2. 江苏省中医药研究院, 中药组分与微生态研究中心, 江苏 南京 210028
2. Multi-component of Traditional Chinese Medicine and Microecology Research Center, Jiangsu Provincial Academy of Chinese Medicine, Nanjing 210028, China
年龄相关性黄斑变性(age-related macular degeneration, AMD) 是一种多发于50岁以上中老年人群的黄斑区视网膜组织退行性疾病, 可导致不可逆的中心视力下降或丧失, 是继白内障、青光眼之后的第三大致盲性眼病[1]。目前防治AMD的新药研发主要聚焦在抗血管内皮生长因子(vascular endothelial growth factor, VEGF)、抗氧化应激损伤、抑制炎症反应、视觉周期修饰、神经保护、减少毒性产物蓄积等环节, 其中以雷珠单抗、阿柏西普等为代表的抗VEGF药物治疗已成为新生血管性AMD的一线疗法[2-4]。但临床应用的抗VEGF药物均为生物大分子, 价格普遍偏高且作用时效短, 需要频繁持久的玻璃体腔注射才能维持疗效, 存在潜在的停药易复发、长期预后不佳、脑卒中、血栓等系统风险[5]。因此, 亟须进一步了解AMD的发病机制, 探寻更加安全有效、经济实用的AMD防治药物或策略。
近年来, 中医药防治AMD正逐渐成为推动眼底病研究进程的一个重要方向。研究表明, 许多来源于活血化瘀、清热解毒、祛痰除湿或补气养血类中药的活性成分具有显著的抗氧化、抗炎、抗衰老、抗凋亡等药理作用, 可通过不同环节、不同途径干预和阻断AMD的发生发展过程, 对防治AMD具有重要意义, 值得进一步研究开发。因此, 本综述将在阐明AMD发病机制的基础上, 归纳总结不同类型的中药活性成分对AMD的调控作用及其作用机制, 以期为AMD的预防和治疗提供新的思路。
1 AMD的发病机制视网膜色素上皮(retinal pigment epithelial, RPE) 层是视网膜营养物质供给和代谢产物清除的主要场所, 在维持神经细胞内外环境、保护视功能、免疫防御等方面发挥重要作用。在光照、衰老、吸烟、肥胖、高脂饮食、高血压等各种致病因素的刺激下, RPE细胞中的线粒体DNA (mitochondrial DNA, mtDNA) 发生损伤, 诱导过量活性氧(reactive oxygen species, ROS) 产生, 进而对RPE细胞造成不可逆性的氧化应激损伤, 影响其各项生理功能[6-8]。最新研究发现, RPE细胞的自噬功能障碍、衰老和异常的免疫炎症反应与AMD的发生发展密切相关, 这些机制相互协同、交叉作用, 最终导致视力丧失(图 1)[9]。此外, 最新的生物医学数据库发掘结果发现, 包括白介素-6 (interleukin-6, IL-6)、血管内皮生长因子-A (vascular endothelial growth factor-A, VEGF-A)、肿瘤蛋白P53 (tumor protein P53, TP53)、白介素-1β (interleukin-1β, IL-1β) 和转化生长因子-β1 (transforming growth factor-β1, TGF-β1) 在内的5个中枢基因在RPE细胞自噬、衰老和炎症反应中起关键作用, 为未来AMD的防治提供了更为明确的分子机制, 也为精准药物开发奠定了基础[10]。

|
Figure 1 The relationship between oxidative stress and RPE cell autophagy dysfunction, cellular senescence, and abnormal immune-inflammatory response in AMD. It is likely that mtDNA damage in the neural retina and RPE cells is associated with many risk factors of AMD and results in ROS overproduction, leading to excessive oxidative stress to RPE cells. The accumulated oxidative stress damage to RPE cells can result in autophagy dysfunction, cellular senescence, and abnormal immune-inflammatory response. These factors interact with each other, causing photoreceptor damage, RPE cell injury or atrophy, drusen formation, lipofuscin deposition and choroid degeneration, and ultimately, loss of vision. RPE: Retinal pigment epithelial; AMD: Age-related macular degeneration; mtDNA: Mitochondrial DNA; ROS: Reactive oxygen species |
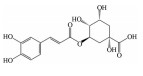
多酚类化合物是一类复杂的具有多个酚羟基的次生代谢产物, 广泛分布于植物中, 其中以白藜芦醇(resveratrol)、姜黄素(curcumin)、木犀草素(luteolin)、漆黄素(fisetin)、绿原酸(chlorogenic acid)、葛根素(puerarin)、没食子酸(gallic acid)、表没食子儿茶素-没食子酸酯(epigallocatechin-3-gallate) 等为代表的中药多酚类成分已被证明可通过多种作用机制预防和治疗AMD (表 1[11-36])。
| Table 1 Effect and mechanism against AMD of polyphenols in traditional Chinese medicine (TCM). HO-1: Heme oxygenase-1; mRNA: Messenger RNA; Bcl-2: B-cell lymphoma-2; PPARα: Peroxisome proliferators-activated receptors α; PPARδ: Peroxisome proliferators-activated receptors δ; IL-6: Interleukin-6; IL-8: Interleukin-8; MCP-1: Monocyte chemotactic protein 1; SIRT1: NAD-dependent deacetylase sirtuin-1; DNMT: DNA methyltransferase; LINE-1: Long interspersed nuclear element-1; HIF-1α: Hypoxia duciblefactors-1α; VEGF-A: Vascular endothelial growth factor-A; ARPE-19 cells: Human retinal pigment epithelial cells; VEGFR2: Vascular endothelial growth factor receptor 2; SOD: Superoxide dismutase; GSH: Glutathione; MDA: Malondialdehyde; Bax: B-cell lymphoma-2 associated x protein; Caspase-3: Cysteinyl aspartate-specific proteinase-3; NF-κB: Nuclear factor kappa-B; IL-1β: Interleukin-1β; PARP: Poly(ADP-ribose) polymerase; THP-1: Human monocytic leukemia cell line; MAPK: Mitogen-activated protein kinase; CREB: cAMP-response element binding protein; CXCL8: Recombinant human C-X-C motif chemokine 8; NFKBIA: Nuclear factor-kappa-B-inhibitor alpha; RELA: v-Rel reticuloendotheliosis viral oncogene homolog A; TRIB3: Tribbles pseudokinase 3; XBP1s: X-box binding protein 1s; COX-2: Cyclooxygenase 2; iNOS: Inducible nitric oxide synthase; HNE: 4-Hydroxynonenal; 8-OHdG: 8-Oxo-2′-deoxyguanosine; NLRP3: NLR family pyrin domain containing 3; ERK: Extracellular regulated protein kinase; UPR: Unfolded protein response; MRPE cells: Mouse retinal pigment epithelial cells; JNK1: c-Jun N-terminal kinase 1 |
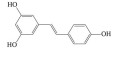
白藜芦醇(resveratrol, RES) 是一种主要存在于中药虎杖、藜芦中的非黄酮多酚类化合物。研究表明, RES可通过清除ROS、增强抗氧化酶活性、改善线粒体功能、激动过氧化物酶增殖剂激活受体α (peroxisome proliferators-activated receptors α, PPARα) 和过氧化物酶增殖剂激活受体δ (peroxisome proliferators-activated receptors δ, PPARδ)、上调抗氧化基因血红素加氧酶-1 (heme oxygenase-1, HO-1) mRNA和B淋巴细胞瘤-2基因(B-cell lymphoma-2, Bcl-2) 表达水平等途径降低RPE细胞的氧化应激损伤水平, 从而抑制早期AMD的发生[11-14]。同时, RES还可通过增强RPE细胞自噬功能, 降低IL-6、IL-8、单核细胞趋化蛋白-1 (monocyte chemotactic protein-1, MCP-1) 等促炎因子的表达和分泌, 减少免疫细胞对炎症部位的化学吸引和招募, 进而阻止AMD的进一步发展[15, 16]。Maugeri等[17]研究发现RES可通过调控NAD-依赖性去乙酰化酶sirtuin-1 (NAD-dependent deacetylase sirtuin-1, SIRT1) 和DNA甲基转移酶(DNA methyltransferase, DNMT) 功能, 恢复长散布核元件1 (long interspersed nuclear element-1, LINE-1) 甲基化水平, 降低ARPE-19细胞的氧化应激和炎症反应水平。此外, RES还可通过激活SIRT1, 下调RPE细胞中缺氧诱导因子-1α (hypoxia duciblefactors-1α, HIF-1α) 的表达水平和VEGF的分泌, 抑制内皮细胞中VEGFR2的磷酸化和激活, 发挥抗AMD效应[18]。
