2. 南京中医药大学药学院, 江苏省中药药效与安全性评价重点实验室, 江苏 南京 210023;
3. 南京中医药大学, 江苏省中医药与再生医学研究国际合作联合实验室, 江苏 南京 210023;
4. 南京中医药大学, 江苏省中医药防治肿瘤协同创新中心, 江苏 南京 210023
2. Jiangsu Key Laboratory for Pharmacology and Safety Evaluation of Chinese Materia Medica, School of Pharmacy, Nanjing University of Chinese Medicine, Nanjing 210023, China;
3. Jiangsu International Cooperative Laboratory of Traditional Chinese Medicine and Regenerative Medicine Research, Nanjing University of Chinese Medicine, Nanjing 210023, China;
4. Jiangsu Collaborative Innovation Center of Traditional Chinese Medicine Prevention and Treatment of Tumor, Nanjing University of Chinese Medicine, Nanjing 210023, China
免疫检查点是对免疫细胞的功能发挥负调控作用的一类分子, 是机体维持免疫稳态的重要途径。肿瘤可以激活免疫检查点, 劫持这类免疫抑制分子, 抵抗肿瘤抗原特异性T细胞的杀伤作用, 实现免疫逃逸。已有研究表明, 常见的免疫检查点分子在恶性肿瘤中的表达往往显著上调, 并与不良预后相关[1, 2]。这些免疫检查点包括: 程序性死亡受体1 (programmed cell death protein 1, PD-1)/程序性死亡配体1 (programmed cell death-ligand 1, PD-L1)、细胞毒性T淋巴细胞相关抗原4 (cytotoxic T lymphocyte-associated antigen-4, CTLA-4)、T细胞表面抑制性分子3 (T cell immunoglobulin domain and mucin domain-3, TIM-3)、吲哚胺2, 3-双加氧化酶(indoleamine-2, 3-dioxygenase, IDO)、淋巴细胞活化基因3蛋白(lymphocyte activation gene 3 protein, LAG-3) 等。目前, 针对免疫检查点使用相应抑制剂的阻断疗法, 已在多种肿瘤治疗中展示出显著疗效[3-5]。然而, 近年来的一些临床数据表明, 免疫检查点抑制剂在肿瘤治疗中也存在响应率低与耐药性的问题[6], 此类问题的出现往往与免疫检查点的异常表达相关。因此, 明确免疫检查点在肿瘤中的表达调控机制及提高免疫检查点抑制剂的疗效逐渐成为肿瘤免疫治疗的研究热点。
肿瘤微环境(tumor microenvironment, TME) 是肿瘤细胞与周围血管系统、免疫细胞、成纤维细胞和细胞外基质相互作用的动态环境[7]。肿瘤的生长代谢异常, 通常具有独特的微环境特征, 如缺氧、低pH和氧化应激等[8]。氧化应激是指暴露在高水平活性氧(reactive oxygen species, ROS) 下细胞氧化还原状态的失衡。ROS是介导氧化应激的重要介质, 同时也是调节免疫检查点表达的重要分子。肿瘤免疫应答过程中肿瘤细胞和免疫细胞的代谢重编程导致胞浆和线粒体ROS产生过多, 这与NADPH氧化酶(NADPH oxidases, NOX) 的激活有关, 可导致氧化应激状态[9]。已有大量研究证明氧化应激会引起免疫细胞的功能改变[10, 11]。ROS可以通过表观遗传修饰及上调核因子κB (nuclear factor-κB, NF-κB)、核因子E2相关因子2 (nuclear factor erythroid 2-related factor 2, Nrf2) 等转录因子, 诱导PD-L1、CTLA-4等免疫检查点的表达[12], 抑制T细胞活化; 此外, ROS也可以改变肿瘤细胞表面主要组织相容性复合体I类(major histocompatibility complex-I, MHC-I)、整合素相关蛋白(integrin-associated protein, 或被称为cluster of differentiation 47, 以下简称为CD47) 与自然杀伤细胞、巨噬细胞等免疫细胞的相互作用, 介导机体免疫系统对肿瘤细胞的杀伤作用。通过查阅近年来相关文献, 本文对ROS调控肿瘤免疫检查点作用及机制进行综述。
1 TME中ROS的来源、功能与特点ROS是以多种形式的氧自由基和非氧自由基形式存在的含氧化合物的总称, 包括超氧阴离子、羟自由基、过氧化氢及脂质过氧化物, 其具有比分子氧更活泼的化学性质[13]。TME中ROS的产生有3种方式, 即线粒体电子传递链、细胞膜上NOX蛋白介导的电子传递和细胞内的芬顿反应(图 1)。线粒体是ROS产生的重要部位。在线粒体电子传递链中, 如果氧供应不足, 那么电子传递将受阻, 部分单电子从复合物I (NADH-泛醌氧化还原酶) 和复合物Ⅲ (泛醌-细胞色素氧化还原酶) 中漏出, 介导分子氧还原为ROS, 进而导致产生的ROS扩散到细胞质中[14]。ROS参与氧化应激和信号转导等过程, 可以调节细胞的增殖、分化、存活甚至凋亡过程[15]。NOX是一类跨膜蛋白, 可以通过生物膜传递电子, 并催化氧转化为超氧化物, 超氧化物歧化酶进一步还原超氧化物以产生ROS[16]。在TME中, 肿瘤细胞产生的大量生长因子、趋化因子、炎症细胞因子等通过激活NOX, 促进肿瘤及其相关免疫细胞产生ROS[17]。由于肿瘤增殖的需氧量远超于其组织内的供氧量, 因此造就了肿瘤生长常见的缺氧微环境特征。缺氧的微环境激活了肿瘤细胞内的缺氧诱导转录因子, 该转录因子经活化后转移至细胞核中启动多种参与葡萄糖代谢、血管生成功能相关基因的转录表达, 以满足肿瘤细胞适应低氧浓度生长[18]。另外, 由于肿瘤组织代谢活性高, 血流灌注不足, 酸性代谢物积聚在TME中。因此, 酸性环境下肿瘤相关免疫细胞内超负荷的铁离子与过氧化氢生成强氧化能力的羟自由基(即芬顿反应), 并引发更多的其他ROS[19]。
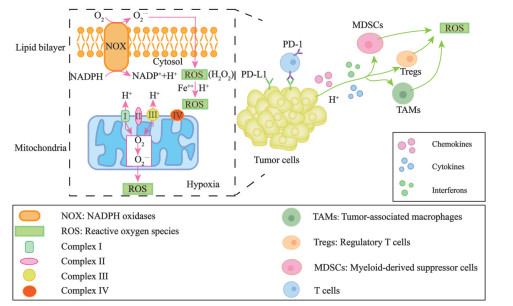
|
Figure 1 The production of ROS in tumor cells in tumor microenvironment (TME). There are three ways: ① NOX in cellular membrane; ② Fenton reaction in which hydrogen peroxide is converted into hydroxyl free radical; ③ Mitochondria electron transport chain |
