2. 杭州市第一人民医院, 浙江 杭州 310006
2. Hangzhou First People's Hospital, Hangzhou 310006, China
炎症性肠病(inflammatory bowel disease, IBD) 是一种影响胃肠道的慢性炎症性疾病, 近年来其发病率在新兴工业化国家迅速上升, 患病率在西方国家持续稳步上升, 已成为一种全球性疾病[1]。IBD包括两种类型: 溃疡性结肠炎(ulcerative colitis, UC) 和克罗恩病(Crohn's disease, CD)。UC患者的黏膜炎症始于直肠, 可连续延伸至近端结肠, 通常表现为血性腹泻[2]。CD可以涉及胃肠道的任何部位, 最常见的是回肠末端和结肠, 典型的临床表现包括腹痛、慢性腹泻、体重减轻和疲劳[3]。尽管IBD确切的发病机制尚不清楚, 但多项研究表明个体的遗传易感性、外部环境、肠道微生物菌群和免疫反应都在IBD发生发展中有重要作用[4]。
药物转运体是介导物质跨膜转运的一类转运蛋白, 对内外源性物质的摄取、分布和排泄有重要作用[5]。药物转运体根据底物跨膜转运的方向分为ATP结合盒(ATP binding cassette, ABC) 转运体和溶质载体(solute carrier, SLC) 转运体。ABC转运体主要介导细胞对内外源性物质的外排, 主要包括多药耐药蛋白(multidrug resistance proteins, MDRs)、多药耐药相关蛋白(multidrug resistance-associated proteins, MRPs)、乳腺癌耐药蛋白(breast cancer resistance protein, BCRP) 等。SLC转运体则主要是介导细胞对内外源性物质的摄取, 主要包括有机阴离子转运体(organic anion transporters, OATs)、有机阴离子转运多肽(organic anion transporting polypeptides, OATPs)、质子偶联寡肽转运体(proton-coupled oligopeptide transporters, POTs) 等。肠道作为物质吸收的主要部位, 在肠上皮细胞中有多种药物转运体表达, 介导了多种内外源性物质的转运, 对维持肠道稳态和人体生理平衡具有重要作用。
研究表明炎症可以调节多种药物转运体的表达和功能[6], 在1型糖尿病、类风湿性关节炎、代谢紊乱和几种神经退行性疾病中均观察到了药物转运体的异常表达[7]。IBD是一种慢性炎症性疾病, 在疾病状态下多种肠道药物转运体的表达和功能发生改变, 从而影响多种内外源性物质的体内行为。
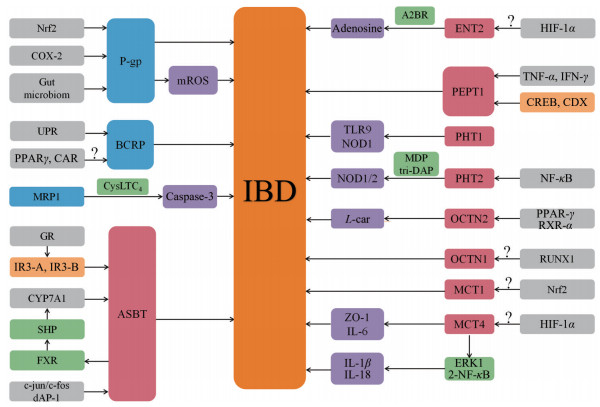
2 研究现状在IBD状态下, 肠道中多种药物转运体的表达发生了改变(表 1[8-23]), 但其确切的调控机制尚不完全清楚, 目前认为促炎细胞因子主要通过相关的炎症信号通路调节转录因子和核受体影响转运体的表达。IBD状态下肠道药物转运体的调控机制见图 1。
| Table 1 Changes in the expression of intestinal drug transporters in inflammatory bowel disease (IBD) |
|
Figure 1 Regulatory mechanism of intestinal drug transporters in inflammatory bowel disease. Nrf2: Nuclear factor erythroid-2 related factor 2; COX-2: Cyclooxygenase-2; mROS: Mitochondrial reactive oxygen species; UPR: Unfolded protein response; PPARγ: Peroxisome proliferators-activated receptor γ; CAR: Constitutive androstane receptor; CysLTC4: Cysteinyl-leukotriene C4; A2BR: Adenosine A2B receptor; HIF-1α: Hypoxia-inducible factor-1α; TNF-α: Tumor necrosis factor-α; IFN-γ: Interferon-γ; TLR9: Toll like receptor 9; NOD1: Nucleotide-binding oligomerisation domain 1; MDP: Muramyl dipeptide; NF-κB: Nuclear factor-κB; L-car: L-Carnitine; RXR-α: Retinoid X receptor α; RUNX1: Runt-related transcription factor; IL-6: Interleukin-6; ERK1/2: Extracellular-regulated kinase 1/2; CYP7A1: Cholesterol 7α-hydroxylase; FXR: Farnesoid X receptor; SHP: Small heterodimer partner; GR: Glucocoriticoid receptor |
ABC转运体在肠道中的表达限制了许多口服药物的吸收, 肠道ABC转运体的表达和功能很容易受到各种因素的调节, 尤其是P-糖蛋白(P-glycoprotein, P-gp)[24]。在IBD活动期的患者中, 已观察到多种促炎细胞因子水平的改变, 这些变化可能会影响疾病期间ABC转运体的表达和功能[25]。
2.1.1 P-糖蛋白(P-gp)P-gp由多药耐药基因1 (人类中的MDR1和啮齿动物中的同系物Mdr1a和Mdr1b) 编码, 是ABC转运体超家族的成员, 在胃肠道中高度表达, 是肠道屏障重要的组成部分[26]。作为一种ATP依赖性外排转运蛋白, P-gp将许多亲脂性阳离子药物和其他有害分子转运出肠黏膜, 在保护组织免受内外源性物质的侵害方面起着重要的作用[27]。
IBD对P-gp表达的影响主要集中在UC, 多项研究结果均表明在UC患者的结肠样本中P-gp蛋白和MDR1 mRNA的表达显著下调[8, 9]。在CD患者的结肠样本中, P-gp表达也显著降低, 而回肠中的P-gp与健康对照组相比没有显著差异[10], 但也有研究发现MDR1在CD患者结肠样本中的表达没有显著变化[11]。
研究发现P-gp表达水平的改变以及MDR1的遗传变异与IBD有关, P-gp外排活性降低可能会促进疾病易感性, 而外排活性增加可能会影响IBD相关药物的吸收[28]。Mdr1a基因敲除小鼠会自发地患上结肠炎症, 其组织病理学特征类似于人类UC[29]。角质形成细胞生长因子-2和益生菌可以诱导肠上皮细胞中P-gp的表达, 从而缓解肠道炎症[30, 31]。小檗碱能够显著减轻葡聚糖硫酸钠(dextran sodium sulfate, DSS) 诱导的结肠炎症, 并改善P-gp介导的屏障功能, 其对P-gp表达和活性的诱导可能是通过激活核因子红细胞2相关因子2 (nuclear factor erythroid-2 related factor 2, Nrf2) 介导的信号通路来实现的[32]。环氧合酶-2 (cyclooxygenase-2, COX-2) 主要在炎症细胞中表达, 在IBD患者的炎症组织中COX-2显著上调[33], 体内外实验均表明COX-2抑制剂能够抑制三硝基苯磺酸诱导的P-gp上调[34], 提示了在IBD中COX-2可能参与了P-gp的调控。肠道菌群对肠道稳态具有重要作用, 研究发现在UC患者中P-gp表达的下调与肠道菌群代谢物相关[35]。另有研究发现MDR1缺陷会导致线粒体功能障碍, 而线粒体活性氧(mitochondrial reactive oxygen species, mROS) 升高会驱动结肠炎的发展[36]。此外, P-gp表达的上调会影响药物的外排, 糖皮质激素治疗失败的IBD患者的外周血淋巴细胞中P-gp表达水平升高[37], 大剂量糖皮质激素给药导致UC患者MDR1 mRNA表达增加[38], 都提示了P-gp在IBD患者耐药性中的重要作用。关于MDR1的遗传变异, 已经有多项研究评估了人类MDR1基因多态性与IBD易感性的潜在关联[39, 40], 这对评估疾病轻重程度、用药易感性及预后等有重要作用。
2.1.2 乳腺癌耐药蛋白(BCRP)BCRP由ABCG2基因编码, 在小肠和结肠上皮的顶膜以及肝细胞的小管膜上高表达[41], 决定了其在内外源性物质转运中的重要作用。与P-gp一样, BCRP也具有非常广泛的底物和抑制剂特异性, 在组织和细胞保护以及介导生理底物的稳态方面具有重要作用[42]。BCRP是影响IBD相关药物体内行为的另一个重要转运蛋白, 在研究P-gp对IBD状态下药物的药代动力学时, BCRP经常也被一起研究[9, 43]。