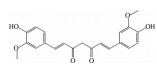
2.1.2 姜黄素姜黄素(curcumin) 是从中药姜黄、郁金、莪术的干燥根茎中提取出的一种有效成分。研究表明, 姜黄素可通过多种途径保护RPE细胞免受氧化应激损伤, 包括: 降低ROS生成水平, 增强超氧化物歧化酶(superoxide dismutase, SOD) 活性, 抑制丙二醛(malondialdehyde, MDA) 生成, 促进谷胱甘肽(glutathione, GSH) 生成, 上调抗凋亡蛋白Bcl-2及细胞保护酶HO-1、硫氧还蛋白的表达水平, 下调促凋亡蛋白Bcl-2相关x蛋白(B-cell lymphoma-2 associated x protein, Bax) 和天冬氨酸特异性半胱氨酸蛋白酶-3 (cysteinyl aspartate-specific proteinase-3, caspase-3) 表达水平等[19-22]; 此外, 姜黄素还可通过调控血小板衍生生长因子(platelet derived growth factor, PDGF)、VEGF、胰岛素样生长因子结合蛋白-2 (insulin-like growth factor-binding protein-2, IGFBP-2)、HO-1、SOD2、谷胱甘肽过氧化物酶1 (glutathione peroxidase 1, GPx1)、核因子κB (nuclear factor kappa-B, NF-κB)、蛋白激酶B (protein kinase B, PKB)、核因子红细胞相关因子2 (nuclear factor erythroid-related factor 2, Nrf2) 等氧化应激-炎症相关基因的表达, 增强RPE细胞的抗氧化防御能力, 抑制炎症反应并保护细胞[23]。在激光诱导的C57BL/6N小鼠AMD模型中, 姜黄素可通过抑制NF-κB和HIF-1α的激活, 下调肿瘤坏死因子-α (tumor necrosis factor-α, TNF-α)、MCP-1、细胞黏附分子-1 (intercellular cell adhesion molecule-1, ICAM-1)、VEGF等炎症-血管生成相关细胞因子的表达, 减少F4/80阳性巨噬细胞和GR-1阳性粒细胞的浸润, 从而阻断脉络膜新生血管(choroidal neovascularization, CNV) 和炎症反应的发生发展过程[24]。
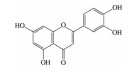
2.2 黄酮类及其糖苷绝大多数中药中都含有黄酮类化合物, 其中槲皮素(quercetin)、黄芩苷(baicalin)、黄芩素(baicalein)、汉黄芩素(wogonin)、芹菜素(apigenin)、白杨素(chrysin)、飞燕草素(delphinidin)、飞燕草素-3-O-葡萄糖苷(delphinidin-3-O-glucoside)、山柰酚(kaempferol)、矢车菊素-3-O-葡萄糖苷(cyanidin-3-O-glucoside)、番石榴苷(guaijaverin)、泽兰黄酮(nepetin)、锦葵素(malvidin)、锦葵素-3-O-葡萄糖苷(malvidin-3-O-glucoside)、橙皮素(hesperetin)、水飞蓟宾(silibinin)、表儿茶素(epicatechin)、染料木素(genistein)、柚皮素(naringenin)、杜鹃素(farrerol) 等已被证实有抗AMD的疗效(表 2[37-64])。
| Table 2 Effect and mechanism against AMD of flavonoids in TCM. PI3K/AKT: Phosphatidylinositol 3-hydroxykinase/protein kinase B; MAPK: Mitogen-activated protein kinases; Keap1: Kelch-1ike ECH-associated protein l; Nrf2: Nuclear factor erythroid-related factor 2; ARE: Antioxidant response element; ER: Endoplasmic reticulum; FADD: Fas associated via death domain; NO: Nitric oxide; COX: Cyclo-oxygenase; PGE-2: Prostaglandin E2; A2E: N-Retinylidene-N-retinyl ethanolamine; Aβ: Amyloid-β; NLRP3: NOD-like receptor protein 3; MMP-2: Matrix metalloproteinase 2; PDGF: Platelet derived growth factor; Fak: Focal adhesion kinase; Syk: Spleen tyrosine kinase; Src: Src proto-oncogene non-receptor tyrosine kinase; MMP-9: Matrix metalloproteinase 9; CREB: cAMP-response element binding protein; TLR4: Toll-like receptor 4; LPS: Lipopolysaccharide; CNV: Choroidal neovascularization; RPE-BM-CC: Retinal pigment epithelial-Bruch's membrane-choroidal capillaries; Nox-1: NADPH oxidase 1; JNK-c-Jun: C-Jun N-terminal kinase; AP-1: Activator protein-1; CYP1A1: Cytochrome P450 family 1 subfamily A member 1; CYP1B1: Cytochrome P450 family 1 subfamily B member 1; MIO-M1 cells: Retinal Müller stem cells; ICAM-1: Intercellular cell adhesion molecule-1; Ets-1: E-Twenty-Six-1 |
槲皮素是一种广泛存在于槐米、侧柏叶、高良姜、款冬花、桑寄生、三七、银杏等中药中的黄酮类成分。研究发现, 槲皮素可通过抑制磷脂酰肌醇3-羟激酶/蛋白激酶B (phosphatidylinositol 3-hydroxykinase/protein kinase B, PI3K/AKT) 信号通路改善ROS介导的线粒体功能障碍, 下调Bax、裂解caspase-3和裂解多聚腺苷二磷酸核糖聚合酶[poly(ADP-ribose) polymerase, PARP] 的表达, 从而保护RPE细胞和视网膜免于细胞凋亡[37]。槲皮素还可通过调控p38和细胞外信号调节激酶/丝裂原活化蛋白激酶(extracellular signal-regulated kinase/mitogen-activated protein kinases, ERK/MAPK) 途径以及环磷腺苷效应元件结合蛋白(cAMP-response element binding protein, CREB) 信号传导, 改善细胞膜的完整性和线粒体功能, 降低促炎因子IL-6、IL-8、MCP-1的分泌, 从而降低4-羟基壬烯醛(4-hydroxynonenal, HEN) 诱导的ARPE-19细胞的毒性和炎症反应[38]。此外, 槲皮素还可通过激活Kelch样ECH相关蛋白1 (Kelch-1ike ECH-associated protein l, Keap1)/Nrf2/抗氧化反应元件(antioxidant response element, ARE) 途径, 抑制内质网应激并靶向抗凋亡蛋白, 保护ARPE-19细胞免于损伤[39, 40]。Cao等[41]研究发现, 在H2O2诱导ARPE-19细胞氧化应激损伤模型中, 槲皮素可通过调控Bcl-2、Bax、Fas相关死亡域蛋白(Fas-associated death domain protein, FADD)、caspase-3、caspase-9等凋亡相关基因的表达发挥保护作用; 在Ccl2-/-/Cx3cr1-/-小鼠模型中, 槲皮素可显著降低血清中一氧化氮(nitric oxide, NO)、环氧酶(cyclo-oxygenase, COX) 和前列腺素E2 (prostaglandin E2, PEG2) 的水平, 同时降低眼内N-亚视黄基-N-视黄基乙醇胺(N-retinyl idene-N-retinyl ethanolamine, A2E) 的含量, 表现出抗AMD的潜力。
2.2.2 黄芩苷、黄芩素、汉黄芩素黄芩苷、黄芩素、汉黄芩素是从中药黄芩根部提取的主要活性成分。研究表明, 黄芩苷可通过诱导miR-223的上调和NOD样受体蛋白3 (NOD-like receptor protein 3, NLRP3) 的下调, 从而抑制NLRP3炎性小体信号传导引发的ARPE-19细胞焦亡[42]; 黄芩苷还可通过下调VEGF、PDGF、基质金属蛋白酶2 (matrix metalloproteinase 2, MMP-2) 的水平, 抑制激光诱导的大鼠眼内CNV的形成[43]。RPE细胞的上皮-间质转化(epithelial-mesenchymal transition, EMT) 与AMD的发生发展密切相关。Park等[44]研究发现, 黄芩素可通过阻断黏附斑激酶(focal adhesion kinase, FAK) 介导的脾酪氨酸激酶(spleen tyrosine kinase, Syk)/Src原癌基因非受体酪氨酸激酶(Src proto-oncogene non-receptor tyrosine kinase, Src) 信号通路, 抑制香烟烟雾提取物诱导的人原代视网膜色素上皮细胞(human primary retinal pigment epithelial cells, HRPEpi) 中EMT相关细胞因子TGF-β1、VEGF分泌, 继而下调EMT标志物E-钙黏蛋白、闭锁小带蛋白(zonula occludens-1, ZO-1)、波形蛋白、α-平滑肌肌动蛋白(α-smooth muscle actin, SMA) 的表达。此外, 黄芩素可通过清除ROS, 下调MMP-9和VEGF的表达, 保护人视网膜色素上皮细胞免受H2O2诱导的氧化应激损伤[45, 46]。汉黄芩素与黄芩素的结构相似, 但其防治AMD的作用机制有所不同。已有研究发现, 汉黄芩素可通过下调PI3K/AKT信号通路抑制H2O2诱导的RPE细胞凋亡[47]; 汉黄芩素还可通过抑制Toll-样受体4 (Toll-like receptor 4, TLR4)/NF-κB信号通路改善脂多糖诱导的ARPE-19细胞的屏障功能障碍和炎症反应[48]。