虽然ROS是肿瘤细胞在缺氧、酸性等环境中重要的信号传递分子, 但是高浓度的ROS亦可以氧化细胞内的脂质、蛋白质和核酸, 从而导致细胞毒性[20]。与癌旁组织相比, TME中的ROS浓度可高达几十倍[21]。TME中免疫细胞和肿瘤细胞持续受到ROS的影响会发生功能失调的情况。肿瘤细胞已经进化出许多抗氧化防御机制来逃避氧化应激引起的损伤[22]。然而, 大多数免疫细胞的抗氧化能力低于肿瘤细胞, 容易受到ROS诱导的损伤, 导致细胞功能障碍和细胞凋亡, 因此, 高浓度的ROS是造成TME中免疫抑制的主要原因之一[23]。此外, ROS诱导的免疫检查点上调也是免疫细胞功能发挥受到抑制的重要因素。已有研究表明, ROS是肿瘤进展的促进剂, 它可以通过抑制免疫细胞的功能来促进肿瘤的发生发展[24]。
2 ROS调控和影响免疫检查点的分布TME中除了肿瘤细胞, 还存在多种免疫细胞。这些免疫细胞上表达的免疫检查点分子PD-1、CTLA-4等可以抑制免疫活化, 抑制机体对肿瘤的免疫监视。肿瘤细胞亦会表达免疫检查点分子, 如PD-L1、IDO[25, 26], 这些免疫检查点阻断了肿瘤免疫中的抗原提呈过程, 使抗原不能被提呈至T细胞, 从而抑制T细胞的免疫功能。
氧化还原信号可以修饰代谢酶和转录因子的活性, 以调节免疫检查点基因的转录表达、运输转运、细胞定位或与其他蛋白的相互作用[27]。PD-L1是典型免疫球蛋白样胞外区的膜蛋白受体, 在多种恶性肿瘤细胞表面上调表达, 负责与胞内区进行相互作用和信号转导[28, 29]。研究表明, 非组蛋白的赖氨酸乙酰化可以影响蛋白质稳定性或亚细胞定位, ROS可能通过氧化还原的方式修饰p300乙酰转移酶, 使PD-L1细胞质结构域的Lys 263处乙酰化, 从而阻断PD-L1的核定位[30]。CTLA-4主要位于FoxP3+调节性T细胞(Treg) 或激活的CD4+、CD8+ T细胞的胞内囊泡中, 并以同源二聚体的形式存在。由于细胞膜的结构性内吞作用, 大约有90%的CTLA-4位于细胞内[31, 32]。CTLA-4细胞质区域中含有酪氨酸的基序, 当酪氨酸残基被氧化发生磷酸化时CTLA-4的内化作用将受到抑制[33]。TIM-3是一种含有免疫球蛋白和黏蛋白结构域的细胞表面分子, 存在于多种类型的免疫细胞中, 包括T细胞、Treg细胞、树突状细胞、B细胞、巨噬细胞、NK细胞和肥大细胞等[34]。TIM-3胞内尾巴具有高度保守的酪氨酸, 当被白介素诱导性T细胞激酶特异性磷酸化, 可参与调节T细胞下游信号传导[35]。TME内产生的ROS具有强氧化性, 可能会影响白介素诱导性T细胞激酶的活性, 阻碍TIM-3参与下游信号转导。吲哚胺2, 3-双加氧酶1 (IDO1) 是L-色氨酸沿犬尿氨酸途径代谢的关键限速酶, 在肿瘤微环境中产生免疫抑制作用, 促进肿瘤生长[36]。研究发现, IDO1与IA类磷酸肌醇3-激酶相互作用, 后者激活可促进IDO1从细胞质转移到早期内体(接受内吞物质并对其进行分类的细胞隔室, 用于囊泡运输到晚期内体和溶酶体, 及将物质再循环到质膜), 这是免疫调节IDO1信号传导所必需的步骤[37], IDO1和IA类磷酸肌醇3-激酶的本质都是蛋白质, 在氧化环境下蛋白质的氨基酸序列可能受影响。LAG-3是一种I型跨膜蛋白, 通过二聚化方式存在于活化的NK细胞和T细胞表面[38]。而在静息状态的细胞中, LAG-3常常在溶酶体内降解。研究表明, LAG-3从溶酶体到细胞表面的运输依赖于其胞质内的结构域, 与蛋白激酶C信号传导相关[39], 该过程可受ROS介导。
3 TME中ROS调控免疫检查点表达的作用及机制TME中免疫检查点的表达受多种因素调节, 如缺氧调节糖酵解途径并驱动上皮-间质转化和其他生物途径, 从而上调CTLA-4、PD-1、PD-L1、LAG-3、TIM-3和其他免疫检查点, 干扰免疫效应细胞的抗肿瘤反应, 并为肿瘤逃避免疫监视提供了便利条件[40]; 在TME中, 浸润的髓系细胞(如肿瘤相关巨噬细胞和肿瘤相关嗜中性粒细胞等) 和Treg细胞对免疫检查点起着重要的调节作用, 以促进肿瘤进展并调节肿瘤浸润淋巴细胞的功能[41, 42]; 此外, 检查点阻断疗法的免疫活性和抗肿瘤作用与患者的肠道菌群及瘤内共生菌有关[43, 44]。ROS在调节免疫检查点表达中同样起到重要作用。ROS可以通过DNA甲基化、组蛋白修饰和转录激活3种主要途径调控免疫检查点的表达(图 2)。下文将从这些途径进行介绍。
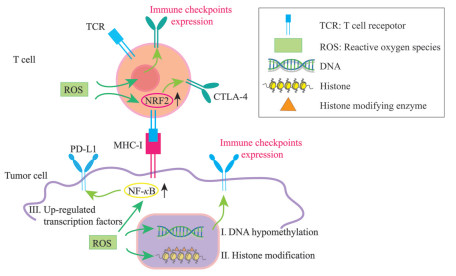
|
Figure 2 Schematic model of regulation of ROS on immune checkpoints in tumor microenvironment. The regulation can be fulfilled through: (I) DNA hypomethylation, (II) histone modification, and (III) up-regulated transcription factors |
氧化应激可引发表观遗传改变, 表观遗传是指DNA序列不发生变化, 单基因表达却发生了可遗传的改变, 在氧化应激诱导的表观遗传变化中, 最重要的机制之一是DNA甲基化[45]。DNA甲基化是指DNA复制后, 经DNA甲基转移酶的催化, 将S-腺苷酰-L-甲硫氨酸上的甲基基团连接到DNA分子腺嘌呤碱基或胞嘧啶碱基上, 进行DNA修饰的过程[46]。ROS的积累与DNA甲基化模式相关, 特别是·OH自由基引起的DNA损伤, 会影响DNA作为甲基转移酶的底物, 从而使基因组整体甲基化水平降低[47]。在各种恶性肿瘤的TME中, 免疫检查点和配体的DNA甲基化是研究最多的表观遗传学改变之一, 这种典型修饰限于胞嘧啶-鸟嘌呤二核苷酸(CpG-oligonucleotide, CpG) 岛。在不同的恶性肿瘤中, 免疫检查点和配体的启动子区域明显存在DNA低甲基化, 并与其在TME中表达的增加有关。在黑色素瘤中, 基因组及其重复序列中整体DNA甲基化的缺失导致PD-L1的结构性表达上调, 从而抑制抗肿瘤免疫反应[48]。另一项关于慢性淋巴细胞白血病的研究表明, PD-1中3个区域(内含子、启动子和增强子) 的低甲基化与其异常表达有关[49]。此外, 与正常组织相比, 非小细胞肺癌中PD-1和CTLA-4都是低甲基化的, 这与它们在肿瘤组织中表达上调呈正相关[50]。这些数据表明, 促进DNA低甲基化的药物与免疫检查点抑制剂联合使用可以增强抗肿瘤反应, 因为前者可能增加免疫检查点在TME中的表达, 这有可能增强免疫检查点抑制剂的作用。
3.2 ROS通过组蛋白修饰调控免疫检查点组蛋白修饰如乙酰化、甲基化和磷酸化等在控制染色质结构和基因活性方面具有不同的功能。有证据表明氧化应激通过影响组蛋白修饰酶的活性, 从而改变组蛋白修饰的模式[51], 如氧化应激下, 半胱氨酸氧化形成的活性醛直接抑制染色质重塑蛋白——I类组蛋白脱乙酰酶, 进而调控组蛋白乙酰化修饰[52-54]。组蛋白甲基化也可以间接地受到影响, 当哺乳动物细胞中主要抗氧化剂谷胱甘肽的氧化还原水平发生失衡, 谷胱甘肽耗竭或其氧化会限制S-腺苷蛋氨酸的生物利用度。因此, 氧化应激会降低细胞中S-腺苷蛋氨酸的水平, 从而降低组蛋白甲基转移酶产生组蛋白甲基化的能力[55-57]。组蛋白修饰是调控TME中基因表达的重要因素, ROS可能通过降低组蛋白脱乙酰酶的活性及组蛋白产生甲基化的能力从而上调免疫检查点的表达。已有报道, 组蛋白乙酰化可增强免疫检查点及其配体在细胞表面的表达, 如组蛋白脱乙酰酶抑制剂上调黑色素瘤中PD-L1的表达[58]。值得注意的是, 对肺癌和黑色素瘤的临床前研究表明, 在组蛋白脱乙酰化酶抑制剂治疗下, T细胞趋化因子的上调表达可以增强PD-1/PD-L1免疫阻断治疗[58, 59]。此外, 组蛋白甲基化也与TME内免疫检查点表达上调有关。在乳腺癌TME中, 与正常组织相比, 抑制性组蛋白H3K9me3和H3K27me3在PD-1、CTLA-4、TIM-3和LAG-3启动子区域的丰度显著降低[60]。此外, 在大肠癌TME中, H3K27me3和H3K9me3与CTLA-4、PD-1、TIGIT启动子区域的结合显著降低[61]。这些研究表明, 免疫检查点启动子区域中抑制性组蛋白甲基化标记的丰度可能是TME内免疫检查点上调的重要原因之一。