与健康受试者相比, 活动性UC患者的炎症组织中BCRP表达显著降低, BCRP阳性染色减少, 同时肠道上皮F-肌动蛋白结构受到破坏[9]。在CD患者中, 回肠炎症组织中的ABCG2 mRNA表达与未受影响的黏膜相比显著下调[12]。有研究发现活动性IBD患者中BCRP介导的黏膜解毒缺失是由于蛋白质折叠受阻导致, 肠道炎症激活了未折叠蛋白质反应, 进而通过破坏BCRP的表达和功能而降低了对异源生物攻击的防御能力[44]。此外, 肠道中BCRP受到过氧化物酶体增殖物激活受体γ (peroxisome proliferators-activated receptor γ, PPARγ) 和组成型雄甾烷受体(constitutive androstane receptor, CAR) 的调节[45], 在UC患者中也观察到PPARγ和CAR表达下调[46, 47], 但还没有实验证明其相关性。
2.1.3 多药耐药相关蛋白(MRPs)MRPs属于ABC转运体家族的C亚族, 由ABCC基因编码。目前已经确定了MRP家族中的9个成员, 但只有MRP1到5被证明在药物运输中具有明确的作用[48]。在肠道中, MRP2在肠细胞的顶端表达, 并作为药物吸收的屏障, MRP1、MRP3、MRP4和MRP5位于肠细胞的基底外侧膜, 促进药物进入循环[49]。MRPs能够转运有机阴离子, 介导多种内外源性物质的转运[50]。
UC患者肠道炎症组织中MRP1蛋白表达显著上调, 但mRNA表达没有显著变化[13]。在Mrp1基因敲除(Mrp1-/-) 小鼠中, 花生四烯酸诱导的炎症刺激反应显著降低[51], 但Mrp1-/-小鼠对DSS诱导的结肠炎肠道损伤显著加重[52]。这表明Mrp1在炎症过程中可能发挥了双重作用, 既可以介导炎症反应, 又可以保护肠道上皮。进一步研究发现Mrp1通过从半胱氨酰白三烯生物合成途径输出促凋亡化合物来保护肠上皮细胞[14]。在结肠样本中, MRP2蛋白由于浓度太低未被检测到, 其mRNA的表达在UC患者结肠的炎症和非炎症组织也没有显著差异, 与MRP2类似, UC患者中MRP3的mRNA表达和蛋白水平与健康组相当[13]。MRP4 mRNA和蛋白的表达在UC患者中存在争议[12, 13], 可能是由于患者的个体差异引起。研究发现炎症期间核激素受体可能参与MRPs的调控[53], 但在IBD状态下MRPs的调控机制还没有报道, 有待进一步研究。
2.2 溶质载体(SLC) 转运体SLC转运体超家族包含400多种转运蛋白, 它们介导离子、核苷酸和糖类等物质的吸收和运输。研究发现超过80种SLC转运体与人类疾病有关, 包括肥胖症和2型糖尿病等, SLC转运体已被认为是治疗代谢性疾病的一个潜在靶点[54]。在IBD患者炎症组织中多种SLC转运体基因的表达显著上调, 如平衡型核苷转运体1 (equilibrative nucleoside transporter 1, ENT1)、浓度型核苷转运体2 (concentration nucleoside transporter 2, CNT2)、肽转运蛋白1 (peptide transporter 1, PEPT1)、OATP2B1和OATP4A1等, 但顶端钠依赖性胆盐转运体(apical sodium dependent bile acid transporter, ASBT) 和肉碱/有机阳离子转运体2 (carnitine/organic cation transporter 2, OCTN2) 的mRNA表达水平显著下调[15]。
2.2.1 核苷转运体(CNTs/ENTs)核苷转运体主要分为两个家族, CNTs和ENTs。CNTs由SLC28基因编码, 依赖于钠离子和氢离子等离子浓度梯度, 具有高亲和性的单向转运核苷及其类似物的作用; ENTs由SLC29基因编码, 介导核苷及其类似物的双向流动[55]。核苷转运蛋白广泛存在于各类生物体内, 通过跨膜转运核苷, 参与和调节生物体内诸多生理、生化反应, 同时通过转运核苷类似物, 在癌症治疗和抗病毒感染方面发挥着重要作用[56]。
在IBD患者结肠组织中ENT1、ENT2和CNT2的mRNA显著上调[15], 推测内源性腺苷的生物利用度降低。双嘧达莫是一种ENT1和ENT2阻滞剂, 对DSS诱导的小鼠结肠炎症具有保护作用, 研究发现ENT1基因的缺失并不能抵消结肠炎的进展, 而ENT2基因的缺失对肠道炎症具有保护作用, 表明ENT2在肠道炎症中具有重要作用, ENT2抑制或缺乏的抗炎作用的潜在机制是由于细胞外腺苷水平的升高, 其通过A2B受体激活发挥保护作用[57]。但是目前还没有关于ENT2的药理学调节在其他IBD小鼠模型中的有益作用的数据, 对ENT2在肠道炎症中的功效还有待进一步研究。嘌呤能系统已被证明是作为治疗免疫介导的炎症性疾病的一个药理学靶点[58], 核苷转运体作为嘌呤能系统的重要组成部分, 也有待为IBD的治疗提供新的方案。此外, 在缺氧条件下, 缺氧诱导因子-1α (hypoxia-inducible factor-1α, HIF-1α) 对ENT2的表达调控有重要作用, HIF-1α依赖性的ENT2抑制减轻了肠道缺氧期间的黏膜炎症[59]。但CD患者炎症组织中HIF-1α表达显著上调[60], 与ENT2的上调是否相关有待进一步研究。
2.2.2 质子偶联寡肽转运体(POTs)POTs由SLC15A基因编码, 是一类表达于细胞膜或细胞器膜上的跨膜转运蛋白, 该家族由4个成员组成, PEPT1 (SLC15A1)、PEPT2 (SLC15A2)、肽/组氨酸转运体1 (peptide/histidine transporter 1, PHT1/SLC15A4) 和PHT2 (SLC15A3), 它们利用质子梯度和负膜电位介导二/三肽和许多拟肽类物质的跨膜转运[61]。大量研究表明POTs在IBD中起到重要作用。
PepT1是质子-寡肽共转运蛋白, 介导二/三肽从肠腔到上皮细胞的转运。PepT1在小鼠和人十二指肠、空肠和回肠的细胞顶膜上大量表达, 在结肠表达水平较低[62], 但在IBD状态下在结肠中表达显著上调[63], 目前尚不清楚这种上调的确切机制, 但已提出了许多合理因素。据报道, 在IBD期间炎症细胞因子和激素水平的变化会影响PepT1的表达和功能, 肿瘤坏死因子-α (tumor necrosis factor-α, TNF-α) 和干扰素-γ (interferon-γ, IFN-γ) 水平的上调导致PepT1表达上调, 因此肠道Caco2-BBE细胞的二肽和三肽摄取增加[64]; 脂肪细胞分泌的激素瘦素也可在Caco2-BBE细胞中上调PepT1的表达并发挥作用, 瘦素介导的PepT1启动子区域的激活取决于转录因子CREB和CDX2的表达[65]。PepT2在肠神经系统的胶质细胞和组织驻留巨噬细胞中表达[66], 但PepT2介导的吸收不太可能涉及胃肠道的神经肌肉层。
PHT1和PHT2主要在细胞内溶酶体膜上表达, 介导寡肽和组氨酸的转运。与结肠非炎症组织相比, IBD患者的炎症组织中SLC15A4 mRNA的表达显著上调, 但在CD患者的回肠末端没有观察到显著差异[16]。利用SLC15A4-/-小鼠研究发现, Pht1通过Toll样受体9和核苷酸结合寡聚化结构域1 (nucleotide-binding oligomerisation domain 1, NOD1) 依赖的先天免疫反应促进结肠炎[67]。因此, 除了PEPT1, PHT1也可能在IBD中有重要作用。Pht2主要表达在肺、脾和胸腺中, 且在巨噬细胞中表达显著, 利用脂多糖(lipopolysaccharide, LPS) 诱导的炎症细胞模型, 发现LPS通过核因子-κB (nuclear factor-κB, NF-κB) 信号通路上调Pht2 mRNA的表达[68]。在DSS诱导的结肠炎小鼠中, 结肠组织和免疫细胞中Pht1基因表达下调而Pht2基因表达上调, 进一步研究发现细菌胞壁酰二肽(muramyl dipeptide, MDP) 和肽类似产物L-Ala-γ-D-Glu-meso-diaminopimelic acid (tri-DAP) 是Pht2的底物, MDP是NOD2的配体, tri-DAP是NOD1的配体, Pht2可能通过参与MDP和tri-DAP的转运来调节小鼠结肠固有层单核细胞和RAW264.7巨噬细胞中NOD依赖的免疫反应, 提示肠道Pht2作为IBD治疗靶标的潜力[69]。但是, 在临床样本中没有报道与PHT2相关的疾病, 故PHT2在人类IBD中的作用有待进一步研究。