2.3 萜类及其糖苷以芍药苷(paeoniflorin)、雷公藤红素(celastrol)、雷公藤甲素(triptolide)、穿心莲内酯(andrographolide)、青蒿素(artemisinin) 等为代表的萜类及其糖苷类化合物也被证明具有防治AMD的潜力(表 3[65-75])。
| Table 3 Effect and mechanism against AMD of terpenoids in TCM. atRAL: All-trans-retinal; AMPK: Adenosine monophosphate-activated protein kinase; Hsp70: Heat shock protein 70; CaMKII: Calcium/calmodulin-dependent protein kinase type Ⅱ; GCLM: Glutamate-cysteine ligase; ICAM-1: Intercellular cell adhesion molecule-1; TNF-α: Tumor necrosis factor-α |
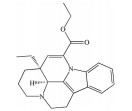
芍药苷是一种单萜糖苷类化合物, 为中药赤芍、白芍的主要有效成分, 也是其质量控制的指标性成分。现代药理学研究表明, 芍药苷可通过抑制氧化亢进、增强抗氧化能力、改善线粒体功能、抑制钙离子超载、抗凋亡、抑制/活化相关信号通路等多途径、多靶点发挥抗氧化应激作用, 是一种高效的抗氧化剂。已有研究发现, 芍药苷可通过触发钙/钙调蛋白依赖性蛋白激酶Ⅱ型(calcium/calmodulin-dependent protein kinase type Ⅱ, Ca2+/CaMKII) 依赖的AMPK激活, 显著降低视黄醛(all-trans-retinal, atRAL) 诱导的ARPE-19细胞的氧化应激、线粒体功能障碍和内质网应激[65]; 同时, 芍药苷还可通过下调p38MAPK和ERK的磷酸化水平, 保护ARPE-19细胞免受H2O2诱导氧化应激损伤[66]。
2.3.2 雷公藤红素雷公藤红素是从中药雷公藤根部提取的一种药理活性较高的五环三萜类成分。研究发现, 雷公藤红素可通过激活Nrf2信号通路, 上调谷氨酸半胱氨酸连接酶(glutamate-cysteine ligase, GCLM) 和HO-1的表达, 保护ARPE-19细胞免于氧化应激诱导的细胞死亡[67]; 雷公藤红素还可通过激活sirtuin 3信号通路, 抑制H2O2介导的ARPE-19细胞的氧化应激、自噬功能障碍和细胞凋亡[68]。最新研究发现, 热休克蛋白70 (heat shock protein 70, Hsp70) 是影响NF-κB活性的关键调节剂, 雷公藤红素可通过调控NF-κB和Hsp70的表达, 抑制脂多糖诱导的ARPE-19细胞的先天免疫应答[69]。
2.4 生物碱类在众多生物碱中, 小檗碱(berberine)、长春西汀(vinpocetine)、川芎嗪(tetramethylpyrazine)、血根碱(sanguinarine) 已被用于防治AMD的研究(表 4[76-81])。其中, 小檗碱又名黄连素, 是从中药黄连、黄柏中分离的一种季铵生物碱。研究表明, 小檗碱能够以时间和剂量依赖性的方式激活人源RPE细胞系D407细胞中AMPK的磷酸化, 从而恢复H2O2诱导的核形态的异常变化, 减少线粒体膜电位的下降和乳酸脱氢酶的释放, 保护细胞免受氧化应激损伤[76]。在光感受器变性的光损伤小鼠模型中, 小檗碱治疗可显著提高视网膜神经上皮层Rho mRNA和RPE层Rpe65 mRNA、Mct3 mRNA的水平, 并降低视网膜氧化应激基因mRNA的水平、小胶质细胞/巨噬细胞的数量和MDA免疫标记, 表明小檗碱可能对与氧化应激相关的视网膜疾病如AMD有防治作用[77]。
| Table 4 Effect and mechanism against AMD of alkaloids in TCM. AMPK: Adenosine monophosphate-activated protein kinase; NSR: Neurosensory retinal; Rho: Rhodopsin; Rpe65: Retinoid isomerohydrolase; Mct3: Monocarboxylate transporter 3; MNU: N-Methyl-N-nitrosourea; MDA: Malondialdehyde; Aβ: β-Amyloid |
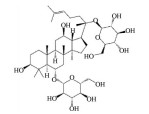
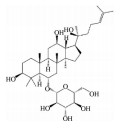
皂苷也是中药中一类重要的活性物质, 黄芪皂苷(astragaloside Ⅳ)、人参皂苷(ginsenoside)、羟基积雪草苷(madecassoside)、甘草甜素(glycyrrhizin) 等已被证明具有良好的抗AMD活性(表 5[82-86])。其中, 黄芪甲苷为羊毛脂醇形的四环三萜皂苷, 是中药黄芪的主要活性成分之一。Chen等[82]研究发现, 黄芪甲苷可通过下调肿瘤坏死因子受体相关因子5 (tumor necrosis factor receptor-associated factor 5, TRAF5) 和NF-κB的表达, 减轻异氟烷诱导的RPE细胞的神经退行性变化。Sun等[83]制备的负载黄芪甲苷的超小型脂质纳米胶囊滴眼液可显著降低干性AMD小鼠模型视网膜组织中ROS的产生和细胞凋亡率, 且对视网膜的形态和功能表现出良好的保护作用。
| Table 5 Effect and mechanism against AMD of saponins in TCM. TRAF: Tumor necrosis factor receptor-associated factor |
丹参酮IIA (tanshinone IIA) 是从中药丹参干燥根部提取的一种醌类成分。研究表明, 在氯化钴诱导的缺氧条件下, 丹参酮IIA能够以剂量依赖的方式抑制ARPE-19细胞中VEGF的分泌和HIF-1α的表达, 表现出抗湿性AMD的潜力[87]; 在H2O2诱导的ARPE-19细胞氧化应激损伤模型中, 丹参酮IIA磺酸钠可通过激活PI3K/AKT/哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin, mTOR) 通路抑制细胞自噬, 并通过调控Bax、MMP、caspase-9、caspase-3和Bcl-2等蛋白的表达抑制细胞凋亡[88]。
2.7 其他除了上述各类化合物外, 丹酚酸A (salvianolic acid A)、叶黄素(lutein)、大蒜素(allicin)、岩藻多糖(fucoidan)、红景天苷(salidroside) 和七叶内酯(esculetin) 也具有防治AMD的作用(表 6[89-97])。丹酚酸A是来源于中药丹参的一种水溶性单体成分, 研究发现丹酚酸A不但可激活Nrf2/HO-1轴, 还可激活AKT/mTORC1信号通路, 保护RPE细胞抵抗H2O2诱导的氧化应激损伤[89]。在氧化-低密度脂蛋白(oxidized-low density lipoprotein, ox-LDL) 诱导的体内外AMD模型中, 丹酚酸A可通过上调Nrf2和下调嘌呤能离子通道型7受体/双链RNA依赖的蛋白质激酶/NOD样受体蛋白3 (purinergic ligand-gated ion channel 7 receptor/double-stranded RNA-dependent protein kinase/NOD-like receptor protein 3, P2x7r-Pkr-Nlrp3) 信号通路, 改善RPE的氧化应激和慢性炎症反应[90]; 丹酚酸A还可通过降低VEGF/PDGF/肿瘤抑制因子(cylindromatosis, CYLD) 表达、提高抗血管生成素水平和促进P62-CYLD-TRAF6相互作用等途径拮抗ox-LDL效应并抑制CNV发展[91]。
| Table 6 Effect and mechanism against AMD of other components in TCM. P2x7r: Purinergic ligand-gated ion channel 7 receptor; Pkr: Double-stranded RNA-dependent protein kinase; CYLD: Cylindromatosis; NAPDH: Nicotinamide adenine dinucleotide phosphate; GSK-3β: Glycogen synthase kinase 3β; TNFR: Tumor necrosis factor receptor; TRAIL: TNF-related apoptosis-inducing ligand |
随着对AMD发病机制的不断阐明, 以血管新生为靶点的抗VEGF治疗已经不能满足当下的治疗需求。血管新生只是湿性AMD的一个核心病理表现, 而AMD是一种复杂的多因素疾病, 抗VEGF治疗只能在一定程度上抑制CNV生成, 改善AMD症状, 但不能阻断诱导AMD病程发生发展的其他致病因素如氧化应激、慢性炎症等的影响, 进而导致其疗效有限、长期预后不佳。从AMD发病的多个环节进行联合阻断的策略有望获得更加广泛和强大的疗效, 也是未来抗AMD治疗最有潜力的发展方向。
基于对AMD发病机制的深入了解, 作者发现目前在研的中药活性成分主要是通过协同调控多个途径发挥作用, 包括抗氧化应激、抗炎、抗血管生成、抗细胞衰老、抗细胞凋亡、改善细胞自噬功能障碍、恢复线粒体功能、保护视网膜神经等。具体而言, 中药活性成分可通过[98-100]①清除ROS、抑制脂质过氧化、提高抗氧化酶活性、激活Nrf2信号通路、增加HO-1等抗氧化蛋白的表达, 保护视网膜RPE层免于氧化应激和线粒体应激诱导的细胞衰老和凋亡; ②改善RPE细胞自噬功能障碍, 增强其清除氧化/糖化蛋白质/脂质、变性线粒体和炎症分子的能力, 抑制细胞外多态性碎片(玻璃膜疣和视网膜下玻璃膜疣) 的积累, 恢复连接RPE与光感受器、布鲁赫氏膜和绒毛膜毛细血管的旁分泌稳态; ③抑制炎症相关蛋白酶(COX-1和COX-2等) 和促炎因子(白介素、肿瘤坏死因子、黏附分子、前列腺素、趋化因子等) 的表达及炎症小体的释放, 减少化学吸引和免疫细胞向炎症部位的募集及补体系统的异常激活, 改善视网膜RPE层异常的免疫炎症反应; ④抑制促血管生成因子和细胞因子(VEGF、PDGF、FGF、TGF-β、MMP、血管生成素-2等) 的分泌, 减少血管内皮细胞的增殖和迁移, 最终阻断CNV的形成, 遏制AMD病程的进一步恶化。
