2. 平度市人民医院, 山东 青岛 266700;
3. 山东中医药大学实验中心, 山东 济南 250355;
4. 山东省中医药基础研究重点实验室, 山东 济南 250355;
5. 山东中医药大学教育部中医药经典理论重点实验室, 山东 济南 250355
2. Pingdu People's Hospital, Qingdao 266700, China;
3. Experimental Center, Shandong University of Traditional Chinese Medicine, Jinan 250355, China;
4. Shandong Provincial Key Laboratory of Traditional Chinese Medicine for Basic Research, Jinan 250355, China;
5. Key Laboratory of Traditional Chinese Medicine Classical Theory, Ministry of Education, Shandong University of Traditional Chinese Medicine, Jinan 250355, China
高血压是影响人类健康的多发慢性疾病, 是引发心脑血管事件的最危险因素[1]。高血压可造成多种靶器官损伤(如脑损伤), 血压波动导致脑内血流减少, 供血区脑组织缺血缺氧, 引起微毛细血管的出血或炎性渗出, 从而导致大脑结构和功能损伤, 且随着血压升高, 这些病理性变化会愈发严重[2-4]。血压的变化受中枢神经系统的调节, 作为中枢神经系统的一部分, 海马是下丘脑-垂体-肾上腺(hypothalamic-pituitary-adrenal axis, HPA) 轴负反馈调节的高位中枢, 能够通过保持兴奋性糖皮质激素受体和抑制性盐皮质激素受体水平的平衡, 抑制HPA轴过度兴奋, 进而维持血压水平稳定[4, 5]。海马神经发生(neurogenesis) 是维持海马功能和结构可塑性的重要因素, 如果海马神经发生受到抑制, 海马神经元对HPA轴的调节作用会减弱, 导致HPA轴反应增强, 而HPA轴反应过度会引起血压升高[6]。海马对应激反应极其敏感, 应激能够影响海马神经元功能, 降低海马对HPA轴的抑制作用, 使HPA轴反应性增强, 造成血压升高[7]。综上, 海马在血压的调节中发挥着重要作用。因此, 研究海马组织中内源性代谢物变化, 分析相关通路和高血压联系, 对于揭示高血压的发病机制和探索降压药物的新靶点至关重要。
异钩藤碱为中药钩藤(Uncaria rhynchophylla) 中的吲哚类生物碱, 是钩藤主要活性成分[8, 9]。异钩藤碱可通过提高NO生成、调节肾素-血管紧张素-醛固酮系统(RAAS)、抑制血管平滑肌增殖和保护血管内皮细胞等作用机制发挥其降压作用[10-12]。本实验采用核磁共振代谢组学技术, 试图从小分子代谢物层次探究异钩藤碱对自发性高血压大鼠(spontaneously hypertensive rat, SHR) 海马组织代谢轮廓与代谢标志物的调控作用, 发现关键代谢通路, 找到其关键靶点蛋白, 并通过分子对接技术分析异钩藤碱与关键蛋白靶点的结合, 为进一步研究异钩藤碱防治高血压作用机制提供依据。
材料与方法实验动物 10周龄雄性Wistar-Kyoto大鼠(WKY) 6只, 10周龄雄性SHR12只, 购自北京维通利华实验动物技术有限公司[动物生产许可证号: SCXK (京) 2016-0006]。所有实验动物均饲养于山东中医药大学动物实验中心SPF级实验室[动物使用许可证号: SYXK (鲁) 20170022], 每笼6只, 每天处于明暗各12 h的饲养环境中。动物实验经山东中医药大学实验动物伦理委员会批准(批准号: SDUTCM20210721002)。
仪器和试剂 AVANCEⅢ 600 MHz型超导傅里叶变换核磁共振波谱仪(德国布鲁克科技有限公司)。BP2010A大小鼠智能无创血压仪(北京软隆生物技术公司)。异钩藤碱对照品(上海源叶生物科技有限公司, 批号: B21526)。含2, 2, 3, 3-D(4)-3-(三甲基硅基) 丙酸钠(TMSP) 内标物的重水(批号DLM-4TPC-25) 购自美国CIL公司。HPLC级甲醇和氯仿购自美国Thermo Fisher公司。
动物分组与给药方法 动物适应性饲养一周后, 12只SHR随机分为模型组和给药组, 每组6只, 6只WKY大鼠为正常对照组。采用生理盐水将异钩藤碱制成混悬液, 用时摇匀, 给药组每日灌胃0.3 mg·kg-1的异钩藤碱, 给药量根据课题组前期研究制定[13]。正常组和模型组大鼠每日均给予等量生理盐水。每天定时给药, 每周给药6天, 连续给药8周。
血压测量 于大鼠给药前和给药8周后, 采用无创套尾法测量各组大鼠清醒状态下尾动脉的收缩压, 每只大鼠连续测量3次。
大鼠海马组织的采集和处理 19周龄的大鼠禁食不禁水24 h, 1.5%戊巴比妥钠麻醉, 打开胸腔, 以生理盐水心脏灌注, 取大鼠全脑并分离出海马, 置于-80 ℃备用。海马组织代谢物采用甲醇/氯仿/水的两相提取法[14-16]。取海马(约50 mg) 置于2 mL EP管中, 精密称定, 按比例加入预冷的甲醇(4 mL·g-1)、氯仿(4 mL·g-1) 和蒸馏水(2 mL·g-1), 涡旋混匀, 冰上匀浆, 4 ℃静置15 min。4 ℃, 12 000 r·min-1离心10 min, 取上清移至EP管中, 氮吹浓缩至干。把样品溶于600 μL添加了TMSP的重水里, 移取550 μL上清液至核磁管中, 即可用于NMR实验。
