2. 山西大学化学生物学与分子工程教育部重点实验室, 山西 太原 030006;
3. 地产中药功效物质研究与利用山西省重点实验室, 山西 太原 030006
2. The Key Laboratory of Chemical Biology and Molecular Engineering of Ministry of Education, Shanxi University, Taiyuan 030006, China;
3. The Key Laboratory of Effective Substances Research and Utilization in TCM of Shanxi Province, Taiyuan 030006, China
脑衰老是一个高度复杂的生物过程, 可导致认知功能下降和神经退行性疾病的发生。神经炎症是神经退行性疾病的主要特征, 与中枢神经系统中小胶质细胞的激活和炎性因子的过度分泌有关[1, 2]。
小胶质细胞是脑内关键的免疫细胞, 小胶质细胞的激活可增加促炎因子和活性氧的生成, 进而引发神经元凋亡或坏死[3, 4]。因此, 抑制小胶质细胞的异常激活可以延缓多种衰老相关疾病的病程。脂多糖(lipopolysaccharide, LPS) 是一种来源于革兰阴性菌的内毒素, 通过诱导促炎因子的分泌, 从而引发神经炎症。因此, LPS诱导的BV-2小胶质细胞和原代小胶质细胞模型常用于神经炎症的研究[5]。
黄芩素是从黄芩根中分离得到的一种黄酮类化合物, 具有抗氧化和抗炎作用[6]。课题组前期研究发现, 黄芩素不仅可减轻D-半乳糖诱导的衰老大鼠认知障碍[7], 还可改善快速老化小鼠SAMP8的学习和记忆功能[8]。同时, 课题组证明黄芩素通过抑制LPS诱导的BV-2细胞促炎因子的释放而表现出抗炎作用[9]。但是, 黄芩素改善神经炎症的具体分子机制尚不明晰。
基因差异表达是指在一定刺激下, 基因的mRNA表达水平发生显著性改变的现象。大量文献报道[10-12], LPS可诱导BV-2细胞的许多基因发生差异表达, 一氧化氮合酶(inducible nitric oxide synthase, iNOS) 和环氧合酶-2 (cyclooxygenase-2, COX-2) 是差异表达最显著的基因之一。iNOS和COX-2是重要的炎症介质, 均受信号转导子和转录激活子1/核因子κB (signal transducer and activator of transcription 1/nuclear factor kappa-B, STAT1/NF-κB) 信号通路的调控[13]。NADPH氧化酶2 (NADPH oxidase-2, NOX2) 是一个关键的酶系统, 当LPS激活小胶质细胞后会迅速导致活性氧的产生增加[14], 进一步激活STAT1/NF-κB信号通路[15, 16]。此外, iNOS和COX-2的过度激活会使前列腺素E2 (prostaglandin E2, PEG2) 和一氧化氮(nitric oxide, NO) 的合成增加, 这也是导致神经元损伤和神经炎症发生的主要原因[17, 18]。
基于文献报道的LPS激活BV-2细胞相关的差异表达基因, 本研究进行了生物信息学分析和网络分析, 寻找在该过程中发挥核心作用的靶点; 同时采用分子对接方法, 根据蛋白质与小分子的形状匹配和能量匹配原理, 计算结合亲和力, 预测小分子化合物的作用靶点[19]。通过上述方法, 预测黄芩素在LPS诱导的BV-2细胞中调节的潜在靶点和通路, 进行实验验证, 以阐释黄芩素抗神经炎症的分子机制。
1 材料与方法药品与试剂 小鼠小胶质细胞株(BV-2细胞)、MEM培养基、特级胎牛血清、青霉素-链霉素溶液(100×) 和BCA试剂盒购自上海生工生物工程有限公司; 黄芩素(批号JZ20150711, 质量分数 > 98%) 购自南京景竹生物科技有限公司; LPS购自Sigma公司; Hoechst 33342、细胞膜/细胞浆蛋白提取试剂盒购自碧云天生物技术(上海) 公司; iNOS、COX-2、NF-κB/p65、phospho - NF - κB (p- NF - κB)、STAT1、phospho-STAT1 (p-STAT1)、gp91phox抗体购自武汉三鹰生物技术有限公司; p47phox抗体购自上海英骏生物技术有限公司; 荧光标记IgG二抗购自美国英杰生命技术有限公司。