综上, 中药活性成分具有多靶点-多通路协同调控的优势, 与当前AMD的防治需求正好吻合, 其在防治AMD方面的潜力也在不断被挖掘和开发, 但距离新药转化和临床应用仍存在一定的差距。首先, 目前对中药活性成分防治AMD的研究大多停留在药物初筛阶段, 只在分子、细胞和实验动物水平对其药效进行确证, 作用机制的阐明也仅局限于某一信号通路的一个或若干个信号蛋白的检测, 对成分来源的易得性、体内吸收、分布、代谢、排泄过程、安全性与毒性缺乏考究, 因此将其转化为新药的可行性和可靠性还有待商榷, 后续研究应不断完善和加强临床前研究和大规模临床验证试验。其次, 许多中药活性成分的生物药剂学性质不佳, 如水溶性差或渗透性不强, 全身给药(口服或静脉注射) 存在体内生物利用度低、组织分布缺乏选择性、血-眼屏障阻碍药物递送、高剂量易产生毒副作用等问题, 难以达到预期疗效; 局部玻璃体注射给药可跨越血眼屏障, 直达病灶, 起效快, 但小分子药物易通过房水周转的前路途径和葡萄膜血流的后路途径被快速清除, 作用时间短, 继而影响其药效发挥。因此, 如何改善这些中药活性成分的理化性质缺陷, 促进其在视网膜病灶部位的精准靶向、持续稳定释药, 延长作用时间, 提高抗AMD疗效, 是广大药剂学工作者需要解决的关键问题。近年来, 以PLGA纳米粒、囊泡、胶束、脂质体、智能化水凝胶等为代表的新型载体系统在眼部药物递送方面表现卓越, 将有望为上述中药活性组分的眼后段视网膜靶向递送提供有利参考[101-103]。
作者贡献: 该文章由刘聪燕收集资料和撰写; 陈彦和瞿鼎为文章提供了重要指导和关键意见。
利益冲突: 所有作者均声明不存在利益冲突。
| [1] |
Datta S, Cano M, Ebrahimi K, et al. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD[J]. Prog Retin Eye Res, 2017, 60: 201-218. DOI:10.1016/j.preteyeres.2017.03.002 |
| [2] |
Kumar Dubey S, Pradhan R, Hejmady S, et al. Emerging innovations in nano-enabled therapy against age-related macular degeneration: a paradigm shift[J]. Int J Pharm, 2021, 600: 120499. DOI:10.1016/j.ijpharm.2021.120499 |
| [3] |
Fleckenstein M, Keenan TDL, Guymer RH, et al. Age-related macular degeneration[J]. Nat Rev Dis Primers, 2021, 1: 31. DOI:10.1038/s43586-021-00035-0 |
| [4] |
Iyer S, Radwan AE, Hafezi-Moghadam A, et al. Long-acting intraocular delivery strategies for biological therapy of age-related macular degeneration[J]. J Control Release, 2019, 296: 140-149. DOI:10.1016/j.jconrel.2019.01.007 |
| [5] |
Ammar MJ, Hsu J, Chiang A, et al. Age-related macular degeneration therapy: a review[J]. Curr Opin Ophthalmol, 2020, 31: 215-221. DOI:10.1097/ICU.0000000000000657 |
| [6] |
Kaarniranta K, Uusitalo H, Blasiak J, et al. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration[J]. Prog Retin Eye Res, 2020, 79: 100858. DOI:10.1016/j.preteyeres.2020.100858 |
| [7] |
Baek A, Son S, Baek YM, et al. KRT8 (keratin 8) attenuates necrotic cell death by facilitating mitochondrial fission-mediated mitophagy through interaction with PLEC (plectin)[J]. Autophagy, 2021. DOI:10.1080/15548627.2021.1897962 |
| [8] |
Mehrzadi S, Hemati K, Reiter RJ, et al. Mitochondrial dysfunction in age-related macular degeneration: melatonin as a potential treatment[J]. Expert Opin Ther Tar, 2020, 24: 359-378. DOI:10.1080/14728222.2020.1737015 |
| [9] |
Wang S, Wang X, Cheng Y, et al. Autophagy dysfunction, cellular senescence, and abnormal immune-inflammatory responses in AMD: from mechanisms to therapeutic potential[J]. Oxid Med Cell Longev, 2019, 2019: 3632169. |
| [10] |
Wang S, Liu C, Ouyang W, et al. Common genes involved in autophagy, cellular senescence and the inflammatory response in AMD and drug discovery identified via biomedical databases[J]. Transl Vis Sci Technol, 2021, 10: 14. |
| [11] |
Delmas D, Cornebise C, Courtaut F, et al. New highlights of resveratrol: a review of properties against ocular diseases[J]. Int J Mol Sci, 2021, 22: 1295. DOI:10.3390/ijms22031295 |
| [12] |
Yang Y, Wu Z, Cheng Y, et al. Resveratrol protects against oxidative damage of retinal pigment epithelium cells by modulating SOD/MDA activity and activating Bcl-2 expression[J]. Eur Rev Med Pharmacol Sci, 2019, 23: 378-388. |
| [13] |
Neal SE, Buehne KL, Besley NA, et al. Resveratrol protects against hydroquinone-induced oxidative threat in retinal pigment epithelial cells[J]. Invest Ophth Vis Sci, 2020, 61: 32. |
| [14] |
Pawlowska E, Szczepanska J, Koskela A, et al. Dietary polyphenols in age-related macular degeneration: protection against oxidative stress and beyond[J]. Oxid Med Cell Longev, 2019, 2019: 9682318. |
| [15] |
Josifovska N, Albert R, Nagymihály R, et al. Resveratrol as inducer of autophagy, pro-survival, and anti-inflammatory stimuli in cultured human RPE cells[J]. Int J Mol Sci, 2020, 21: 813. DOI:10.3390/ijms21030813 |
| [16] |
Lançon A, Frazzi R, Latruffe N. Anti-oxidant, anti-inflammatory and anti-angiogenic properties of resveratrol in ocular diseases[J]. Molecules, 2016, 21: 304. DOI:10.3390/molecules21030304 |
| [17] |
Maugeri A, Barchitta M, Mazzone MG, et al. Resveratrol modulates SIRT1 and DNMT functions and restores LINE-1 methylation levels in ARPE-19 cells under oxidative stress and inflammation[J]. Int J Mol Sci, 2018, 19: 2118. DOI:10.3390/ijms19072118 |
| [18] |
Zhang H, He S, Spee C, et al. SIRT1 mediated inhibition of VEGF/VEGFR2 signaling by resveratrol and its relevance to choroidal neovascularization[J]. Cytokine, 2015, 76: 549-552. DOI:10.1016/j.cyto.2015.06.019 |
| [19] |
Franzone F, Nebbioso M, Pergolizzi T, et al. Anti-inflammatory role of curcumin in retinal disorders (Review)[J]. Exp Ther Med, 2021, 22: 790. DOI:10.3892/etm.2021.10222 |