1H NMR数据采集和处理 1H NMR测定频率为600.13 MHz, 检测温度为298 K, 调用zg30脉冲序列进行1H NMR图谱采集, 谱宽为12 019.23 Hz, 扫描次数为128次, 空扫次数为2次, 接收器增益因子为226, 采样点数为64 K, 采样时间为2.7 s, 弛豫时间为2 s, 自由感应衰减(free induction decay, FID) 分辨率为0.37 Hz, FID信号经过64 K傅里叶变换转为1H NMR图谱。
以TMSP为化学位移参考峰位置, 设为0 ppm, 校正化学位移, 对图谱进行相位校正和基线校正, 将1H谱按默认值, 以每段为0.04进行分段并积分, 扣除δ 4.5~5.0处重水中残余H2O的影响。将数据进行归一化处理[17], 所得数据Excel文件贮存。
采用SIMCA-P 14.0软件(瑞典Umetrics公司) 进行主成分分析(principal components analysis, PCA) 和偏最小二乘-判别分析(partial least squares-discriminant analysis, PLS-DA)。根据PLS-DA相应的VIP值(variable importance in the projection) 结果寻找对分类贡献较大的变量(化学位移), 确定各组的共性代谢物和差异的生物标记物。根据差异代谢物的化学位移信息, 查阅文献并参考HMDB数据库(http://www.hmdb.ca/), 根据化合物化学位移、峰裂分情况和耦合常数对图谱进行物质指认。对VIP值> 1的变量, 通过SPSS 22.0 (美国IBM公司) 软件, 采用t检验筛选差异代谢物, 以P < 0.05为差异有统计学意义。
差异性代谢物相关靶点筛选 将差异代谢物导入MetaboAnalyst 3.0 (http://www.metaboanalyst.ca/MetaboAnalyst) 中进行通路分析。将与差异性代谢物相关的代谢通路输入京都基因与基因组百科全书(KEGG) 数据库进行分析, 寻找相关通路上的关键靶点。
异钩藤碱与靶蛋白的分子对接 将海马差异性代谢物代谢通路上的关键靶点输入蛋白质结构数据库(protein data bank, PDB), 下载靶点蛋白的3D结构。已有研究证明异钩藤碱可通过血脑屏障[18], 所以直接采用AutoDock软件, 运行Autodock vina模式进行将异钩藤碱小分子与靶蛋白进行分子对接, 通过vina计算的结合能验证靶点与化合物的结合活性, 然后通过Pymol和Discovery Studio进行结果可视化分析[19]。
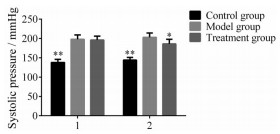
结果 1 各组大鼠血压测定为了确定异钩藤碱对SHR血压的影响, 对大鼠的收缩压检测并详细记录。如图 1所示, 模型组大鼠的收缩压指标与对照组比较显著增高, 而异钩藤碱干预后的SHR血压显著下降(P < 0.05)。因此, 异钩藤碱能显著发挥降低高血压的效果。
|
Figure 1 The systolic blood pressure of rats before and after 8 weeks intervention. A: before intervention; B: After 8 weeks intervention. The rats in treatment group were administered with isorhyncophylline (0.3 mg·kg-1) while the rats in other two groups were treated with the same amount of sterilized saline solution. *P < 0.05, **P < 0.01 vs the model group |
根据化学位移、峰裂分情况、耦合常数等信息, 通过查阅文献[20-24]并查询HMDB (http:/www.hmdb.ca) 数据库, 共鉴定出21个化合物, 化合物鉴定结果见表 1。
| Table 1 Metabolites found in 1H NMR analysis of hippocampus extract |
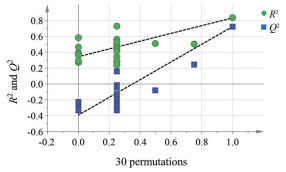
采用无监督的PCA分析, 如散点图(图 2A) 所示, 每个点分别代表一个样品。从图 2A可以看出, 3组样品均呈现出较为明显的分离, 累计的解释率R2X = 0.963 cum, Q2 = 0.763 cum。随后, 为寻找贡献较大的差异性代谢物, 采用有监督的PLS-DA方法对各组海马组织样本数据矩阵进行判别分析, 分析结果见图 2B。模型的R2X、R2Y和Q2cum分别为0.812、0.721和0.498, 说明模型的拟合能力和预测能力良好。使用置换检验(permutation test) 验证模型稳定性, 经过30次随机排列的结果见图 3, R2和Q2的截距分别为0.295, -0.338, 表明模型可靠[25]。PCA和PLS-DA结果提示异钩藤碱会显著改变SHR大鼠海马区的代谢轮廓, 使其向正常大鼠转变, 从整体水平上干预SHR海马区的代谢模式。