3.3 ROS通过转录因子调控免疫检查点ROS通过激活或抑制众多转录因子的各种信号通路改变了宿主的分子、结构和功能特性[62]。如Nrf2是一种典型的应激响应型转录因子, 通过与富含半胱氨酸的KELCH样ECH关联蛋白1 (recombinant Kelch like ECH associated protein 1, KEAP1) 重组蛋白结合感知细胞氧化还原状态的变化。KEAP1结合可导致Nrf2泛素化和蛋白酶体降解。在ROS水平升高时, KEAP1中半胱氨酸的氧化诱导构象变化, 其释放Nrf2并允许其易位到细胞核[63]。研究表明, 氧化应激状态下Nrf2/抗氧化响应元件(antioxidant response element, ARE) 信号通路被激活, Nrf2会结合到CTLA-4上游的ARE元件上, 进而启动ARE调控CTLA-4的表达升高[64]。另外, NF-κB可以被抑制性复合物IκB解体的亚基所激活, 这是在受到氧化应激的刺激下, 活化的激酶磷酸化IκB并经历泛素化, 引发其降解, 导致P65和P50亚基的释放, P65和P50二聚化单元进入细胞核并与其靶基因启动子中存在的κB基序结合, 刺激靶基因转录[65]。研究表明, NF-κB通过与PD-L1启动子结合直接诱导PD-L1基因转录, 并且还可以通过间接途径在转录后上调PD-L1[66]。
研究表明, ROS还可以促进芳香烃受体(aryl hydrocarbon receptor, AhR) 的表达[67], 通过AhR信号直接诱导人胶质母细胞瘤和肝细胞癌细胞系中的PD-L1及IDO1的表达[68, 69]。在重复刺激T细胞的体外模型中, 细胞能量供应所必需的烟酰胺可以防止T细胞耗竭。研究发现, 烟酰胺可能作为NAD+/NADH前体参与许多氧化还原反应, 通过氧化磷酸化过程降低ROS的水平, 从而下调ROS介导的胸腺细胞选择相关HMG盒蛋白(thymocyte selection-associated HMG box protein, TOX) 的表达, 导致抑制性受体如LAG-3、TIM-3、PD-1发生下调[70]。髓源抑制性细胞(myeloid-derived suppressor cells, MDSCs) 通过TIGIT/CD155信号通路抑制NK细胞的增殖、杀伤功能[71]。有研究显示在不同的肿瘤模型中, MDSCs通过增加NADPH氧化酶活性显著上调ROS, 从而发生氧化应激[72], CD155基因启动子包含核呼吸因子1 (nuclear respiratory factor-1, NRF-1) 的结合位点, NRF-1是一种受氧化应激调节的转录因子, CD155的表达可能间接受到氧化应激的控制[73], 因此, 逆转MDSCs的抑制作用可通过阻断NK细胞上的TIGIT或抑制MDSCs产生ROS。
3.4 ROS通过其他途径调控免疫检查点除以上讨论, 还有研究指出了原癌基因K-ras通过氧化还原介导的机制显著增强PD-L1的表达。K-ras突变是与侵袭性恶性表型相关的癌症发生的主要基因事件, 研究通过激活K-ras促进ROS的产生, 生成的ROS可以激活成纤维细胞生长因子受体1 (fibroblast growth factor receptor 1, FGFR1), 导致PD-L1的表达显著上调[74]。ROS与钙离子之间存在相互作用, TME中ROS可氧化修饰线粒体、内质网等的钙离子通道, 升高钙离子水平。反过来, 钙离子信号可以参与ROS水平升高的调节[75]。钙离子结合钙调蛋白(calmodulin, CaM) 激活钙调磷酸酶可诱导静息状态下T细胞上调CTLA-4的表达, 这提示ROS可能参与Ca2+/CaM信号转导途径上调CTLA-4的表达[76]。MHC-I在包括肿瘤细胞在内的所有细胞上均有表达, 并在免疫应答过程中参与抗原识别, 研究表明人癌细胞通过降低自身表面的MHC-I表达水平逃避T细胞攻击[77]。在卵巢癌模型中, ROS刺激树突细胞发生脂质过氧化反应形成脂质过氧化产物。脂质过氧化产物激活内质网应激反应因子X-box结合蛋白1 (X-box binding protein 1, X-box 1), 诱导脂质合成和脂滴异常聚集[78]。最近的数据表明, 在肿瘤小鼠模型中, 树突细胞积聚的脂质通过隔离分子伴侣HSP70和阻止MHC-I-肽复合物转移到细胞表面来阻断交叉呈递, 因而抑制CD8+ T细胞的抗肿瘤免疫应答[79]。CD47主要表达于肿瘤细胞表面, 通常被认为是肿瘤细胞免于宿主免疫系统攻击的保护性受体, 通过与巨噬细胞表面的信号调节蛋白α (signal regulatory protein-α, SIRP-α) 相互结合抑制巨噬细胞的吞噬功能, 传递了一个“别吃我(don't eat me)”的信号, 可以阻止癌细胞被免疫清除[80]。光动力疗法是一种监管批准的癌症治疗方法, 可以通过产生ROS介导的内质网应激来引起“吃我(eat me)”和“don't eat me”信号的失调, 能够促进免疫原性细胞死亡。肿瘤细胞受到外界刺激发生死亡的同时, 由非免疫原性转为免疫原性而介导机体产生抗肿瘤免疫的过程称为免疫原性细胞死亡。肿瘤细胞发生免疫原性细胞死亡的同时, 会产生一系列的信号分子, 此类物质被称为损伤相关分子模式(damage-associated molecular patterns, DAMPs), DAMPs转移至细胞表面后下调肿瘤细胞上CD47的表达[81]。
此外, 纤维蛋白原相关蛋白1 (fibrinogen like protein 1, FGL1) 是免疫抑制分子, 主要由肝细胞分泌, 通过充当LAG-3的主要配体来抑制抗原特异性T细胞活化。当辐射及其他刺激介导的氧化应激诱导肝细胞损伤时, 结果发现细胞表面的FGL1表达上调。由于辐射激活了多种信号, 包括α平滑肌肌动蛋白(alpha-smooth muscle actin, α-SMA)、成纤维细胞特异性蛋白-1 (fibroblast specific protein-1, FSP-1) 介导的纤维化, caspase-3介导的细胞凋亡和自噬相关蛋白3 (microtubule-associated proteins 3, LC3) 介导的自噬, 这些损伤信号与体内和体外的FGL1表达密切相关, 至于具体作用机制以上研究尚未明确解释, 关于FGL1在肿瘤微环境中受ROS调控的研究尚未见报道[82]。