| [20] |
Radomska-Leśniewska DM, Osiecka-Iwan A, Hyc A, et al. Therapeutic potential of curcumin in eye diseases[J]. Cent Eur J Immunol, 2019, 44: 181-189. DOI:10.5114/ceji.2019.87070 |
| [21] |
Mandal MNA, Patlolla JMR, Zheng L, et al. Curcumin protects retinal cells from light-and oxidant stress-induced cell death[J]. Free Radic Biol Med, 2009, 46: 672-679. DOI:10.1016/j.freeradbiomed.2008.12.006 |
| [22] |
Zhu W, Wu Y, Meng YF, et al. Effect of curcumin on aging retinal pigment epithelial cells[J]. Drug Des Devel Ther, 2015, 9: 5337-5344. |
| [23] |
Chang YC, Chang WC, Kuo-Hsuan H, et al. The generation of induced pluripotent stem cells for macular degeneration as a drug screening platform: identification of curcumin as a protective agent for retinal pigment epithelial cells against oxidative stress[J]. Front Aging Neurosci, 2014, 6: 191. |
| [24] |
Xie P, Zhang W, Yuan S, et al. Suppression of experimental choroidal neovascularization by curcumin in mice[J]. PLoS One, 2012, 7: e53329. DOI:10.1371/journal.pone.0053329 |
| [25] |
Huang W, Liou C, Shen S, et al. Luteolin attenuates IL-1β-induced THP-1 adhesion to ARPE-19 cells via suppression of NF-κB and MAPK pathways[J]. Mediators Inflamm, 2020, 2020: 9421340. |
| [26] |
Hytti M, Piippo N, Korhonen E, et al. Fisetin and luteolin protect human retinal pigment epithelial cells from oxidative stress-induced cell death and regulate inflammation[J]. Sci Rep, 2015, 5: 17645. |
| [27] |
Govindaraju VK, Bodas M, Vij N, et al. Cigarette smoke induced autophagy-impairment regulates AMD pathogenesis mechanisms in ARPE-19 cells[J]. PLoS One, 2017, 12: e0182420. DOI:10.1371/journal.pone.0182420 |
| [28] |
Pham TNM, Shin C, Park SH, et al. Solanum melongena L. extract protects retinal pigment epithelial cells from blue light-induced phototoxicity in in vitro and in vivo models[J]. Nutrients, 2021, 13: 359. DOI:10.3390/nu13020359 |
| [29] |
Wang Y, Zhao L, Wang C, et al. Protective effect of quercetin and chlorogenic acid, two polyphenols widely present in edible plant varieties, on visible light-induced retinal degeneration in vivo[J]. J Funct Foods, 2017, 33: 103-111. DOI:10.1016/j.jff.2017.02.034 |
| [30] |
Wang K, Zhu X, Zhang K, et al. Puerarin inhibits amyloid β-induced NLRP3 inflammasome activation in retinal pigment epithelial cells via suppressing ROS-dependent oxidative and endoplasmic reticulum stresses[J]. Exp Cell Res, 2017, 2: 335-340. |
| [31] |
Shanmuganathan S, Angayarkanni N. Chebulagic acid and gallic acid, the active principles of triphala, inhibit TNFα induced pro-angiogenic and pro-inflammatory activities in retinal capillary endothelial cells by inhibiting p38, ERK and NFκB phosphorylation[J]. Vascul Pharmacol, 2018, 108: 23-35. DOI:10.1016/j.vph.2018.04.005 |
| [32] |
Cao G, Chen M, Song Q, et al. EGCG protects against UVB-induced apoptosis via oxidative stress and the JNK1/c-Jun pathway in ARPE19 cells[J]. Mol Med Rep, 2012, 5: 54. |
| [33] |
Li C, Yao J, Tao Z, et al. Epigallocatechin-gallate (EGCG) regulates autophagy in human retinal pigment epithelial cells: a potential role for reducing UVB light-induced retinal damage[J]. Biochem Biophys Res Commun, 2013, 438: 739-745. DOI:10.1016/j.bbrc.2013.07.097 |
| [34] |
Chan CM, Huang JH, Lin HH, et al. Protective effects of (-)-epigallocatechin gallate on UVA-induced damage in ARPE19 cells[J]. Mol Vis, 2008, 14: 2528-2534. |
| [35] |
Karthikeyan B, Harini L, Krishnakumar V, et al. Insights on the involvement of (-)-epigallocatechin gallate in ER stress-mediated apoptosis in age-related macular degeneration[J]. Apoptosis, 2017, 22: 72-85. DOI:10.1007/s10495-016-1318-2 |
| [36] |
Xu J, Tu Y, Wang Y, et al. Prodrug of epigallocatechin-3-gallate alleviates choroidal neovascularization via down-regulating HIF-1alpha/VEGF/VEGFR2 pathway and M1 type macrophage/microglia polarization[J]. Biomed Pharmacother, 2020, 121: 109606. DOI:10.1016/j.biopha.2019.109606 |
| [37] |
Chang Y, Lee Y, Hsu M, et al. Protective effect of quercetin on sodium iodate-induced retinal apoptosis through the reactive oxygen species-mediated mitochondrion-dependent pathway[J]. Int J Mol Sci, 2021, 22: 4056. DOI:10.3390/ijms22084056 |
| [38] |
Hytti M, Piippo N, Salminen A, et al. Quercetin alleviates 4-hydroxynonenal-induced cytotoxicity and inflammation in ARPE-19 cells[J]. Exp Eye Res, 2015, 132: 208-215. DOI:10.1016/j.exer.2015.02.001 |
| [39] |
Weng S, Mao L, Gong Y, et al. Role of quercetin in protecting ARPE-19 cells against H2O2-induced injury via nuclear factor erythroid 2 like 2 pathway activation and endoplasmic reticulum stress inhibition[J]. Mol Med Rep, 2017, 16: 3461-3468. DOI:10.3892/mmr.2017.6964 |
| [40] |
Zhu Q, Liu M, He Y, et al. Quercetin protect cigarette smoke extracts induced inflammation and apoptosis in RPE cells[J]. Artif Cells Nanomed Biotechnol, 2019, 47: 2010-2015. DOI:10.1080/21691401.2019.1608217 |