2.2.3 肉碱/有机阳离子转运体(OCTNs)OCTNs属于SLC22A家族, 包括人和动物体内的OCTN1 (SLC22A4) 和OCTN2 (SLC22A5) 和仅在小鼠体内表达的Octn3 (Slc22a21)。OCTN1和OCTN2表达于肠细胞顶膜[49], 转运左旋肉碱(L-carnitine, L-Car) 和其他阳离子化合物, 在维持机体内源性化合物的分布平衡和阳离子化合物在体内的药代动力学中起着重要作用[70, 71]。
在研究IBD中肠道OCTNs与遗传多态性相关的功能时发现, OCTN1和OCTN2蛋白水平在CD患者结肠组织和对照中没有显著差异, 但在突变纯合或杂合基因型的CD患者中, OCTN1表达更高; 同样, 在CD患者和健康人群回肠中L-Car的转运功能相似, 但在具有OCTN1和OCTN2基因突变的受试者中观察到了较高的L-Car转运趋势[17]。但是也有研究发现UC患者结肠黏膜中OCTN1和OCTN2的mRNA表达均显著降低[18], IBD患者和小鼠发炎的结肠组织中OCTN2表达水平和L-Car含量均降低, 其机制是促炎性细胞因子通过PPAR-γ/类视黄醇X受体-α (retinoid X receptor α, RXR-α) 途径在IBD中降低OCTN2的表达, 从而降低了L-Car的浓度, 进而引起IBD的恶化, 提示OCTN2可以作为IBD的潜在治疗靶标[19]。与OCTN2不同, OCTN1不是肉碱转运蛋白, 非生理代谢物麦角硫因[72]和生理性阳离子乙酰胆碱[73]是其良好的底物。研究发现OCTN1基因突变(L503F) 的CD患者中乙酰胆碱外排功能缺陷[74], 在Octn1-/-小鼠中, 检测到组织中麦角硫因水平的显著降低, 即使动物存活且没有明显的表型变化, 但Octn1-/-小鼠对肠道氧化应激的耐受性显著降低[75], 这一发现可能有助于解释人类CD患者中典型的肠道炎症状态。关于OCTN1的调控, 有研究报道在类风湿性关节炎中Runt相关转录因子1可能参与OCTN1的转录调控[76], 但在IBD中OCTN1的调控机制还没有报道。
2.2.4 单羧酸转运体(MCTs)单羧酸转运体(monocarboxylte transporters, MCTs) 属于SLC16A亚家族成员, 目前已发现该家族有14个成员。MCTs介导丙酮酸、乳酸、酮体以及短链脂肪酸等单羧酸类化合物的转运, 在保证碳水化合物、脂质及氨基酸正常代谢中发挥重要作用[77]。MCT1在结肠上皮的顶膜和基底外侧膜中均有表达, 而MCT4在基底外侧膜中特异性表达, 两者被认为在维持结肠稳态中有重要作用[78]。
研究发现IBD患者肠黏膜上皮细胞中MCT4表达上调, MCT4介导乳酸和质子外流到细胞外环境, MCT4的表达上调与IBD患者血液中乳酸水平升高一致, 提示MCT4在IBD中作为标志物的潜在用途[20]。从机制上讲, MCT4有助于NF-κB p65核转位并增加其与白细胞介素-6 (interleukin-6, IL-6) 启动子区域的结合, 通过抑制ZO-1和诱导IL-6促进IBD的发展[79]。进一步研究还发现MCT4的异位表达会导致肠上皮细胞的细胞焦亡, MCT4通过ERK1/2 (extracellular-regulated kinase 1/2)-NF-κB轴介导的炎性NLRP3活化, 导致caspase-1激活, 促进IL-1β和IL-18的成熟, 从而加重肠道炎症, 抑制ERK1/2-NF-κB活性可以克服MCT4对细胞焦亡的影响[80]。这些发现表明了MCT4在IBD中的新作用, 可能作为IBD治疗的潜在靶标。此外, 研究表明在缺氧条件下HIF-1α介导MCT4的上调[81], 这与CD患者炎症组织中HIF-1α表达上调相符合, 但两者是否相关有待进一步证实。
短链脂肪酸如乙酸盐、丙酸盐和丁酸盐是维持肠道稳态的重要代谢物, 其中丁酸盐(MCT1的底物) 具有重要的免疫调节功能[82]。研究发现IBD患者和DSS诱导的结肠炎大鼠的结肠黏膜中MCT1 mRNA和蛋白质水平与正常组相比显著降低, 用IFN-γ和TNF-α刺激肠上皮细胞, MCT1 mRNA和蛋白表达下调, 同时丁酸盐摄取减少[21]。环孢菌素用于治疗重度UC患者, 研究发现其可以上调肠上皮细胞中MCT1 mRNA的表达, 从而增加丁酸盐的摄取, 缓解结肠炎症[83], 提示了MCT1在IBD中的重要作用。在人结肠癌细胞中Nrf2能够诱导MCT1表达上调, 在IBD患者的结肠组织中MCT1和Nrf2也共表达[84], 提示了在IBD状态下Nrf2可能参与MCT1的调控。
2.2.5 顶端钠依赖性胆盐转运体(ASBT)ASBT由SLC10A2基因编码, 主要表达于回肠上皮细胞的顶膜, 少量表达于胆管细胞上皮, 负责在回肠末端吸收胆汁酸(bile acids, BAs)[85]。ASBT是决定BAs浓度的主要因素, 也在调节脂质和胆固醇稳态中具有重要作用[86]。
在UC患者结肠中ASBT蛋白的表达显著下调, 而肝脏中胆固醇7α-羟化酶(cholesterol 7α-hydroxylase, CYP7A1) 水平代偿性升高, 此外, 法尼醇受体(farnesoid X receptor, FXR) 降低, 其下游靶基因小异二聚体伴侣(small heterodimer partner, SHP) 也下调, 揭示了结肠炎中的腹泻与胆汁酸转运蛋白表达之间的相关性[22]。在CD患者炎症组织中的ASBT mRNA表达显著低于健康人, 并且在患者恢复期ASBT表达也下调, 主要是因为BAs的肝肠循环受到ASBT降低的干扰, 减慢了药物的吸收, 导致药物作用时间缩短, 药效降低[12]。IBD中ASBT的下调机制与炎症因子的水平升高有关, 炎症因子通过c-jun/c-fos异二聚体和死亡相关蛋白-1 (death-associated protein-1, DAP-1) 的组合抑制ASBT的表达[87]。布地奈德是一种具有糖皮质激素作用的高效药物, 可特异性逆转CD患者中ASBT表达的降低, 缓解ASBT降低引起的胆汁酸吸收障碍, 其机制是调节ASBT启动子上的两个GC反应元件IR3-A和IR3-B, 与糖皮质激素受体(glucocoriticoid receptor, GR) 结合以反式激活ASBT表达[23]。因此, 基于上述原则, 以ASBT为靶点, 在IBD期间适当诱导ASBT的表达, 减少BAs在结肠内的积累对IBD的治疗是有益的。
2.2.6 其他SLC类转运体CD和UC患者的回肠和结肠组织中的OATP2B1和OATP4A1表达上调[15], 在UC患者结肠中有机溶质转运体α/β (organic solute transporter α/β, OST α/β) 的mRNA表达下调, 但在CD患者中未发现明显差异[12], 有机阳离子转运体3 (organic cation transporter 3, OCT3) mRNA在UC患者结肠中表达下调[13], 但迄今还没有明确的迹象表明上述转运体可能参与人类IBD的病理过程。OCT1在人类肠道中少量表达, 但其在肠上皮细胞顶端或基底外侧膜的细胞定位存在争议[88], 质膜单胺转运体(plasma membrane monoamine transporter, PMAT) 在人类肠道各个部分的高水平表达, 位于极化上皮细胞的顶端膜[89], 这些转运体在IBD病理状态下的变化还没有被报道, 有待进一步研究。
3 总结和展望IBD状态下多种药物转运体的表达和功能发生了改变, 这不仅影响了多种药物的体内行为, 而且也对多种内源性物质产生影响。阐明药物转运体在IBD状态下的变化和相关机制, 对寻找治疗IBD的新策略和临床合理用药具有重要意义。目前对药物转运体在IBD状态下的研究很多还停留在转运体的表达上, 具体机制还尚未阐明, 故对药物转运体在IBD状态下的调控机制进行研究是下一步方向, 这也为寻找治疗IBD的新策略提供理论基础。此外, 炎症性肠病不仅仅影响肠道, 而且作用于全身, 故在IBD状态下不仅肠道中的药物转运体发生了改变, 其他部位的转运体也可能发生改变, 研究其他部位转运体在IBD状态下的调控也可能对IBD的治疗有一定作用。
作者贡献: 董敏磊负责查阅文献、论文撰写; 罗筠负责论文内容核对; 蒋惠娣负责对论文进行整体的指导和修改; 李萍负责论文选题、设计及论文修改。
利益冲突: 本文所有作者均声明不存在利益冲突。
| [1] |
Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease[J]. Gastroenterology, 2017, 152: 313-321. DOI:10.1053/j.gastro.2016.10.020 |
| [2] |
Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis[J]. Lancet, 2017, 389: 1756-1770. DOI:10.1016/S0140-6736(16)32126-2 |