4 靶向ROS协同免疫检查点抑制剂的应用鉴于肿瘤免疫治疗近年来取得的巨大成功, 越来越多的研究开始关注ROS和免疫治疗的协同效应。例如在乳腺癌、胰腺癌和结直肠癌的小鼠模型中, 大剂量抗坏血酸作为抗氧化剂与免疫检查点抑制剂联合使用, 促进T细胞浸润至肿瘤[83]; 绿茶提取物中表没食子儿茶素-3-没食子酸酯(epigallocatechin gallate, EGCG) 具有减少细胞内ROS的能力, 可降低肺癌细胞系中PD-L1的表达, 所以EGCG可成为一种替代免疫检查点抑制剂的疗法, 介导T细胞活化[84]。然而, 在靶向ROS与免疫检查点抑制剂发挥协同作用时, 不能片面地强调清除ROS。有时, ROS的存在也可以提高免疫检查点抑制剂的疗效, 如钙调素拮抗剂三氟拉嗪已被证明可以提高结直肠癌细胞中的ROS水平, 进而诱导这些癌细胞中PD-L1的表达及肿瘤浸润CD4+和CD8+ T细胞中PD-1的表达[85], 三氟拉嗪诱导的PD-L1上调是钙调蛋白结合肽CBP501与抗PD-L1单克隆抗体联合使用疗效增加的原因[86]。因此, 正确调控ROS的浓度是与免疫治疗的协同增效时的难点。这需要研究者更深入地了解ROS对免疫检查点调控的复杂生物学作用。
5 总结与展望作为信号分子或细胞毒物, ROS几乎参与肿瘤发生发展过程中的方方面面, 包括调节免疫检查点的转录表达或改变免疫检查点与配体的结合活性。由于免疫检查点抑制剂疗法在临床仍具有响应率低与耐药性等问题, 为此探索ROS与免疫检查点的调控关系具有重要的科学意义, 并有可能为ICIs临床疗效的提高提供理论依据与实验基础。使用ROS调节剂与ICIs联合用药有可能为未来的肿瘤免疫治疗提供新思路与方法。
作者贡献: 李晓风负责文献、资料的收集整理及初稿的撰写工作; 王圆、韦淑颖、邹伟、罗欣、李佳怡参与文献资料的分析、整理; 韦忠红、余苏云、李晓曼、陈文星、王爱云、赵杨、陆茵参与讨论; 吴媛媛负责文章构思、稿件撰写与修改。
利益冲突: 所有作者均声明无任何利益冲突。
| [1] |
Liu S, Wang F, Tan W, et al. CTLA4 has a profound impact on the landscape of tumor-infiltrating lymphocytes with a high prognosis value in clear cell renal cell carcinoma (ccRCC)[J]. Cancer Cell Int, 2020, 20: 519. DOI:10.1186/s12935-020-01603-2 |
| [2] |
Ma J, Zheng B, Goswami S, et al. PD1 CD8 T cells correlate with exhausted signature and poor clinical outcome in hepatocellular carcinoma[J]. J Immunother Cancer, 2019, 7: 331. DOI:10.1186/s40425-019-0814-7 |
| [3] |
Hamanishi J, Mandai M, Ikeda T, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer[J]. J Clin Oncol, 2015, 33: 4015-4022. DOI:10.1200/JCO.2015.62.3397 |
| [4] |
Di Nunnvo V, Franceschi E, Tosoni A, et al. Immune-checkpoint inhibitors in pituitary malignancies[J]. Anticancer Drugs, 2022, 33: e28-e35. DOI:10.1097/CAD.0000000000001157 |
| [5] |
Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial[J]. Lancet Oncol, 2017, 18: 312-322. DOI:10.1016/S1470-2045(17)30065-7 |
| [6] |
Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study[J]. J Clin Oncol, 2016, 34: 2460-2467. DOI:10.1200/JCO.2015.64.8931 |
| [7] |
Giraldo NA, Sanchez-Salas R, Peske JD, et al. The clinical role of the TME in solid cancer[J]. Br J Cancer, 2019, 120: 45-53. DOI:10.1038/s41416-018-0327-z |
| [8] |
Augustin RC, Delgoffe GM, Najjar YG. Characteristics of the tumor microenvironment that influence immune cell functions: hypoxia, oxidative stress, metabolic alterations[J]. Cancers (Basel), 2020, 12: 3802. DOI:10.3390/cancers12123802 |
| [9] |
Sun L, Wang X, Saredy J, et al. Innate-adaptive immunity interplay and redox regulation in immune response[J]. Redox Biol, 2020, 37: 101759. DOI:10.1016/j.redox.2020.101759 |
| [10] |
Bu FX, Zheng YZ, Zhou JP, et al. Research process of reactive oxygen species-based tumor immunomodulation[J]. Acta Pharm Sin (药学学报), 2022, 57: 296-302. |
| [11] |
Wang L, Kuang Z, Zhang D, et al. Reactive oxygen species in immune cells: a new antitumor target[J]. Biomed Pharmacother, 2021, 133: 110978. DOI:10.1016/j.biopha.2020.110978 |
| [12] |
Wang J, Liu N, Jiang H, et al. Reactive oxygen species in anticancer immunity: a double-edged sword[J]. Front Bioeng Biotechnol, 2021, 9: 784612. DOI:10.3389/fbioe.2021.784612 |
| [13] |
Shadel GS, Horvath TL. Mitochondrial ROS signaling in organismal homeostasis[J]. Cell, 2015, 163: 560-569. DOI:10.1016/j.cell.2015.10.001 |
| [14] |
Fuhrmann DC, Brüne B. Mitochondrial composition and function under the control of hypoxia[J]. Redox Biol, 2017, 12: 208-215. DOI:10.1016/j.redox.2017.02.012 |
| [15] |
Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling[J]. Cell Signal, 2012, 24: 981-990. DOI:10.1016/j.cellsig.2012.01.008 |