| [41] |
Cao X, Liu M, Tuo J, et al. The effects of quercetin in cultured human RPE cells under oxidative stress and in Ccl2/Cx3cr1 double deficient mice[J]. Exp Eye Res, 2010, 91: 15-25. DOI:10.1016/j.exer.2010.03.016 |
| [42] |
Sun H, Jin X, Xu J, et al. Baicalin alleviates age-related macular degeneration via miR-223/NLRP3-regulated pyroptosis[J]. Pharmacology, 2020, 105: 28-38. DOI:10.1159/000502614 |
| [43] |
Yang SJ, Jo H, Kim J, et al. Baicalin attenuates laser-induced choroidal neovascularization[J]. Curr Eye Res, 2014, 39: 745-751. DOI:10.3109/02713683.2013.868908 |
| [44] |
Park G, Kim D. Cigarette smoke-induced EGFR activation promotes epithelial mesenchymal migration of human retinal pigment epithelial cells through regulation of the FAK-mediated Syk/Src pathway[J]. Mol Med Rep, 2017, 17: 3563-3574. |
| [45] |
Liu JH, Wann H, Chen MM, et al. Baicalein significantly protects human retinal pigment epithelium cells against H2O2-induced oxidative stress by scavenging reactive oxygen species and downregulating the expression of matrix metalloproteinase-9 and vascular endothelial growth factor[J]. J Ocul Pharmacol Ther, 2010, 5: 421-429. |
| [46] |
Hanneken A, Lin F, Johnson J, et al. Flavonoids protect human retinal pigment epithelial cells from oxidative-stress-induced death[J]. Invest Ophth Vis Sci, 2006, 47: 3164-3177. DOI:10.1167/iovs.04-1369 |
| [47] |
Yan T, Bi H, Wang Y. Wogonin modulates hydroperoxide-induced apoptosis via PI3K/Akt pathway in retinal pigment epithelium cells[J]. Diagn Pathol, 2014, 9: 154-154. DOI:10.1186/s13000-014-0154-3 |
| [48] |
Chen C, Guo D, Lu G. Wogonin protects human retinal pigment epithelium cells from LPS-induced barrier dysfunction and inflammatory responses by regulating the TLR4/NF-κB signaling pathway[J]. Mol Med Rep, 2017, 4: 2289-2295. |
| [49] |
Zhang Y, Yang Y, Yu H, et al. Apigenin protects mouse retina against oxidative damage by regulating the Nrf2 pathway and autophagy[J]. Oxid Med Cell Longev, 2020, 2020: 9420704. |
| [50] |
Zou Y, Chiou GC. Apigenin inhibits laser-induced choroidal neovascularization and regulates endothelial cell function[J]. J Ocul Pharmacol Ther, 2006, 22: 425. DOI:10.1089/jop.2006.22.425 |
| [51] |
Song JH, Moon KY, Lee SC, et al. Inhibition of hypoxia-inducible factor-1α and vascular endothelial growth factor by chrysin in a rat model of choroidal neovascularization[J]. Int J Mol Sci, 2020, 21: 2842. DOI:10.3390/ijms21082842 |
| [52] |
Park SM, Lee K, Huh M, et al. Development of an in vitro 3D choroidal neovascularization model using chemically induced hypoxia through an ultra-thin, free-standing nanofiber membrane[J]. Mater Sci Eng C Mater Biol Appl, 2019, 104: 109964. DOI:10.1016/j.msec.2019.109964 |
| [53] |
Ni T, Yang W, Xing Y. Protective effects of delphinidin against H2O2-induced oxidative injuries in human retinal pigment epithelial cells[J]. Bioscience Rep, 2019, 39: BSR20190689. DOI:10.1042/BSR20190689 |
| [54] |
Silván JM, Reguero M, de Pascual-Teresa S. A protective effect of anthocyanins and xanthophylls on UVB-induced damage in retinal pigment epithelial cells[J]. Food Funct, 2016, 7: 167-176. |
| [55] |
Du W, An Y, He X, et al. Protection of kaempferol on oxidative stress-induced retinal pigment epithelial cell damage[J]. Oxid Med Cell Longev, 2018, 2018: 1610751. |
| [56] |
Jin X, Wang C, Wu W, et al. Cyanidin-3-glucoside alleviates 4-hydroxyhexenal-induced NLRP3 inflammasome activation via JNK-c-Jun/AP-1 pathway in human retinal pigment epithelial cells[J]. J Immunol Res, 2018, 2018: 5604610. |
| [57] |
Chen X, Han R, Hao P, et al. Nepetin inhibits IL-1β induced inflammation via NF-κB and MAPKs signaling pathways in ARPE-19 cells[J]. Biomed Pharmacother, 2018, 101: 87-93. DOI:10.1016/j.biopha.2018.02.054 |
| [58] |
Huang W, Wu H, Li D, et al. Protective effects of blueberry anthocyanins against H2O2-induced oxidative injuries in human retinal pigment epithelial cells[J]. J Agr Food Chem, 2018, 66: 1638-1648. DOI:10.1021/acs.jafc.7b06135 |
| [59] |
Zhu C, Dong Y, Liu H, et al. Hesperetin protects against H2O2-triggered oxidative damage via upregulation of the Keap1-Nrf2/HO-1 signal pathway in ARPE-19 cells[J]. Biomed Pharmacother, 2017, 88: 124-133. DOI:10.1016/j.biopha.2016.11.089 |
| [60] |
Lin CH, Li CH, Liao PL, et al. Silibinin inhibits VEGF secretion and age-related macular degeneration in a hypoxia-dependent manner through the PI-3 kinase/Akt/mTOR pathway[J]. Br J Pharmacol, 2013, 4: 920-931. |
| [61] |
Mansoor S, Gupta N, Luczy-Bachman G, et al. Protective effects of memantine and epicatechin on catechol-induced toxicity on Müller cells in vitro[J]. Toxicology, 2010, 271: 107-114. DOI:10.1016/j.tox.2010.03.013 |
| [62] |