|
Figure 2 Principal components analysis (A) and partial least squares discriminant analysis (B) score plots of hippocampus tissues metabolites from the control group, model group and treatment group |
|
Figure 3 The permutation test for PLS-DA model |
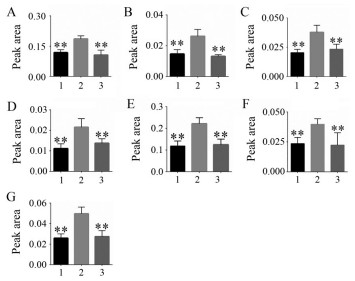
为寻求对分类最有贡献的变量, 选择VIP > 1的变量作为潜在生物标志物, 共筛选出45个VIP值> 1的变量。结合t检验结果和1H NMR谱鉴定结果, 从这45个变量中分析出7个差异性代谢物, 分别为: 乳酸、丙氨酸、谷氨酸、谷氨酰胺、肌酸、牛磺酸、葡萄糖, 这7种代谢物含量在模型组中均显著升高, 而经异钩藤碱干预后, 各代谢物均呈明显的下降趋势(图 4)。
|
Figure 4 The change trends of differential metabolites among three groups. 1: Control group; 2: Model group; 3: Treatment group. A: Lactate; B: Alanine; C: Glutamate; D: Glutamine; E: Creatine; F: Taurine; G: Glucose. **P < 0.01 vs model group |
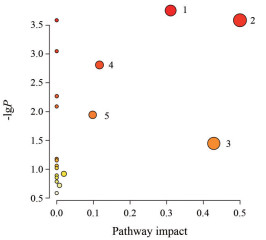
利用MetaboAnalyst 3.0对所得的差异性代谢物进行通路分析, 5条通路发生显著性变化: 丙氨酸、天冬氨酸和谷氨酸代谢, 谷氨酰胺和谷氨酸代谢, 牛磺酸和亚牛磺酸代谢, 精氨酸生物合成, 精氨酸和脯氨酸代谢, 这些代谢通路主要涉及谷氨酸代谢和糖代谢等(图 5)。将相关通路导入KEGG数据库中分析, 共得到9个与谷氨酸代谢和糖代谢密切相关的靶点, 分别是兴奋性氨基酸转运体1 (glutamate-aspartate transporter-1, GLAST-1)、兴奋性氨基酸转运体2 (glutamate transporter-1, GLT-1)、谷氨酸脱氢酶(glutamate dehydrogenase, GDH)、谷氨酸合成酶(glutamate synthase, GOGAT)、谷氨酸氧化酶(glutamate oxidase, GLUOX)、葡萄糖-1-磷酸酶(glucose-1-phosphatase, AGPE)、葡萄糖转运体1 (glucose transporter-1, GLUT-1)、丙酮酸激酶(pyruvate kinase M, PKM) 和乳酸脱氢酶(lactate dehydrogenase, LDH)。相关代谢通路网络图见图 6。
|
Figure 5 Metabolic pathway analysis of the altered metabolic network generated by MetaboAnalyst solftware. 1: Alanine, aspartate and glutamate metabolism; 2: Glutamine and glutamate metabolism; 3: Taurine and hypotaurine metabolism; 4: Arginine biosynthesis; 5: Arginine and proline metabolism |
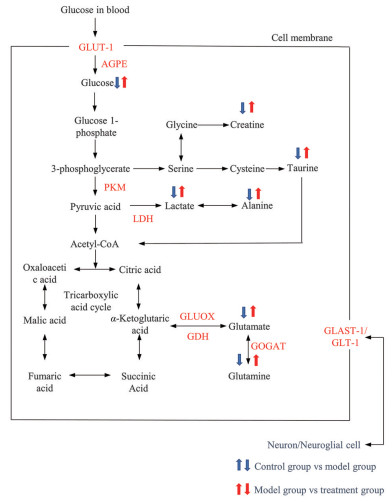
|
Figure 6 The metabolic network of the altered biomarkers in three groups. Red markers represented target proteins which was performed by docking verification. GLAST-1: Glutamate-aspartate transporter-1; GLT-1: Glutamate transporter-1; GDH: Glutamate dehydrogenase; GOGAT: Glutamate synthase; GLUOX: Glutamate oxidase; AGPE: Glucose-1-phosphatase; GLUT-1: Glucose transporter-1; PKM: Pyruvate kinase M; LDH: Lactate dehydrogenase |
异钩藤碱与9个靶点对应的蛋白结构对接结果见图 7。分子亲和力结果见表 2, 结合能越低, 表明分子与蛋白结合能力越佳, 最低结合能≤ -5.0 kJ·mol-1即表明药效分子与蛋白对接效果良好[26]。结果表明, 异钩藤碱与各靶点之间具有良好的对接活性, 其中结合活性最好的是葡萄糖转运体1 (GLUT-1)。

|