| [3] |
Torres J, Mehandru S, Colombel JF, et al. Crohn's disease[J]. Lancet, 2017, 389: 1741-1755. DOI:10.1016/S0140-6736(16)31711-1 |
| [4] |
Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis[J]. World J Gastroenterol, 2014, 20: 91-99. DOI:10.3748/wjg.v20.i1.91 |
| [5] |
Czuba LC, Hillgren KM, Swaan PW. Post-translational modifications of transporters[J]. Pharmacol Ther, 2018, 192: 88-99. DOI:10.1016/j.pharmthera.2018.06.013 |
| [6] |
Zeng H, Bi HC, Huang M. A review on regulation of drug transporters during inflammation[J]. Acta Pharm Sin (药学学报), 2011, 46: 773-779. |
| [7] |
Wu KC, Lin CJ. The regulation of drug-metabolizing enzymes and membrane transporters by inflammation: evidences in inflammatory diseases and age-related disorders[J]. J Food Drug Anal, 2019, 27: 48-59. DOI:10.1016/j.jfda.2018.11.005 |
| [8] |
Ufer M, Hasler R, Jacobs G, et al. Decreased sigmoidal ABCB1 (P-glycoprotein) expression in ulcerative colitis is associated with disease activity[J]. Pharmacogenomics, 2009, 10: 1941-1953. DOI:10.2217/pgs.09.128 |
| [9] |
Englund G, Jacobson A, Rorsman F, et al. Efflux transporters in ulcerative colitis: decreased expression of BCRP (ABCG2) and Pgp (ABCB1)[J]. Inflamm Bowel Dis, 2007, 13: 291-297. DOI:10.1002/ibd.20030 |
| [10] |
Wilson A, Urquhart BL, Ponich T, et al. Crohn's disease is associated with decreased CYP3A4 and P-glycoprotein protein expression[J]. Mol Pharm, 2019, 16: 4059-4064. DOI:10.1021/acs.molpharmaceut.9b00459 |
| [11] |
Langmann T, Moehle C, Mauerer R, et al. Loss of detoxification in inflammatory bowel disease: dysregulation of pregnane X receptor target genes[J]. Gastroenterology, 2004, 127: 26-40. DOI:10.1053/j.gastro.2004.04.019 |
| [12] |
Jahnel J, Fickert P, Hauer AC, et al. Inflammatory bowel disease alters intestinal bile acid transporter expression[J]. Drug Metab Dispos, 2014, 42: 1423-1431. DOI:10.1124/dmd.114.058065 |
| [13] |
Erdmann P, Bruckmueller H, Martin P, et al. Dysregulation of mucosal membrane transporters and drug-metabolizing enzymes in ulcerative colitis[J]. J Pharm Sci, 2019, 108: 1035-1046. DOI:10.1016/j.xphs.2018.09.024 |
| [14] |
Blokzijl H, van Steenpaal A, Vander Borght S, et al. Up-regulation and cytoprotective role of epithelial multidrug resistance-associated protein 1 in inflammatory bowel disease[J]. J Biol Chem, 2008, 283: 35630-35637. DOI:10.1074/jbc.M804374200 |
| [15] |
Wojtal KA, Eloranta JJ, Hruz P, et al. Changes in mRNA expression levels of solute carrier transporters in inflammatory bowel disease patients[J]. Drug Metab Dispos, 2009, 37: 1871-1877. DOI:10.1124/dmd.109.027367 |
| [16] |
Lee J, Tattoli I, Wojtal KA, et al. pH-dependent internalization of muramyl peptides from early endosomes enables Nod1 and Nod2 signaling[J]. J Biol Chem, 2009, 284: 23818-23829. DOI:10.1074/jbc.M109.033670 |
| [17] |
Girardin M, Dionne S, Goyette P, et al. Expression and functional analysis of intestinal organic cation/L-carnitine transporter (OCTN) in Crohn's disease[J]. J Crohns Colitis, 2012, 6: 189-197. DOI:10.1016/j.crohns.2011.08.003 |
| [18] |
Yamamoto-Furusho JK, Mendivil EJ, Villeda-Ramirez MA, et al. Gene expression of carnitine organic cation transporters 1 and 2 (OCTN) is downregulated in patients with ulcerative colitis[J]. Inflamm Bowel Dis, 2011, 17: 2205-2206. DOI:10.1002/ibd.21621 |
| [19] |
Li P, Wang YQ, Luo J, et al. Downregulation of OCTN2 by cytokines plays an important role in the progression of inflammatory bowel disease[J]. Biochem Pharmacol, 2020, 178: 114115. DOI:10.1016/j.bcp.2020.114115 |
| [20] |
He LY, Wang HL, Zhang YH, et al. Evaluation of monocarboxylate transporter 4 in inflammatory bowel disease and its potential use as a diagnostic marker[J]. Dis Markers, 2018, 2018: 2649491. |
| [21] |