Kinoshita S, Noda K, Tagawa Y, et al. Genistein attenuates choroidal neovascularization[J]. J Nutr Biochem, 2014, 25: 1177-1182. DOI:10.1016/j.jnutbio.2014.06.004 |
| [63] |
Xu XR, Yu HT, Hang L, et al. Preparation of naringenin/ beta-cyclodextrin complex and its more potent alleviative effect on choroidal neovascularization in rats[J]. Biomed Res Int, 2014, 2014: 623509. |
| [64] |
Ma N, Yang X, Qi C, et al. Farrerol enhances Nrf2-mediated defense mechanisms against hydrogen peroxide-induced oxidative damage in human retinal pigment epithelial cells by activating Akt and MAPK[J]. Oxid Med Cell Longev, 2021, 2021: 8847844. |
| [65] |
Zhu X, Wang K, Zhou F, et al. Paeoniflorin attenuates atRAL-induced oxidative stress, mitochondrial dysfunction and endoplasmic reticulum stress in retinal pigment epithelial cells via triggering Ca2+/CaMKII-dependent activation of AMPK[J]. Arch Pharm Res, 2018, 41: 1009-1018. DOI:10.1007/s12272-018-1059-6 |
| [66] |
Xie WK, Yu WZ, Zhao M, et al. Protective effect of paeoniflorin against oxidative stress in human retinal pigment epithelium in vitro[J]. Mol Vis, 2011, 17: 3512-3522. |
| [67] |
Zhou Y, Zhou L, Zhou K, et al. Celastrol protects RPE cells from oxidative stress-induced cell death via activation of Nrf2 signaling pathway[J]. Curr Mol Med, 2019, 19: 172. DOI:10.2174/1566524019666190424131704 |
| [68] |
Du Z, Zhang W, Wang S, et al. Celastrol protects human retinal pigment epithelial cells against hydrogen peroxide mediated oxidative stress, autophagy, and apoptosis through sirtuin 3 signal pathway[J]. J Cell Biochem, 2019, 120: 10413-10420. DOI:10.1002/jcb.28326 |
| [69] |
Paimela T, Hyttinen JMT, Viiri J, et al. Celastrol regulates innate immunity response via NF-κB and Hsp70 in human retinal pigment epithelial cells[J]. Pharmacol Res, 2011, 64: 501-508. DOI:10.1016/j.phrs.2011.05.027 |
| [70] |
Lai K, Gong Y, Zhao W, et al. Triptolide attenuates laser-induced choroidal neovascularization via M2 macrophage in a mouse model[J]. Biomed Pharmacother, 2020, 129: 110312. DOI:10.1016/j.biopha.2020.110312 |
| [71] |
Lai K, Li Y, Gong Y, et al. Triptolide-nanoliposome-APRPG, a novel sustained-release drug delivery system targeting vascular endothelial cells, enhances the inhibitory effects of triptolide on laser-induced choroidal neovascularization[J]. Biomed Pharmacother, 2020, 131: 110737. DOI:10.1016/j.biopha.2020.110737 |
| [72] |
Cui K, Liu J, Huang L, et al. Andrographolide attenuates choroidal neovascularization by inhibiting the HIF-1α/VEGF signaling pathway[J]. Biochem Bioph Res Commun, 2020, 530: 60-66. DOI:10.1016/j.bbrc.2020.06.130 |
| [73] |
Li S, Chaudhary SC, Zhao X, et al. Artemisinin protects human retinal pigmented epithelial cells against hydrogen peroxide-induced oxidative damage by enhancing the activation of AMP-active protein kinase[J]. Int J Biol Sci, 2019, 9: 2016-2028. |
| [74] |
Yan F, Wang H, Gao Y, et al. Artemisinin protects retinal neuronal cells against oxidative stress and restores rat retinal physiological function from light exposed damage[J]. Acs Chem Neurosci, 2017, 8: 1713-1723. DOI:10.1021/acschemneuro.7b00021 |
| [75] |
Chong C, Zheng W. Artemisinin protects human retinal pigment epithelial cells from hydrogen peroxide-induced oxidative damage through activation of ERK/CREB signaling[J]. Redox Biol, 2016, 9: 50-56. DOI:10.1016/j.redox.2016.06.002 |
| [76] |
Li S, Gaur U, Chong C, et al. Berberine protects human retinal pigment epithelial cells from hydrogen peroxide-induced oxidative damage through activation of AMPK[J]. Int J Mol Sci, 2018, 19: 1736. DOI:10.3390/ijms19061736 |
| [77] |
Song D, Song J, Wang C, et al. Berberine protects against light-induced photoreceptor degeneration in the mouse retina[J]. Exp Eye Res, 2016, 145: 1-9. DOI:10.1016/j.exer.2015.10.005 |
| [78] |
Liu RT, Wang A, To E, et al. Vinpocetine inhibits amyloid-beta induced activation of NF-κB, NLRP3 inflammasome and cytokine production in retinal pigment epithelial cells[J]. Exp Eye Res, 2014, 127: 49-58. DOI:10.1016/j.exer.2014.07.003 |
| [79] |
Zou Y, Jiang W, Chiou GC. Effect of tetramethylpyrazine on rat experimental choroidal neovascularization in vivo and endothelial cell cultures in vitro[J]. Curr Eye Res, 2007, 32: 71-75. DOI:10.1080/02713680601088787 |
| [80] |
Yang J, Xu J, Chen J, et al. Protective effects of ligustrazine against photoreceptor cell injury induced by N-methyl-N-nitrosourea and its mechanism[J]. Acta Pharm Sin (药学学报), 2005, 40: 690-694. |
| [81] |
Zhang J, Mao K, Gu Q, et al. The antiangiogenic effect of sanguinarine chloride on experimental choroidal neovacularization in mice via inhibiting vascular endothelial growth factor[J]. Front Pharmacol, 2021, 12: 638215. DOI:10.3389/fphar.2021.638215 |
| [82] |