Figure 7 The molecule docking model of isorhynchophylline and target proteins. A: Isorhynchophylline and GLAST-1; B: Isorhynchophylline and GLT-1; C: Isorhynchophylline and GDH; D: Isorhynchophylline and GOGAT; E: Isorhynchophylline and GLUOX; F: Isorhynchophylline and AGPE; G: Isorhynchophylline and GLUT-1; H: Isorhynchophylline and PKM; I: Isorhynchophylline and LDH |
| Table 2 The molecule docking result of isorhynchophylline and target proteins |
本研究将自发性高血压大鼠作为研究对象, 发现经异钩藤碱干预后, 大鼠血压有较为明显的降低, 同时, 1H NMR结果表明, 异钩藤碱可以改善自发性高血压大鼠海马组织代谢谱。
在本研究中, 模型组大鼠海马组织中葡萄糖和乳酸的含量均显著高于对照组大鼠, 表明SHR的大脑长期处于高能耗状态。SHR海马区的高能耗状态使脑组织缺血缺氧, 导致氧化应激、免疫激活、细胞凋亡等病理改变[27]。而经异钩藤碱干预后, 给药组大鼠海马组织中葡萄糖和乳酸的含量均有回归正常组趋势。分子对接结果显示, 异钩藤碱与糖酵解相关的关键靶点葡萄糖转运体-1、葡萄糖-1-磷酸酶、丙酮酸激酶和乳酸脱氢酶结合紧密, 表明异钩藤碱可以影响海马组织中的能量。
本研究表明, 模型组大鼠海马组织谷氨酸及与其合成有关的丙氨酸、谷氨酰胺含量均有所上升。作为一种兴奋性神经递质, 当谷氨酸兴奋性过高时, 其突触传递通过兴奋性氨基酸转运体(EAATs) 激发中枢神经系统中神经元及交感神经的兴奋性, 血管收缩作用加强, 升高血压[28, 29]。谷氨酸在脑组织能量代谢中也扮演着非常重要的角色, 当脑组织神经元能量代谢增加时, 谷氨酸可以显著增加脑神经元细胞对葡萄糖的摄取[30]。本研究也发现, 模型组大鼠海马组织中谷氨酸和葡萄糖的含量均显著上升。随着能量代谢的增加, 由葡萄糖代谢产生的兴奋性神经递质谷氨酸开始大量堆积, 并产生具有细胞毒性的活性氧(ROS), 严重影响神经元细胞的功能, 使其无法正常发挥调节血压的作用[31, 32]。结果表明, 异钩藤碱干预后, 自发性高血压大鼠海马区谷氨酸、谷氨酰胺、丙氨酸含量均显著下降, 在进一步的分子对接分析中, 与谷氨酸转运和代谢相关的兴奋性氨基酸转运体1/2、谷氨酸合成酶、谷氨酸氧化酶和谷氨酸脱羧酶结合较好。上述结果表明, 异钩藤碱通过可通过调节谷氨酸代谢而起到保护海马区和预防高血压的作用。
肌酸在能量代谢中扮演着重要角色, 通过刺激线粒体的呼吸作用, 给机体供给更多能量[33]。通过与对照组大鼠比较, 发现自发性高血压大鼠海马组织中肌酸的含量处于较高水平, 再次证明自发性高血压大鼠能量消耗偏高, 异钩藤碱则可以降低肌酸水平, 调节海马区的能量代谢异常。
牛磺酸可以通过阻止钙离子内流来抵抗谷氨酸的神经毒性[34], 同时能降低乳酸的堆积, 减少酸中毒[35]。在本研究中, 模型组大鼠海马组织牛磺酸的含量显著上升, 这表明自发性高血压大鼠在19周龄前已经出现了明显的代谢紊乱, 乳酸和谷氨酸水平的显著提升导致海马区牛磺酸水平代偿性升高, 而在异钩藤碱发挥了治疗作用后, 由于自发性高血压大鼠各种症状的减轻, 导致其海马组织牛磺酸水平下降。
综上所述, 对SHR所进行的1H NMR代谢组学分析结果显示其存在能量代谢、谷氨酸代谢、牛磺酸代谢紊乱, 异钩藤碱干预可回调葡萄糖、乳酸、谷氨酸、谷氨酰胺、丙氨酸肌酸、牛磺酸的水平。分子对接结果表明异钩藤碱可通过影响海马组织的能量代谢和谷氨酸代谢, 改善自发性高血压大鼠的代谢紊乱。异钩藤碱在防治高血压和保护海马组织学习记忆功能作用还有待进一步深入探讨。
作者贡献: 王冠杰负责实验操作、数据处理和文章撰写; 田振华负责指导核磁实验及文章修改; 蒋海强负责整体设计及文章修改; 杜镇、杨文杰、王玥琛协助完成动物实验; 郑海涛协助指导实验和数据处理。
利益冲突: 所有作者均声明不存在利益冲突。
| [1] |
Joint Committee for Guideline Revision. 2018 Chinese guidelines for the management of hypertension[J]. Chin J Cardiovasc Med (中国心血管杂志), 2019, 24: 24-56. |
| [2] |
Liu XJ, Li J, Guan YX. Inhibiting microRNA-27b regulation of Nrf2/ARE signaling pathway on brain injury in rat dodel of hypertensive cerebral hemorrhage[J]. Hebei Med (河北医学), 2020, 26: 76-80. |
| [3] |
Qin YF, Tian HH, Sun F, et al. Effect of losartan on the protection of the kidney and PRCP-kallikrein axis of the two-kidney, one-clipped renovascular hypertensive rats[J]. Acta Pharm Sin (药学学报), 2013, 48: 59-65. |