仪器 Infinite M200 Pro多功能酶标仪(瑞士Tecan公司); ECLIPSE Ti荧光显微镜(日本Nikon公司); 电泳仪、光成像系统Chemi Doc TM XRS + (Bio-Rad公司); CytationTM 5细胞成像多功能检测仪(美国伯腾仪器有限公司)。
靶点收集 在PubMed[20] (https://www.ncbi.nlm.nih.gov/pubmed) 数据库以关键词“LPS、BV- 2 cell、differentially expressed genes”收集LPS激活BV-2细胞相关差异表达基因。将所得差异基因汇总, 并删除重复基因。使用SwissTargetPrediction数据库[21] (http://www.swisstargetprediction.ch/) 和SEA数据库[22] (http://sea.bkslab.org/) 预测黄芩素的靶点, 并与LPS激活BV-2细胞的差异基因取交集, 从而得到黄芩素抗神经炎症的潜在靶点。
蛋白- 蛋白互作 (protein-protein interaction, PPI) 网络构建 使用STRING11.0数据库[23] (https://string-db.org/) 获得靶点与靶点之间的作用关系, 并用Cytoscape 3.7.2软件构建蛋白与蛋白互作网络[24]。应用Network Analyzer[25]插件计算网络中的拓扑参数, 并使用MCODE插件获得关键子网络[26]。
分子对接 使用ZINC数据库[27] (http://zinc.docking.org/) 获得黄芩素的结构, RCSB PDB数据库[28] (https://www.rcsb.org/) 获得COX-2 (PDB ID: 5KIR) 和iNOS (PDB ID: 3E7G) 蛋白的3D结构。采用AutoDock Vina (version 1.1.2) 软件[29]预测黄芩素与COX-2、iNOS的结合能值和结合模式, 并用PyMOL (version 0.99)[30]可视化对接结果。
GO富集分析 采用DAVID数据库[31] (https://david.ncifcrf.gov/) 预测黄芩素发挥抗神经炎症作用涉及的重要生物过程。
细胞培养和药物处理 将BV-2细胞以每毫升1.2×106个细胞的密度接种于含10%胎牛血清(fetal bovine serum, FBS) 的MEM培养基中, 并在含5% CO2的37 ℃培养箱中培养。根据前期研究结果[9], 给予4 μmol·L-1黄芩素预处理2 h后, 再加入0.1 μg·mL-1 LPS共孵育24 h进行后续实验。
Western blot实验 收集细胞样品, 按照制造商的说明书提取细胞膜蛋白与胞浆蛋白, 具体步骤如下: 向细胞中加入1 mL含1%苯甲基磺酰氟(phenyl methanesulfonyl fluoride, PMSF) 的裂解缓冲液A, 反复冻融5次破坏细胞后, 置冰上裂解15 min, 700 ×g离心10 min, 收集上清液, 再以12 000 ×g离心10 min, 收集胞浆蛋白。之后将200 μL裂解缓冲液B加入细胞膜沉淀物中, 置冰上裂解10 min, 12 000 ×g离心5 min, 收集膜蛋白。通过BCA蛋白质测定法确定蛋白质浓度。
收集细胞样品, 加入RIPA裂解液在冰上裂解30 min, 每10 min涡旋一次, 用BCA法测定蛋白浓度。通过10%或12% SDS-PAGE凝胶电泳分离蛋白(每孔上样量为50 μg), 并转移到PVDF膜上。用5%脱脂牛奶封闭2 h后, 分别加入iNOS、COX-2、NF-κB/p65、p-NF-κB、STAT1、p-STAT1、p47phox、gp91phox抗体, 并在4 ℃孵育过夜。TBST洗去一抗后, 加入二抗在37 ℃孵育条带1 h, 再次用TBST洗涤3次后, 在凝胶成像仪上显影成像, 并运用Image J对条带进行分析。
免疫荧光染色 将BV-2细胞以每毫升1.2×106个细胞的密度接种于96孔板中, 4 μmol·L-1黄芩素预处理2 h后用0.1 μg·mL-1 LPS刺激24 h。弃去上清液后, 用4%多聚甲醛固定细胞20 min, 并用PBS洗涤。使用0.1% TritonX-100透化细胞, 并封闭1 h, 加入一抗p47phox在4 ℃孵育过夜。清洗掉一抗后, 加入荧光二抗置于摇床上在37 ℃孵育2 h。最后加入10 μmol·L-1 Hoechst 33342与细胞共孵育15 min。使用CytationTM 5细胞成像多功能检测仪检测荧光强度和拍摄荧光图像。