Thibault R, De Coppet P, Daly K, et al. Down-regulation of the monocarboxylate transporter 1 is involved in butyrate deficiency during intestinal inflammation[J]. Gastroenterology, 2007, 133: 1916-1927. DOI:10.1053/j.gastro.2007.08.041 |
| [22] |
Hou RG, Fan L, Liu JJ, et al. Bile acid malabsorption is associated with diarrhea in acute phase of colitis[J]. Can J Physiol Pharmacol, 2018, 96: 1328-1336. DOI:10.1139/cjpp-2018-0017 |
| [23] |
Jung D, Fantin AC, Scheurer U, et al. Human ileal bile acid transporter gene ASBT (SLC10A2) is transactivated by the glucocorticoid receptor[J]. Gut, 2004, 53: 78-84. DOI:10.1136/gut.53.1.78 |
| [24] |
Murakami T, Bodor E, Bodor N. Modulation of expression/function of intestinal P-glycoprotein under disease states[J]. Expert Opin Drug Metab Toxicol, 2020, 16: 59-78. DOI:10.1080/17425255.2020.1701653 |
| [25] |
Verma N, Ahuja V, Paul J. Profiling of ABC transporters during active ulcerative colitis and in vitro effect of inflammatory modulators[J]. Dig Dis Sci, 2013, 58: 2282-2292. DOI:10.1007/s10620-013-2636-7 |
| [26] |
Huls M, Russel FG, Masereeuw R. The role of ATP binding cassette transporters in tissue defense and organ regeneration[J]. J Pharmacol Exp Ther, 2009, 328: 3-9. DOI:10.1124/jpet.107.132225 |
| [27] |
Sharom FJ. The P-glycoprotein multidrug transporter[J]. Essays Biochem, 2011, 50: 161-178. DOI:10.1042/bse0500161 |
| [28] |
Cario E. P-glycoprotein multidrug transporter in inflammatory bowel diseases: more questions than answers[J]. World J Gastroenterol, 2017, 23: 1513-1520. DOI:10.3748/wjg.v23.i9.1513 |
| [29] |
Wilk JN, Bilsborough J, Viney JL. The mdr1a-/- mouse model of spontaneous colitis: a relevant and appropriate animal model to study inflammatory bowel disease[J]. Immunol Res, 2005, 31: 151-159. DOI:10.1385/IR:31:2:151 |
| [30] |
Saksena S, Priyamvada S, Kumar A, et al. Keratinocyte growth factor-2 stimulates P-glycoprotein expression and function in intestinal epithelial cells[J]. Am J Physiol Gastrointest Liver Physiol, 2013, 304: G615-G622. DOI:10.1152/ajpgi.00445.2012 |
| [31] |
Saksena S, Goyal S, Raheja G, et al. Upregulation of P-glycoprotein by probiotics in intestinal epithelial cells and in the dextran sulfate sodium model of colitis in mice[J]. Am J Physiol Gastrointest Liver Physiol, 2011, 300: G1115-G1123. DOI:10.1152/ajpgi.00027.2011 |
| [32] |
Jing WH, Safarpour Y, Zhang T, et al. Berberine upregulates P-glycoprotein in human Caco-2 cells and in an experimental model of colitis in the rat via activation of Nrf2-dependent mechanisms[J]. J Pharmacol Exp Ther, 2018, 366: 332-340. DOI:10.1124/jpet.118.249615 |
| [33] |
Singer II, Kawka DW, Schloemann S, et al. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease[J]. Gastroenterology, 1998, 115: 297-306. DOI:10.1016/S0016-5085(98)70196-9 |
| [34] |
Zrieki A, Farinotti R, Buyse M. Cyclooxygenase-2 inhibitors prevent trinitrobenzene sulfonic acid-induced P-glycoprotein up-regulation in vitro and in vivo[J]. Eur J Pharmacol, 2010, 636: 189-197. DOI:10.1016/j.ejphar.2010.03.039 |
| [35] |
Foley SE, Tuohy C, Dunford M, et al. Gut microbiota regulation of P-glycoprotein in the intestinal epithelium in maintenance of homeostasis[J]. Microbiome, 2021, 9: 183. DOI:10.1186/s40168-021-01137-3 |
| [36] |
Ho GT, Aird RE, Liu B, et al. MDR1 deficiency impairs mitochondrial homeostasis and promotes intestinal inflammation[J]. Mucosal Immunol, 2018, 11: 120-130. DOI:10.1038/mi.2017.31 |
| [37] |
Farrell RJ, Murphy A, Long A, et al. High multidrug resistance (P-glycoprotein 170) expression in inflammatory bowel disease patients who fail medical therapy[J]. Gastroenterology, 2000, 118: 279-288. DOI:10.1016/S0016-5085(00)70210-1 |
| [38] |
Hirano T, Onda K, Toma T, et al. MDR1 mRNA expressions in peripheral blood mononuclear cells of patients with ulcerative colitis in relation to glucocorticoid administration[J]. J Clin Pharmacol, 2004, 44: 481-486. DOI:10.1177/0091270004264162 |