| [16] |
Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species[J]. Nat Rev Immunol, 2013, 13: 349-361. DOI:10.1038/nri3423 |
| [17] |
Kennel KB, Greten FR. Immune cell-produced ROS and their impact on tumor growth and metastasis[J]. Redox Biol, 2021, 42: 101891. DOI:10.1016/j.redox.2021.101891 |
| [18] |
Schito L, Semenza GL. Hypoxia-inducible factors: master regulators of cancer progression[J]. Trends Cancer, 2016, 2: 758-770. DOI:10.1016/j.trecan.2016.10.016 |
| [19] |
Zhou Y, Que KT, Zhang Z, et al. Iron overloaded polarizes macrophage to proinflammation phenotype through ROS/acetyl-p53 pathway[J]. Cancer Med, 2018, 7: 4012-4022. DOI:10.1002/cam4.1670 |
| [20] |
Xie A, Li H, Hao Y, et al. Tuning the toxicity of reactive oxygen species into advanced tumor therapy[J]. Nanoscale Res Lett, 2021, 16: 142. DOI:10.1186/s11671-021-03599-8 |
| [21] |
Surikova EI, Goroshinskaja IA, Nerodo GA, et al. The activity of redox-regulatory systems in the tumor and its surrounding tissues in various histological types of tumor[J]. Biomed Khim, 2016, 62: 187-192. DOI:10.18097/PBMC20166202187 |
| [22] |
Castaldo SA, Freitas JR, Conchinha NV, et al. The tumorigenic roles of the cellular redox regulatory systems[J]. Oxid Med Cell Longev, 2016, 2016: 8413032. |
| [23] |
Yang Y, Bazhin AV, Werner J, et al. Reactive oxygen species in the immune system[J]. Int Rev Immunol, 2013, 32: 249-270. DOI:10.3109/08830185.2012.755176 |
| [24] |
Weinberg F, Ramnath N, Nagrath D. Reactive oxygen species in the tumor microenvironment: an overview[J]. Cancers, 2019, 11: 1191. DOI:10.3390/cancers11081191 |
| [25] |
Wang X, Teng F, Kong L, et al. PD-L1 expression in human cancers and its association with clinical outcomes[J]. Onco Targets Ther, 2016, 9: 5023-5039. DOI:10.2147/OTT.S105862 |
| [26] |
Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance[J]. Trends Immunol, 2016, 37: 193-207. DOI:10.1016/j.it.2016.01.002 |
| [27] |
Lennicke C, Cochemé HM. Redox metabolism: ROS as specific molecular regulators of cell signaling and function[J]. Mol Cell, 2021, 81: 3691-3707. DOI:10.1016/j.molcel.2021.08.018 |
| [28] |
Syed Khaja AS, Toor SM, El Salhat H, et al. Intratumoral FoxP3+Helios+ regulatory T cells upregulating immunosuppressive molecules are expanded in human colorectal cancer[J]. Front Immunol, 2017, 8: 619. DOI:10.3389/fimmu.2017.00619 |
| [29] |
Syed Khaja AS, Toor SM, El Salhat H, et al. Preferential accumulation of regulatory T cells with highly immunosuppressive characteristics in breast tumor microenvironment[J]. Oncotarget, 2017, 8: 33159-33171. DOI:10.18632/oncotarget.16565 |
| [30] |
Gao Y, Nihira NT, Bu X, et al. Acetylation-dependent regulation of PD-L1 nuclear translocation dictates the efficacy of anti-PD-1 immunotherapy[J]. Nat Cell Biol, 2020, 22: 1064-1075. DOI:10.1038/s41556-020-0562-4 |
| [31] |
Rowshanravan B, Halliday N, Sansom DM. CTLA-4: a moving target in immunotherapy[J]. Blood, 2018, 131: 58-67. DOI:10.1182/blood-2017-06-741033 |
| [32] |
Walker LS, Sansom DM. Confusing signals: recent progress in CTLA-4 biology[J]. Trends Immunol, 2015, 36: 63-70. DOI:10.1016/j.it.2014.12.001 |
| [33] |