统计学方法 实验结果采用x ± SEM表示, 使用GraphPad Prism 5.0软件进行统计分析。采用单因素方差分析和Tukey's检验比较组间差异, P < 0.05表明有统计学意义。
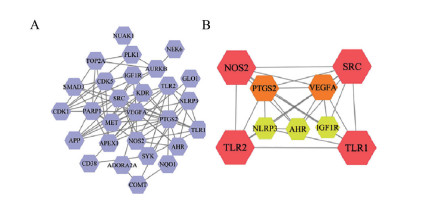
2 结果 1 黄芩素抗神经炎症靶点PPI网络构建从PubMed数据库共收集到2 463个差异表达基因, 利用SwissTargetPrediction和SEA数据库预测得到黄芩素的作用靶点139个。通过取交集, 得到38个共同靶点, 见表 1[10-12]所示。基于STRING数据库构建蛋白-蛋白互作网络, 删除网络中未连接的节点, 得到黄芩素抗神经炎症的靶点网络(图 1A)。网络聚类分析显示, 核心子网络[top 1, molecular complex detection (MCODE) 得分= 6.75] 由9个节点组成(图 1B), 其中COX-2和iNOS在核心子网络中具有较高的度值(degree), 且已报道这两个基因在LPS诱导的BV-2细胞中发生明显的差异表达。
| Table 1 The fold changes of the 38 differentially expressed genes in lipopolysaccharide (LPS) -stimulated BV-2 cells |
|
Figure 1 Protein-protein interaction network constructed by Cytoscape software. A: Graphical illustration of 38 targets; B: A core sub-network visualization of 9 targets. The higher the MCODE node score (the larger distribution a node has), the bigger the node size and the darker the node color are in the network |
为了进一步探索黄芩素与COX-2 (PDB ID: 5KIR)、iNOS (PDB ID: 3E7G) 的结合模式, 采用AutoDock Vina对结合能进行预测, 并与相应的阳性小分子(罗非昔布和AR-C102222) 进行比较。所有对接结果的均方根偏差(RMSD) 均小于2, 表明对接结果是可靠的[32]。Morris等[33]研究发现, 当结合能小于-5.0 kcal·mol-1时, 代表对接良好。结果显示, 黄芩素和阳性小分子罗非昔布与COX-2的结合能分别为-9.9和-8.4 kcal·mol-1, 黄芩素和阳性小分子AR-C102222与iNOS的结合能分别为-7.2和-7.1 kcal·mol-1, 表明黄芩素与COX-2和iNOS对接良好。黄芩素与iNOS、COX-2的最佳结合模式如图 2所示。

|
Figure 2 Molecular docking modes between baicalein and cyclooxygenase-2 (COX-2, A), baicalein and inducible nitric oxide synthase (iNOS, B) |
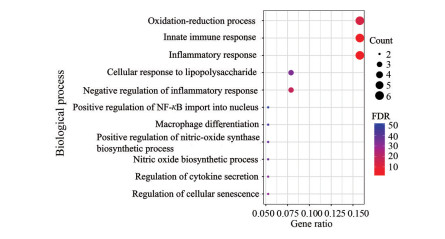
运用DAVID数据库对38个靶点进行GO富集分析, 根据P < 0.05, 得到11个显著富集的生物过程。结果显示, 黄芩素可调节氧化还原过程(oxidation-reduction process)、炎症反应(inflammatory response)、NF-κB入核的正向调节(positive regulation of NF-κB import into nucleus)、iNOS生物合成过程的正向调节(positive regulation of nitric-oxide synthase biosynthetic process), 这些生物过程与氧化应激和神经炎症的发生密切相关(图 3)。
|
Figure 3 Eleven enriched biological processes predicted by DAVID database and visualized by RStudio. The color scales represented the different false discovery rate (FDR) and the sizes of the dots indicated the target counts of each term |