| [39] |
Brinar M, Cukovic-Cavka S, Bozina N, et al. MDR1 polymorphisms are associated with inflammatory bowel disease in a cohort of Croatian IBD patients[J]. BMC Gastroenterol, 2013, 13: 57. DOI:10.1186/1471-230X-13-57 |
| [40] |
Mijac D, Vukovic-Petrovic I, Mijac V, et al. MDR1 gene polymorphisms are associated with ulcerative colitis in a cohort of Serbian patients with inflammatory bowel disease[J]. PLoS One, 2018, 13: e0194536. DOI:10.1371/journal.pone.0194536 |
| [41] |
Maliepaard M, Scheffer GL, Faneyte IF, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues[J]. Cancer Res, 2001, 61: 3458-3464. |
| [42] |
Jonker JW, Buitelaar M, Wagenaar E, et al. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria[J]. Proc Natl Acad Sci U S A, 2002, 99: 15649-15654. DOI:10.1073/pnas.202607599 |
| [43] |
Østergaard M, Ernst A, Labouriau R, et al. Cyclooxygenase-2, multidrug resistance 1, and breast cancer resistance protein gene polymorphisms and inflammatory bowel disease in the Danish population[J]. Scand J Gastroenterol, 2009, 44: 65-73. DOI:10.1080/00365520802400826 |
| [44] |
Deuring JJ, de Haar C, Koelewijn CL, et al. Absence of ABCG2-mediated mucosal detoxification in patients with active inflammatory bowel disease is due to impeded protein folding[J]. Biochem J, 2012, 441: 87-93. DOI:10.1042/BJ20111281 |
| [45] |
Gorczyca L, Aleksunes LM. Transcription factor-mediated regulation of the BCRP/ABCG2 efflux transporter: a review across tissues and species[J]. Expert Opin Drug Metab Toxicol, 2020, 16: 239-253. DOI:10.1080/17425255.2020.1732348 |
| [46] |
Dubuquoy L, Jansson EA, Deeb S, et al. Impaired expression of peroxisome proliferator-activated receptor γ in ulcerative colitis[J]. Gastroenterology, 2003, 124: 1265-1276. DOI:10.1016/S0016-5085(03)00271-3 |
| [47] |
Hudson GM, Flannigan KL, Erickson SL, et al. Constitutive androstane receptor regulates the intestinal mucosal response to injury[J]. Br J Pharmacol, 2017, 174: 1857-1871. DOI:10.1111/bph.13787 |
| [48] |
Liu YH, Di YM, Zhou ZW, et al. Multidrug resistance-associated proteins and implications in drug development[J]. Clin Exp Pharmacol Physiol, 2010, 37: 115-120. DOI:10.1111/j.1440-1681.2009.05252.x |
| [49] |
Estudante M, Morais JG, Soveral G, et al. Intestinal drug transporters: an overview[J]. Adv Drug Deliv Rev, 2013, 65: 1340-1356. DOI:10.1016/j.addr.2012.09.042 |
| [50] |
Zhou SF, Wang LL, Di YM, et al. Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development[J]. Curr Med Chem, 2008, 15: 1981-2039. DOI:10.2174/092986708785132870 |
| [51] |
Wijnholds J, Evers R, van Leusden MR, et al. Increased sensitivity to anticancer drugs and decreased inflammatory response in mice lacking the multidrug resistance-associated protein[J]. Nat Med, 1997, 3: 1275-1279. DOI:10.1038/nm1197-1275 |
| [52] |
ten Hove T, Drillenburg P, Wijnholds J, et al. Differential susceptibility of multidrug resistance protein-1 deficient mice to DSS and TNBS-induced colitis[J]. Dig Dis Sci, 2002, 47: 2056-2063. DOI:10.1023/A:1019629013945 |
| [53] |
Teng S, Piquette-Miller M. Regulation of transporters by nuclear hormone receptors: implications during inflammation[J]. Mol Pharm, 2008, 5: 67-76. DOI:10.1021/mp700102q |
| [54] |
Schumann T, Konig J, Henke C, et al. Solute carrier transporters as potential targets for the treatment of metabolic disease[J]. Pharmacol Rev, 2020, 72: 343-379. DOI:10.1124/pr.118.015735 |
| [55] |
Young JD, Yao SY, Baldwin JM, et al. The human concentrative and equilibrative nucleoside transporter families, SLC28 and SLC29[J]. Mol Aspects Med, 2013, 34: 529-547. DOI:10.1016/j.mam.2012.05.007 |
| [56] |
Baldwin SA, Beal PR, Yao SY, et al. The equilibrative nucleoside transporter family, SLC29[J]. Pflugers Arch, 2004, 447: 735-743. DOI:10.1007/s00424-003-1103-2 |