| [4] |
Tan HJ, Yang WJ, Wu CG, et al. Assessment of the role of intracranial hypertension and stress on hippocampal cell apoptosis and hypothalamic-pituitary dysfunction after TBI[J]. Sci Rep, 2017, 7: 3805. DOI:10.1038/s41598-017-04008-w |
| [5] |
Michailidou Z, Carter RN, Marshall E, et al. Glucocorticoid receptor haploinsufficiency causes hypertension and attenuates hypothalamic-pituitary-adrenal axis and blood pressure adaptions to high-fat diet[J]. FASEB J, 2008, 22: 3896-3907. DOI:10.1096/fj.08-111914 |
| [6] |
Snyder JS, Soumier A, Brewer M, et al. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour[J]. Nature, 2011, 476: 458-461. DOI:10.1038/nature10287 |
| [7] |
Kitayama N, Vaccarino V, Kutner M, et al. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis[J]. J Affect Disord, 2005, 88: 79-86. DOI:10.1016/j.jad.2005.05.014 |
| [8] |
Gao XY, Ding R, Wang DP, et al. Research progress on chemical constituents and pharmacological effects of Uncaria[J]. J Tianjin Med Univ (天津医科大学学报), 2017, 23: 380-382. DOI:10.1007/s12209-017-0057-y |
| [9] |
Yang W, Ip SP, Liu L, et al. Uncaria rhynchophylla and its major constituents on central nervous system: a review on their pharmacological actions[J]. Curr Vasc Pharmacol, 2020, 18: 346-357. DOI:10.2174/1570161117666190704092841 |
| [10] |
Zhang W, Zhou YF, Hao X, et al. Effects of combination of aspirin and isorhynchophylline on antiplatelet aggregation[J]. Chin J Mod Appl Pharm (中国现代应用药学), 2015, 32: 295-297. |
| [11] |
Tian LN, Gao HW, Long ZJ, et al. Antihypertensive effect and vascular regulation mechanism of rhynchophylline on spontaneously hypertensive rats[J]. Chin Tradit Herb Drugs (中草药), 2014, 45: 2210-2213. DOI:10.7501/j.issn.0253-2670.2014.15.017 |
| [12] |
Zheng Q, Zhang Y, Zhao Z, et al. Isorhynchophylline ameliorates paraquat-induced acute kidney injury by attenuating oxidative stress and mitochondrial damage via regulating toll-interacting expression[J]. Toxicol Appl Pharmacol, 2021, 420: 115521. DOI:10.1016/j.taap.2021.115521 |
| [13] |
Li Y, Yu RX, Zhang D, et al. Deciphering the mechanism of the anti-hypertensive effect of isorhynchophylline by targeting neurotransmitters metabolism of hypothalamus in spontaneously hypertensive rats[J]. ACS Chem Neurosci, 2020, 11: 1563-1572. DOI:10.1021/acschemneuro.9b00699 |
