 2020, 196(10): 879-887. DOI:10.1007/s00066-020-01625-9 |
| [17] |
Wisnivesky JP, Mushlin AI, Sicherman N, et al. The cost-effectiveness of low-dose CT screening for lung cancer: preliminary results of baseline screening[J]. Chest, 2003, 124(2): 614-621. DOI:10.1378/chest.124.2.614 |
| [18] |
Oudkerk M, Liu SY, Heuvelmans MA, et al. Lung cancer LDCT screening and mortality reduction - evidence, pitfalls and future perspectives[J]. Nat Rev Clin Oncol, 2021, 18(3): 135-151. DOI:10.1038/s41571-020-00432-6 |
| [19] |
Yuan JM, Sun Y, Wang K, et al. Cost effectiveness of lung cancer screening with low-dose CT in heavy smokers in China[J]. Cancer Prev Res (Phila), 2022, 15(1): 37-44. DOI:10.1158/1940-6207.CAPR-21-0155 |
| [20] |
Azar FE, Azami-Aghdash S, Pournaghi-Azar F, et al. Cost-effectiveness of lung cancer screening and treatment methods: a systematic review of systematic reviews[J]. BMC Health Serv Res, 2017, 17(1): 413. DOI:10.1186/s12913-017-2374-1 |
| [21] |
Lam S. Lung cancer screening in never-smokers[J]. J Thorac Oncol, 2019, 14(3): 336-337. DOI:10.1016/j.jtho.2018.12.019 |
| [22] |
Wood DE. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for Lung Cancer Screening[J]. Thorac Surg Clin, 2015, 25(2): 185-197. DOI:10.1016/j.thorsurg.2014.12.003 |
| [23] |
Godoy MCB, Odisio EGLC, Erasmus JJ, et al. Understanding Lung-RADS 1.0: A case-based review[J]. Semin Ultrasound CT MRI, 2018, 39(3): 260-272. DOI:10.1053/j.sult.2018.03.001 |
| [24] |
Matos Figueroa JR, Vázquez Torres OL, Hernández I, et al. PET-CT scan positive pulmonary nodule revealing histoplasmosis: a case report[J]. Bol Asoc Med P R, 2010, 102(4): 47-50. |
| [25] |
Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer[J]. J Thorac Oncol, 2016, 11(1): 39-51. DOI:10.1016/j.jtho.2015.09.009 |
| [26] |
Jonas DE, Reuland DS, Reddy SM, et al. Screening for lung cancer with low-dose computed tomography: Updated evidence report and systematic review for the US Preventive Services Task Force[J]. JAMA, 2021, 325(10): 971-987. DOI:10.1001/jama.2021.0377 |
| [27] |
唐威, 吴宁, 黄遥, 等. 4690例无症状健康体检者低剂量CT早期肺癌筛查研究[J]. 中华肿瘤杂志, 2014, 36(7): 549-554. Tang W, Wu N, Huang Y, et al. Results of low-dose computed tomography (LDCT)screening for early lung cancer: prevalence in 4 690 asymptomatic participants[J]. Chin J Oncol, 2014, 36(7): 549-554. DOI:10.3760/cma.j.issn.0253-3766.2014.07.016 |
| [28] |
张勇, 洪群英, 施伟斌, 等. 低剂量螺旋CT在肺癌筛查中的应用价值[J]. 中华医学杂志, 2013, 93(38): 3011-3014. Zhang Y, Hong QY, Shi WB, et al. Value of low-dose spiral computed tomography in lung cancer screening[J]. Nat Med J China, 2013, 93(38): 3011-3014. DOI:10.3760/cma.j.issn.0376-2491.2013.38.002 |
| [29] |
谢欣, 许慧琳, 严玉洁, 等. 低剂量螺旋CT对7496名社区肺癌高危人群的筛查研究[J]. 中国肿瘤, 2015, 24(10): 811-814. Xie X, Xu HL, Yan YJ, et al. Lung cancer screening using low-dose computed tomography in 7496 community residents with high-risk[J]. Chin Cancer, 2015, 24(10): 811-814. DOI:10.11735/j.issn.1004-0242.2015.10.A002 |
| [30] |
Bonney A, Malouf R, Marchal C, et al. Impact of low-dose computed tomography (LDCT) screening on lung cancer-related mortality[J]. Cochrane Database Syst Rev, 2022, 8(8): CD013829. DOI:10.1002/14651858.CD013829.pub2 |