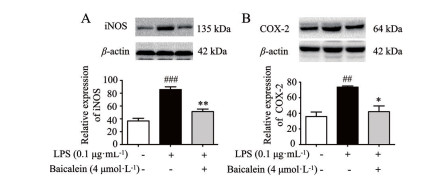
为了验证黄芩素对炎症介质iNOS、COX-2的抑制作用, 采用Western blot技术进行检测。结果显示, 0.1 μg·mL-1 LPS处理细胞后, iNOS和COX-2表达水平分别显著升高132.94%和105.52%。然而, 用4 μmol·L-1黄芩素预处理后可使iNOS表达水平显著降低40.09% (图 4A), COX-2表达水平显著降低42.80% (图 4B), 这表明黄芩素可以显著抑制iNOS和COX-2的表达。
|
Figure 4 Effects of baicalein on protein levels of iNOS (A) and COX-2 (B) in LPS-induced BV-2 cells. n = 3, x ± SEM. ##P < 0.01, ###P < 0.001 vs control group; *P < 0.05, **P < 0.01 vs LPS group |
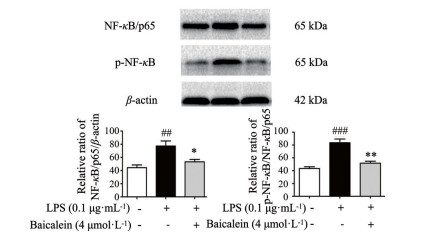
iNOS和COX-2是NF-κB信号通路中两种重要的分子, iNOS和COX-2蛋白的过表达可激活NF-κB信号通路, 诱导神经炎症反应。本课题组前期采用免疫荧光技术发现4 μmol·L-1黄芩素可使LPS诱导的NF-κB/p65表达显著降低且有效抑制NF-κB/p65的核易位[9]。本研究采用Western blot对NF-κB信号通路的关键蛋白进行检测。结果显示, LPS诱导BV-2细胞后, NF-κB/p65和p-NF-κB表达水平分别上调74.03%和92.63%。然而, 黄芩素给药后可使NF-κB/p65和p-NF-κB的表达水平显著下调31.12%和38.22% (图 5), 这表明黄芩素能有效抑制NF-κB/p65的表达和NF-κB的磷酸化。
|
Figure 5 Effects of baicalein on protein levels of nuclear factor kappa-B (NF-κB) /p65 and p- NF - κB in LPS-induced BV-2 cells. n = 3, x ± SEM. ##P < 0.01, ###P < 0.001 vs control group; *P < 0.05, **P < 0.01 vs LPS group |
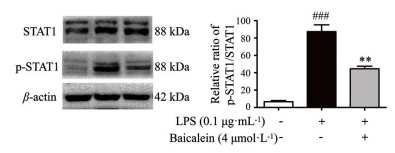
LPS可诱导NF-κB蛋白过表达, 并快速激活其上游蛋白STAT1的表达, 从而加剧神经炎症。采用Western blot技术对STAT1信号通路的关键蛋白p-STAT1进行检测。结果显示, LPS诱导BV-2细胞后能使p-STAT1的表达显著上调1 222.45%。然而, 黄芩素处理后可使p-STAT1的表达水平显著降低48.97% (图 6), 这表明黄芩素能明显下调LPS诱导的BV-2细胞STAT1蛋白的磷酸化。
|
Figure 6 Effects of baicalein on the level of p-signal transducer and activator of transcription 1 (STAT1) protein in LPS-stimulated BV-2 cells. n = 3, x ± SEM. ###P < 0.001 vs control group; **P < 0.01 vs LPS group |
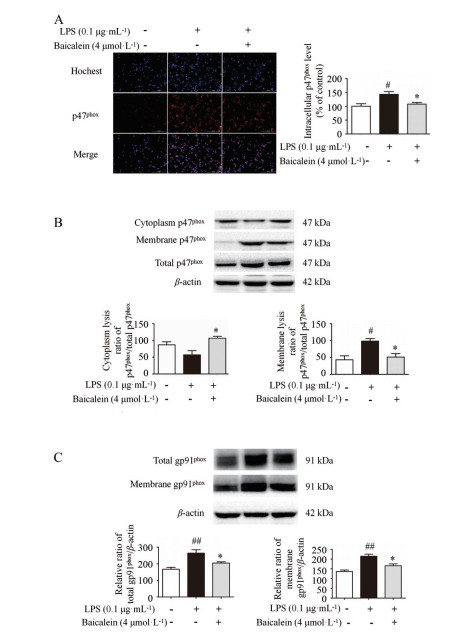
STAT1介导的炎症反应需要NOX2 (gp91phox/p47phox) 依赖性生成ROS, 而过量的ROS对于激活NF-κB信号通路, 促进STAT1磷酸化和核易位至关重要。采用免疫荧光和Western blot技术对p47phox、gp91phox蛋白表达进行检测。免疫荧光结果表明, LPS处理后p47phox的表达显著增加42.89%。然而, 黄芩素使p47phox的表达显著降低24.57% (图 7A)。Western blot结果表明, LPS刺激后使胞膜p47phox的表达增加了128.55%, 然而, 黄芩素处理后使胞膜p47phox水平显著下调了48.12% (图 7B)。此外, LPS刺激使总蛋白水平和胞膜gp91phox水平分别显著增加57.80%和57.21% (图 7C)。然而, 黄芩素处理使gp91phox总蛋白和膜蛋白表达显著降低了35.76%和35.48%。以上结果表明, LPS可加快p47phox从细胞质到细胞膜的转移, 并上调膜蛋白gp91phox的表达, 但给予黄芩素后能抑制p47phox从胞质到胞膜的转移, 并抑制总gp91phox蛋白和膜gp91phox蛋白的表达。