Shiratori T, Miyatake S, Ohno H, et al. Tyrosine phosphorylation controls internalization of CTLA-4 by regulating its interaction with clathrin-associated adaptor complex AP-2[J]. Immunity, 1997, 6: 583-589. DOI:10.1016/S1074-7613(00)80346-5 |
| [34] |
Sheng CC, Han FY. Immunoregulation effects of TIM-3 on tumors[J]. Neoplasma, 2019, 66: 167-175. DOI:10.4149/neo_2018_180610N385 |
| [35] |
Das M, Zhu C, Kuchroo VK. Tim-3 and its role in regulating anti-tumor immunity[J]. Immunol Rev, 2017, 276: 97-111. DOI:10.1111/imr.12520 |
| [36] |
Li F, Zhang R, Li S, et al. IDO1: an important immunotherapy target in cancer treatment[J]. Int Immunopharmacol, 2017, 47: 70-77. DOI:10.1016/j.intimp.2017.03.024 |
| [37] |
Iacono A, Pompa A, De Marchis F, et al. Class IA PI3Ks regulate subcellular and functional dynamics of IDO1[J]. EMBO Rep, 2020, 21: e49756. |
| [38] |
Andrews LP, Marciscano AE, Drake CG, et al. LAG3 (CD223) as a cancer immunotherapy target[J]. Immunol Rev, 2017, 276: 80-96. DOI:10.1111/imr.12519 |
| [39] |
Bae J, Lee SJ, Park CG, et al. Trafficking of LAG-3 to the surface on activated T cells via its cytoplasmic domain and protein kinase C signaling[J]. J Immunol, 2014, 193: 3101-3112. DOI:10.4049/jimmunol.1401025 |
| [40] |
Hu M, Li Y, Lu Y, et al. The regulation of immune checkpoints by the hypoxic tumor microenvironment[J]. PeerJ, 2021, 9: e11306. DOI:10.7717/peerj.11306 |
| [41] |
Kitamura T, Qian BZ, Soong D, et al. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages[J]. J Exp Med, 2015, 212: 1043-1059. DOI:10.1084/jem.20141836 |
| [42] |
Huang B, Lei Z, Zhao J, et al. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers[J]. Cancer Lett, 2007, 252: 86-92. DOI:10.1016/j.canlet.2006.12.012 |
| [43] |
Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy[J]. Science, 2015, 350: 1084-1089. DOI:10.1126/science.aac4255 |
| [44] |
Vétizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota[J]. Science, 2015, 350: 1079-1084. DOI:10.1126/science.aad1329 |
| [45] |
García-Guede Á, Vera O, Ibáñez-de-Caceres I. When oxidative stress meets epigenetics: implications in cancer development[J]. Antioxidants (Basel), 2020, 9: 468. DOI:10.3390/antiox9060468 |
| [46] |
Shi J, Xu J, Chen YE, et al. The concurrence of DNA methylation and demethylation is associated with transcription regulation[J]. Nat Commun, 2021, 12: 5285. DOI:10.1038/s41467-021-25521-7 |
| [47] |
Liu XX, Li Q. Role of genetic and DNA methylation alterations caused by oxidative stress in tumorigenesis[J]. J Radiat Res Radiat Process (辐射研究与辐射工艺学报), 2013, 31: 3-10. |
| [48] |
Chatterjee A, Rodger EJ, Ahn A, et al. Marked global DNA hypomethylation is associated with constitutive PD-L1 expression in melanoma[J]. iScience, 2018, 4: 312-325. DOI:10.1016/j.isci.2018.05.021 |
| [49] |
Eun Joon L, Jimei L, Ethan S, et al. DNA hypomethylation leads to aberrant expression of PD-1 in chronic lymphocytic leukemia[J]. Blood, 2012, 120: 3504. DOI:10.1182/blood.V120.21.3504.3504 |
| [50] |
Marwitz S, Scheufele S, Perner S, et al. Epigenetic modifications of the immune-checkpoint genes CTLA4 and PDCD1 in non-small cell lung cancer results in increased expression[J]. Clin Epigenetics, 2017, 9: 51. DOI:10.1186/s13148-017-0354-2 |
| [51] |