| [14] |
Ni ZT, Ji H, Gao HC, et al. NMR-based metabolomics analysis of brain region-specific metabolic changes in diabetic rats[J]. J Wenzhou Med Univ (温州医科大学学报), 2020, 50: 364-370, 376. |
| [15] |
Beckonert O, Keun HC, Ebbels TM, et al. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts[J]. Nat Protoc, 2007, 2: 2692-2703. DOI:10.1038/nprot.2007.376 |
| [16] |
Peng GJ, Shi BY, Tian JS, et al. 1H NMR based metabonomics study on the antidepressant effect of genipin in rat hippocampus[J]. Acta Pharm Sin (药学学报), 2014, 49: 209-216. |
| [17] |
Wen JB, Yang SY, Xiao X, et al. NMR based metabonomic data preprocessing[J]. J Xiamen Univ (Nat Sci) (厦门大学学报·自然科学版), 2007, 46: 783-787. |
| [18] |
Zhang YN, Yang YF, Xu W, et al. The blood-brain barrier permeability of six indole alkaloids from Uncariae Ramulus Cum Uncis in the MDCK-pHaMDR cell monolayer model[J]. Molecules, 2017, 22: 1944. DOI:10.3390/molecules22111944 |
| [19] |
Cao CH, Chen CS, Zhao ZH, et al. Biomolecular mechanism of lingzhiol against chronic kidney disease based on network pharmacology[J]. Immunol J (免疫学杂志), 2021, 37: 910-916. |
| [20] |
Liu XJ, Zheng XY, Li ZY, et al. 1H-NMR metabonomic study on brain tissues of chronic unpredicted mild stress model of depression in rats[J]. Drug Eval Res (药物评价研究), 2019, 42: 612-621. |
| [21] |
Abreu AC, Navas MM, Fernández CP, et al. NMR-based metabolomics approach to explore brain metabolic changes induced by prenatal exposure to autisminducing chemicals[J]. ACS Chem Biol, 2021, 16: 753-765. DOI:10.1021/acschembio.1c00053 |
| [22] |
Liu X, Ruan Z, Shao XC, et al. Protective effects of 28-O-caffeoyl betulin (B-CA) on the cerebral cortex of ischemic rats revealed by a NMR-based metabolomics analysis[J]. Neurochem Res, 2021, 46: 686-698. DOI:10.1007/s11064-020-03202-z |
| [23] |
Martin F, Rezzi S, Montoliu I, et al. Metabolic assessment of gradual development of moderate experimental colitis in IL-10 deficient mice[J]. J Proteome Res, 2009, 8: 2376-2387. DOI:10.1021/pr801006e |
| [24] |
Zhang TT, Zheng H, Fan K, et al. NMR-based metabolomics characterizes metabolic changes in different brain regions of streptozotocin-induced diabetic mice with cognitive decline[J]. Metab Brain Dis, 2020, 35: 1165-1173. DOI:10.1007/s11011-020-00598-z |
| [25] |