| [57] |
Aherne CM, Collins CB, Rapp CR, et al. Coordination of ENT2-dependent adenosine transport and signaling dampens mucosal inflammation[J]. JCI Insight, 2018, 3: e121521. DOI:10.1172/jci.insight.121521 |
| [58] |
Antonioli L, Blandizzi C, Pacher P, et al. The purinergic system as a pharmacological target for the treatment of immune-mediated inflammatory diseases[J]. Pharmacol Rev, 2019, 71: 345-382. DOI:10.1124/pr.117.014878 |
| [59] |
Morote-Garcia JC, Rosenberger P, Nivillac NM, et al. Hypoxia-inducible factor-dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia[J]. Gastroenterology, 2009, 136: 607-618. DOI:10.1053/j.gastro.2008.10.037 |
| [60] |
Mimouna S, Goncalves D, Barnich N, et al. Crohn disease-associated Escherichia coli promote gastrointestinal inflammatory disorders by activation of HIF-dependent responses[J]. Gut Microbes, 2011, 2: 335-346. DOI:10.4161/gmic.18771 |
| [61] |
Smith DE, Clemencon B, Hediger MA. Proton-coupled oligopeptide transporter family SLC15: physiological, pharmacological and pathological implications[J]. Mol Aspects Med, 2013, 34: 323-336. DOI:10.1016/j.mam.2012.11.003 |
| [62] |
Groneberg DA, Doring F, Eynott PR, et al. Intestinal peptide transport: ex vivo uptake studies and localization of peptide carrier PEPT1[J]. Am J Physiol Gastrointest Liver Physiol, 2001, 281: G697-G704. DOI:10.1152/ajpgi.2001.281.3.G697 |
| [63] |
Merlin D, Si-Tahar M, Sitaraman SV, et al. Colonic epithelial hPepT1 expression occurs in inflammatory bowel disease: transport of bacterial peptides influences expression of MHC class 1 molecules[J]. Gastroenterology, 2001, 120: 1666-1679. DOI:10.1053/gast.2001.24845 |
| [64] |
Vavricka SR, Musch MW, Fujiya M, et al. Tumor necrosis factor-alpha and interferon-gamma increase PepT1 expression and activity in the human colon carcinoma cell line Caco-2/bbe and in mouse intestine[J]. Pflugers Arch, 2006, 452: 71-80. DOI:10.1007/s00424-005-0007-8 |
| [65] |
Nduati V, Yan YT, Dalmasso G, et al. Leptin transcriptionally enhances peptide transporter (hPepT1) expression and activity via the cAMP-response element-binding protein and Cdx2 transcription factors[J]. J Biol Chem, 2007, 282: 1359-1373. DOI:10.1074/jbc.M604267200 |
| [66] |
Ruhl A, Hoppe S, Frey I, et al. Functional expression of the peptide transporter PEPT2 in the mammalian enteric nervous system[J]. J Comp Neurol, 2005, 490: 1-11. DOI:10.1002/cne.20617 |
| [67] |
Sasawatari S, Okamura T, Kasumi E, et al. The solute carrier family 15A4 regulates TLR9 and NOD1 functions in the innate immune system and promotes colitis in mice[J]. Gastroenterology, 2011, 140: 1513-1525. DOI:10.1053/j.gastro.2011.01.041 |
| [68] |
Wang YQ, Sun DL, Song FF, et al. Expression and regulation of the proton-coupled oligopeptide transporter PhT2 by LPS in macrophages and mouse spleen[J]. Mol Pharm, 2014, 11: 1880-1888. DOI:10.1021/mp500014r |
| [69] |
Wang YQ, Hu YJ, Li P, et al. Expression and regulation of proton-coupled oligopeptide transporters in colonic tissue and immune cells of mice[J]. Biochem Pharmacol, 2018, 148: 163-173. |
| [70] |
Ingoglia F, Visigalli R, Rotoli BM, et al. Functional activity of L-carnitine transporters in human airway epithelial cells[J]. Biochim Biophys Acta, 2016, 1858: 210-219. DOI:10.1016/j.bbamem.2015.11.013 |
| [71] |
Nakamichi N, Shima H, Asano S, et al. Involvement of carnitine/organic cation transporter OCTN1/SLC22A4 in gastrointestinal absorption of metformin[J]. J Pharm Sci, 2013, 102: 3407-3417. DOI:10.1002/jps.23595 |
| [72] |
Nakamura T, Yoshida K, Yabuuchi H, et al. Functional characterization of ergothioneine transport by rat organic cation/carnitine transporter Octn1 (slc22a4)[J]. Biol Pharm Bull, 2008, 31: 1580-1584. DOI:10.1248/bpb.31.1580 |