García-Giménez JL, Romá-Mateo C, Pallardó FV. Oxidative post-translational modifications in histones[J]. Biofactors, 2019, 45: 641-650. DOI:10.1002/biof.1532 |
| [52] |
Kato T, Shimono Y, Hasegawa M, et al. Characterization of the HDAC1 complex that regulates the sensitivity of cancer cells to oxidative stress[J]. Cancer Res, 2009, 69: 3597-3604. DOI:10.1158/0008-5472.CAN-08-4368 |
| [53] |
Chuang JY, Chang WC, Hung JJ. Hydrogen peroxide induces Sp1 methylation and thereby suppresses cyclin B1 via recruitment of Suv39H1 and HDAC1 in cancer cells[J]. Free Radic Biol Med, 2011, 51: 2309-2318. DOI:10.1016/j.freeradbiomed.2011.10.001 |
| [54] |
Doyle K, Fitzpatrick FA. Redox signaling, alkylation (carbonylation) of conserved cysteines inactivates class I histone deacetylases 1, 2, and 3 and antagonizes their transcriptional repressor function[J]. J Biol Chem, 2010, 285: 17417-17424. DOI:10.1074/jbc.M109.089250 |
| [55] |
García-Giménez JL, Romá-Mateo C, Pérez-Machado G, et al. Role of glutathione in the regulation of epigenetic mechanisms in disease[J]. Free Radic Biol Med, 2017, 112: 36-48. |
| [56] |
Cyr AR, Domann FE. The redox basis of epigenetic modifications: from mechanisms to functional consequences[J]. Antioxidants Redox Signal, 2011, 15: 551-589. DOI:10.1089/ars.2010.3492 |
| [57] |
Hitchler MJ, Domann FE. Redox regulation of the epigenetic landscape in cancer: a role for metabolic reprogramming in remodeling the epigenome[J]. Free Radic Biol Med, 2012, 53: 2178-2187. DOI:10.1016/j.freeradbiomed.2012.09.028 |
| [58] |
Woods DM, Sodré AL, Villagra A, et al. HDAC inhibition upregulates PD-1 ligands in melanoma and augments immunotherapy with PD-1 blockade[J]. Cancer Immunol Res, 2015, 3: 1375-1385. DOI:10.1158/2326-6066.CIR-15-0077-T |
| [59] |
Zheng H, Zhao W, Yan C, et al. HDAC inhibitors enhance T-cell chemokine expression and augment response to PD-1 immunotherapy in lung adenocarcinoma[J]. Clin Cancer Res, 2016, 22: 4119-4132. DOI:10.1158/1078-0432.CCR-15-2584 |
| [60] |
Sasidharan Nair V, Toor SM, Taha RZ, et al. DNA methylation and repressive histones in the promoters of PD-1, CTLA-4, TIM-3, LAG-3, TIGIT, PD-L1, and galectin-9 genes in human colorectal cancer[J]. Clin Epigenetics, 2018, 10: 104. DOI:10.1186/s13148-018-0539-3 |
| [61] |
Sasidharan Nair V, El Salhat H, Taha RZ, et al. DNA methylation and repressive H3K9 and H3K27 trimethylation in the promoter regions of PD-1, CTLA-4, TIM-3, LAG-3, TIGIT, and PD-L1 genes in human primary breast cancer[J]. Clin Epigenetics, 2018, 10: 78. DOI:10.1186/s13148-018-0512-1 |
| [62] |
Priya Dharshini LC, Vishnupriya S, Sakthivel KM, et al. Oxidative stress responsive transcription factors in cellular signalling transduction mechanisms[J]. Cell Signal, 2020, 72: 109670. DOI:10.1016/j.cellsig.2020.109670 |
| [63] |
Sajadimajd S, Khazaei M. Oxidative stress and cancer: the role of Nrf2[J]. Curr Cancer Drug Targets, 2018, 18: 538-557. DOI:10.2174/1568009617666171002144228 |
| [64] |
Xiao Y, Zheng YT. Oxidative stress up-regulates the expression of CTLA-4 through Nrf2/ARE signal pathway resulting in immunosuppression [C] // The 13th National Congress on Immunology (第十三届全国免疫学学术大会摘要汇编). Shanghai: Chinese Immunology Society, 2018: 317.