Chen PJ, Shang AQ, Wang WW, et al. Astragaloside suppresses tumor necrosis factor receptor‐associated factor 5 signaling pathway and alleviates neurodegenerative changes in retinal pigment epithelial cells induced by isoflurane[J]. J Cell Biochem, 2019, 120: 1028-1037. DOI:10.1002/jcb.27599 |
| [83] |
Sun R, Zhang A, Ge Y, et al. Ultra-small-size astragaloside-Ⅳ loaded lipid nanocapsules eye drops for the effective management of dry age-related macular degeneration[J]. Expert Opin Drug Del, 2020, 17: 1305-1320. DOI:10.1080/17425247.2020.1783236 |
| [84] |
Lee Y, Hussain AA, Seok J, et al. Modulating the transport characteristics of Bruch's membrane with steroidal glycosides and its relevance to age-related macular degeneration (AMD)[J]. Invest Ophth Vis Sci, 2015, 56: 8403-8418. DOI:10.1167/iovs.15-16936 |
| [85] |
Zhou J, Chen F, Yan A, et al. Madecassoside protects retinal pigment epithelial cells against hydrogen peroxide-induced oxidative stress and apoptosis through the activation of Nrf2/HO-1 pathway[J]. Bioscience Rep, 2020, 40: BSR20194347. DOI:10.1042/BSR20194347 |
| [86] |
He H, Wei D, Liu H, et al. Glycyrrhizin protects against sodium iodate‐induced RPE and retinal injury though activation of AKT and Nrf2/HO‐1 pathway[J]. J Cell Mol Med, 2019, 23: 3495-3504. DOI:10.1111/jcmm.14246 |
| [87] |
Alzhrani RM, Alhadidi Q, Bachu RD, et al. Tanshinone IIA inhibits VEGF secretion and HIF-1alpha expression in cultured human retinal pigment epithelial cells under hypoxia[J]. Curr Eye Res, 2017, 42: 1667-1673. DOI:10.1080/02713683.2017.1355467 |
| [88] |
Han D, Wu X, Liu L, et al. Sodium tanshinone IIA sulfonate protects ARPE-19 cells against oxidative stress by inhibiting autophagy and apoptosis[J]. Sci Rep, 2018, 8: 15137. DOI:10.1038/s41598-018-33552-2 |
| [89] |
Zhang H, Liu Y, Jiang Q, et al. Salvianolic acid A protects RPE cells against oxidative stress through activation of Nrf2/HO-1 signaling[J]. Free Radic Biol Med, 2014, 69: 219-228. DOI:10.1016/j.freeradbiomed.2014.01.025 |
| [90] |
Mao K, Shu W, Qiu Q, et al. Salvianolic acid A protects retinal pigment epithelium from OX-LDL-induced inflammation in an age-related macular degeneration model[J]. Discov Med, 2017, 23: 129. |
| [91] |
Mao K, Shu W, Liu L, et al. Salvianolic acid A inhibits OX-LDL effects on exacerbating choroidal neovascularization via downregulating CYLD[J]. Oxid Med Cell Longev, 2017, 2017: 6210694. |
| [92] |
Frede K, Ebert F, Kipp AP, et al. Lutein activates the transcription factor Nrf2 in human retinal pigment epithelial cells[J]. J Agr Food Chem, 2017, 65: 5944-5952. DOI:10.1021/acs.jafc.7b01929 |
| [93] |
Tu G, Zhang YF, Wei W, et al. Allicin attenuates H2O2-induced cytotoxicity in retinal pigmented epithelial cells by regulating the levels of reactive oxygen species[J]. Mol Med Rep, 2016, 13: 2320-2326. DOI:10.3892/mmr.2016.4797 |
| [94] |
Dörschmann P, Mikkelsen MD, Thi TN, et al. Effects of a newly developed enzyme-assisted extraction method on the biological activities of fucoidans in ocular cells[J]. Mar Drugs, 2020, 18: 282. DOI:10.3390/md18060282 |
| [95] |
Dörschmann P, Klettner A. Fucoidans as potential therapeutics for age-related macular degeneration-current evidence from in vitro research[J]. Int J Mol Sci, 2020, 21: 9272. DOI:10.3390/ijms21239272 |
| [96] |
Yin Y, Liu D, Tian D. Salidroside prevents hydroperoxide-induced oxidative stress and apoptosis in retinal pigment epithelium cells[J]. Exp Ther Med, 2018, 16: 2363-2368. |
| [97] |
Ozal SA, Turkekul K, Gurlu V, et al. Esculetin protects human retinal pigment epithelial cells from lipopolysaccharide-induced inflammation and cell death[J]. Curr Eye Res, 2018, 43: 1169-1176. DOI:10.1080/02713683.2018.1481517 |
| [98] |
Pinelli R, Biagioni F, Limanaqi F, et al. A re-appraisal of pathogenic mechanisms bridging wet and dry age-related macular degeneration leads to reconsider a role for phytochemicals[J]. Int J Mol Sci, 2020, 21: 5563. DOI:10.3390/ijms21155563 |
| [99] |
Huynh TP, Mann SN, Mandal NA. Botanical compounds: effects on major eye diseases[J]. Evid Based Complement Alternat Med, 2013, 2013: 549174. |
| [100] |
Yang T, Zhang LJ, Huang R, et al. Research progress in the regulation of angiogenesis by active ingredients of traditional Chinese medicine[J]. Acta Pharm Sin (药学学报), 2020, 55: 1995-2007. |
| [101] |
Campos PM, Petrilli R, Lopez RFV. The prominence of the dosage form design to treat ocular diseases[J]. Int J Pharm, 2020, 586: 119577. DOI:10.1016/j.ijpharm.2020.119577 |
| [102] |
Durak S, Esmaeili Rad M, Alp Yetisgin A, et al. Niosomal drug delivery systems for ocular disease-recent advances and future prospects[J]. Nanomaterials, 2020, 10: 1191. DOI:10.3390/nano10061191 |
| [103] |
Zhang YY, Gao X, Jiang K, et al. Gene therapy and delivery strategies for ocular diseases[J]. Acta Pharm Sin (药学学报), 2018, 53: 518-528. |
 2022, Vol. 57
2022, Vol. 57