| [73] |
Ishimoto T, Nakamichi N, Hosotani H, et al. Organic cation transporter-mediated ergothioneine uptake in mouse neural progenitor cells suppresses proliferation and promotes differentiation into neurons[J]. PLoS One, 2014, 9: e89434. DOI:10.1371/journal.pone.0089434 |
| [74] |
Pochini L, Scalise M, Galluccio M, et al. The human OCTN1 (SLC22A4) reconstituted in liposomes catalyzes acetylcholine transport which is defective in the mutant L503F associated to the Crohn's disease[J]. Biochim Biophys Acta, 2012, 1818: 559-565. DOI:10.1016/j.bbamem.2011.12.014 |
| [75] |
Kato Y, Kubo Y, Iwata D, et al. Gene knockout and metabolome analysis of carnitine/organic cation transporter OCTN1[J]. Pharm Res, 2010, 27: 832-840. DOI:10.1007/s11095-010-0076-z |
| [76] |
Tokuhiro S, Yamada R, Chang XT, et al. An intronic SNP in a RUNX1 binding site of SLC22A4, encoding an organic cation transporter, is associated with rheumatoid arthritis[J]. Nat Genet, 2003, 35: 341-348. DOI:10.1038/ng1267 |
| [77] |
Halestrap AP. The monocarboxylate transporter family-structure and functional characterization[J]. IUBMB Life, 2012, 64: 1-9. DOI:10.1002/iub.573 |
| [78] |
Sivaprakasam S, Bhutia YD, Yang SP, et al. Short-chain fatty acid transporters: role in colonic homeostasis[J]. Compr Physiol, 2017, 8: 299-314. |
| [79] |
Zhang SX, Xu WF, Wang HL, et al. Inhibition of CREB-mediated ZO-1 and activation of NF-κB-induced IL-6 by colonic epithelial MCT4 destroys intestinal barrier function[J]. Cell Prolif, 2019, 52: e12673. |
| [80] |
Wang YD, Zhou XR, Zou KJ, et al. Monocarboxylate transporter 4 triggered cell pyroptosis to aggravate intestinal inflammation in inflammatory bowel disease[J]. Front Immunol, 2021, 12: 644862. DOI:10.3389/fimmu.2021.644862 |
| [81] |
Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1α-dependent mechanism[J]. J Biol Chem, 2006, 281: 9030-9037. DOI:10.1074/jbc.M511397200 |
| [82] |
Parada Venegas D, De la Fuente MK, Landskron G, et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases[J]. Front Immunol, 2019, 10: 277. DOI:10.3389/fimmu.2019.00277 |
| [83] |
Ota S, Sakuraba H, Hiraga H, et al. Cyclosporine protects from intestinal epithelial injury by modulating butyrate uptake via upregulation of membrane monocarboxylate transporter 1 levels[J]. Biochem Biophys Rep, 2020, 24: 100811. |
| [84] |
Diehl K, Dinges LA, Helm O, et al. Nuclear factor E2-related factor-2 has a differential impact on MCT1 and MCT4 lactate carrier expression in colonic epithelial cells: a condition favoring metabolic symbiosis between colorectal cancer and stromal cells[J]. Oncogene, 2018, 37: 39-51. DOI:10.1038/onc.2017.299 |
| [85] |
Li M, Wang Q, Li Y, et al. Apical sodium-dependent bile acid transporter, drug target for bile acid related diseases and delivery target for prodrugs: current and future challenges[J]. Pharmacol Ther, 2020, 212: 107539. DOI:10.1016/j.pharmthera.2020.107539 |
| [86] |
Han XP, Sun J, Wang YJ, et al. PepT1, ASBT-linked prodrug strategy to improve oral bioavailability and tissue targeting distribution[J]. Curr Drug Metab, 2015, 16: 71-83. DOI:10.2174/1389200216666150401110754 |
| [87] |
Yang N, Dong YQ, Jia GX, et al. ASBT (SLC10A2): a promising target for treatment of diseases and drug discovery[J]. Biomed Pharmacother, 2020, 132: 110835. DOI:10.1016/j.biopha.2020.110835 |
| [88] |
Wenzel C, Drozdzik M, Oswald S. Organic cation transporter 1 an intestinal uptake transporter: fact or fiction?[J]. Front Pharmacol, 2021, 12: 648388. DOI:10.3389/fphar.2021.648388 |
| [89] |
Xia L, Engel K, Zhou MY, et al. Membrane localization and pH-dependent transport of a newly cloned organic cation transporter (PMAT) in kidney cells[J]. Am J Physiol Renal Physiol, 2007, 292: F682-F690. DOI:10.1152/ajprenal.00302.2006 |
 2022, Vol. 57
2022, Vol. 57