|
| [65] |
Lingappan K. NF-κB in oxidative stress[J]. Curr Opin Toxicol, 2018, 7: 81-86. DOI:10.1016/j.cotox.2017.11.002 |
| [66] |
Antonangeli F, Natalini A, Garassino MC, et al. Regulation of PD-L1 expression by NF-κB in cancer[J]. Front Immunol, 2020, 11: 584626. DOI:10.3389/fimmu.2020.584626 |
| [67] |
Kubli SP, Bassi C, Roux C, et al. AhR controls redox homeostasis and shapes the tumor microenvironment in BRCA1-associated breast cancer[J]. Proc Natl Acad Sci U S A, 2019, 116: 3604-3613. DOI:10.1073/pnas.1815126116 |
| [68] |
Wang LT, Chiou SS, Chai CY, et al. Intestine-specific homeobox gene integrates IL6 signaling, tryptophan catabolism, and immune suppression[J]. Cancer Res, 2017, 77: 4065-4077. |
| [69] |
Takenaka MC, Gabriely G, Rothhammer V, et al. Control of tumor-associated macrophages and T cells in glioblastoma via AhR and CD39[J]. Nat Neurosci, 2019, 22: 729-740. DOI:10.1038/s41593-019-0370-y |
| [70] |
Alavi S, Emran AA, Tseng HY, et al. Nicotinamide inhibits T cell exhaustion and increases differentiation of CD8 effector T cells[J]. Cancers (Basel), 2022, 14: 323. DOI:10.3390/cancers14020323 |
| [71] |
Sarhan D, Cichocki F, Zhang B, et al. Adaptive NK cells with low TIGIT expression are inherently resistant to myeloid-derived suppressor cells[J]. Cancer Res, 2016, 76: 5696-5706. DOI:10.1158/0008-5472.CAN-16-0839 |
| [72] |
Corzo CA, Cotter MJ, Cheng P, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells[J]. J Immunol, 2009, 182: 5693-5701. DOI:10.4049/jimmunol.0900092 |
| [73] |
Solecki D, Bernhardt G, Lipp M, et al. Identification of a nuclear respiratory factor-1 binding site within the core promoter of the human polio virus receptor/CD155 gene[J]. J Biol Chem, 2000, 275: 12453-12462. DOI:10.1074/jbc.275.17.12453 |
| [74] |
Glorieux C, Xia X, He YQ, et al. Regulation of PD-L1 expression in K-ras-driven cancers through ROS-mediated FGFR1 signaling[J]. Redox Biol, 2021, 38: 101780. DOI:10.1016/j.redox.2020.101780 |
| [75] |
Hempel N, Trebak M. Crosstalk between calcium and reactive oxygen species signaling in cancer[J]. Cell Calcium, 2017, 63: 70-96. DOI:10.1016/j.ceca.2017.01.007 |
| [76] |
Vendetti S, Riccomi A, Sacchi A, et al. Cyclic adenosine 5'-monophosphate and calcium induce CD152 (CTLA-4) up-regulation in resting CD4+ T lymphocytes[J]. J Immunol, 2002, 169: 6231-6235. DOI:10.4049/jimmunol.169.11.6231 |
| [77] |
Garrido F, Aptsiauri N, Doorduijn EM, et al. The urgent need to recover MHC class I in cancers for effective immunotherapy[J]. Curr Opin Immunol, 2016, 39: 44-51. DOI:10.1016/j.coi.2015.12.007 |
| [78] |
Zhang RG. Therapeutic approaches for ARID1A-mutated ovarian cancer [C] // Proceedings of the AACR Special Conference on Advances in Ovarian Cancer Research. Atlanta: AACR, 2020: Abstract IA23.
|
| [79] |
Veglia F, Tyurin VA, Mohammadyani D, et al. Lipid bodies containing oxidatively truncated lipids block antigen cross-presentation by dendritic cells in cancer[J]. Nat Commun, 2017, 8: 2122. DOI:10.1038/s41467-017-02186-9 |
| [80] |
Huang CY, Ye ZH, Huang MY, et al. Regulation of CD47 expression in cancer cells[J]. Transl Oncol, 2020, 13: 100862. DOI:10.1016/j.tranon.2020.100862 |
| [81] |
Zheng Y, Yin G, Le V, et al. Photodynamic-therapy activates immune response by disrupting immunity homeostasis of tumor cells, which generates vaccine for cancer therapy[J]. Int J Biol Sci, 2016, 12: 120-132. DOI:10.7150/ijbs.12852 |
| [82] |
Han NK, Jung MG, Jeong YJ, et al. Plasma fibrinogen-like 1 as a potential biomarker for radiation-induced liver injury[J]. Cells, 2019, 8: 1042. DOI:10.3390/cells8091042 |
| [83] |
Magrì A, Germano G, Lorenzato A, et al. High-dose vitamin C enhances cancer immunotherapy[J]. Sci Transl Med, 2020, 12: eaay8707. DOI:10.1126/scitranslmed.aay8707 |
| [84] |
Rawangkan A, Wongsirisin P, Namiki K, et al. Green tea catechin is an alternative immune checkpoint inhibitor that inhibits PD-L1 expression and lung tumor growth[J]. Molecules, 2018, 23: 2071. DOI:10.3390/molecules23082071 |
| [85] |
Xia Y, Jia C, Xue Q, et al. Antipsychotic drug trifluoperazine suppresses colorectal cancer by inducing G0/G1 arrest and apoptosis[J]. Front Pharmacol, 2019, 10: 1029. DOI:10.3389/fphar.2019.01029 |
| [86] |
Sakakibara K, Sato T, Kufe DW, et al. CBP501 induces immunogenic tumor cell death and CD8 T cell infiltration into tumors in combination with platinum, and increases the efficacy of immune checkpoint inhibitors against tumors in mice[J]. Oncotarget, 2017, 8: 78277-78288. DOI:10.18632/oncotarget.20968 |
 2022, Vol. 57
2022, Vol. 57