Andersson PM, Sjöström M, Lundstedt T. Preprocessing peptide sequences for multivariate sequence-property analysis[J]. Chemometr Intell Lab Syst, 1998, 42: 41-50. DOI:10.1016/S0169-7439(98)00062-8 |
| [26] |
Gong PY, Guo YJ, Li XP, et al. Exploring active compounds of Jinhua Qinggan Granules for prevention of COVID-19 based on network pharmacology and molecular docking[J]. Chin Tradit Herb Drugs (中草药), 2020, 51: 1685-1693. |
| [27] |
Qiao S, Feng JC, Yang J, et al. Comparative study on neuroethology and pathology in rat model with spontaneous hypertension and persistent hypoperfusion[J]. Med J Chin People's Lib Army (解放军医学杂志), 2008, 33: 725-729. DOI:10.1016/S1872-2040(08)60071-7 |
| [28] |
Singh A, Jenkins MA, Burke KJ, et al. Glutamatergic tuning of hyperactive striatal projection neurons controls the motor response to dopamine replacement in parkinsonian primates[J]. Cell Rep, 2018, 22: 941-952. DOI:10.1016/j.celrep.2017.12.095 |
| [29] |
Xu XH, Chen Y, Zheng XX. Protective effects of breviscapine against cultured rat hippocampal neuronal toxicity induced by glutamate[J]. Acta Pharm Sin (药学学报), 2007, 42: 583-588. |
| [30] |
Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions[J]. Nature, 2004, 431: 195-199. DOI:10.1038/nature02827 |
| [31] |
Luo C, Xia ZY, Zhao YH. Research progress on the coupling of energy metabolism between neurons-astrocytes in the brain[J]. Chongqing Med J (重庆医学), 2018, 47: 1244-1247. |
| [32] |
Zhang DW. Identification and Mechanism of RIP3 as a Molecular Switch Between Apoptosis and Necrosis (RIP3作为细胞凋亡与细胞坏死相互转换的分子开关的发现及机理研究) [D]. Xiamen: Xiamen University, 2009.
|
| [33] |
Chen HJ, Shen YC, Lin CY, et al. Metabolomics study of Buyang Huanwu Tang Decoction in ischemic stroke mice by 1H NMR[J]. Metabolomics, 2012, 8: 974-984. DOI:10.1007/s11306-011-0394-0 |
| [34] |
Morland C, Pettersen MN, Hassel B. Hyperosmolar sodium chloride is toxic to cultured neurons and causes reduction of glucose metabolism and ATP levels, an increase in glutamate uptake, and a reduction in cytosolic calcium[J]. Neurotoxicology, 2016, 54: 34-43. DOI:10.1016/j.neuro.2016.03.005 |
| [35] |
Sun M, Zhao YM, Xu C. Taurine reduces the disorder of energy metabolism and oxidative damage due to focal cerebral ischemia in rats[J]. J Apoplexy Nerv Dis (中风与神经疾病杂志), 2008, 25: 577-579. DOI:10.1016/S1872-2075(08)60026-6 |
 2022, Vol. 57
2022, Vol. 57