|
Figure 7 Effects of baicalein on the levels of p47phox, gp91phox in LPS-induced BV-2 cells. A: Intracellular p47phox level obtained by immunofluorescence assay; B: The levels of membrane and cytosol p47phox; C: The levels of total and membrane gp91phox. n = 3, x ± SEM. #P < 0.05, ##P < 0.01 vs control group; *P < 0.05 vs LPS group |
差异表达基因是在疾病发生发展过程中发挥重要作用的基因, 这些基因对于阐述疾病病理机制具有重要意义。大量文献报道, LPS可诱导BV-2细胞内大量基因发生差异表达, 这些基因为神经炎症相关靶点的发现提供了依据。对这些基因进行生物信息挖掘, 通过网络构建和网络聚类分析寻找核心靶点, 是药物靶点发现的新途径[34]。分子对接可用于模拟药物小分子和受体蛋白的结合模式, 将分子对接和生物信息分析相结合, 为黄芩素抗神经炎症潜在靶点和通路的预测提供了新的思路。预测结果显示, 黄芩素可能通过与关键靶点COX-2和iNOS结合发挥抗神经炎症的作用。
LPS可诱导小胶质细胞的过度活化, 从而引起神经变性, 导致神经元功能受损[35]。此外, 小胶质细胞的激活将引起多种炎性介质水平的升高, 包括COX-2和iNOS[36-38]。iNOS的过度累积加速NO的生成, 同时增强COX-2的活性, 进一步加重炎症的级联反应和神经系统的退行性病变[39-41]。本研究发现, LPS显著上调iNOS和COX-2的表达。然而, 给予黄芩素后可显著下调iNOS和COX-2的蛋白表达, 表明黄芩素抑制LPS诱导的BV-2细胞炎症。
NF-κB信号通路与慢性炎症和神经退行性疾病的发展高度相关。NF-κB信号通路的激活可导致炎症介质iNOS和COX-2的生成增加[42-44]。同时, NF-κB转录因子表达受胞质内STAT1的调控[45], STAT1可与NF-κB结合并激活下游基因的表达, 最终导致神经炎症和神经退行性疾病[46]。因此, 通过抑制NF-κB信号通路来调节炎症因子的生成可减轻神经毒性和减少神经元死亡, 进而延缓衰老相关疾病的发生[47, 48]。本研究发现, LPS显著增加NF-κB/p65的表达和NF-κB的磷酸化, 但给予黄芩素后显著降低NF-κB/p65的表达和NF-κB的磷酸化, 表明黄芩素可通过NF-κB信号通路抑制LPS诱导的BV-2细胞炎症。
STAT1受Toll样受体刺激后迅速磷酸化, 而磷酸化的STAT1参与调节促炎因子的释放和介导免疫反应[49]。通过抑制STAT1的磷酸化可显著减少COX-2和iNOS的生成[50]。本研究发现, LPS刺激后STAT1的磷酸化显著增加。给予黄芩素后显著抑制STAT1的磷酸化, 表明黄芩素可通过STAT1信号通路抑制LPS诱导的BV-2细胞炎症。本研究结果与前期发现黄芩素抑制STAT1信号通路减少快速老化小鼠炎症因子分泌相一致[51]。
NF-κB和STAT1信号通路的激活受ROS信号调控[52]。NOX是加快细胞中ROS产生的重要物质。当胞质组分p47phox转移到细胞膜, 并在细胞膜与gp91phox结合后, NOX的表达会迅速增加, 进而诱导细胞内产生高水平的ROS[53, 54]。因此, 抑制依赖于NOX产生ROS的通路可调控NF-κB和STAT1信号通路激活介导的炎症反应。本研究结果表明, LPS处理后胞质组分p47phox在细胞膜上的表达显著增加, 且促使胞膜gp91phox蛋白表达增加, 有利于NOX2的形成, 进而加速ROS的产生。然而, 给予黄芩素后抑制gp91phox和p47phox的表达, 从而减轻LPS诱导的BV-2细胞炎症反应。
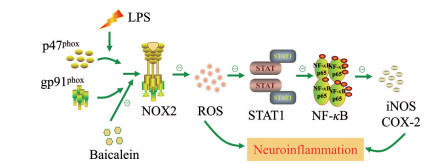
综上, 通过结合生物信息分析和分子对接技术, 本研究结果揭示黄芩素可能通过抑制NOX2 (gp91phox/p47phox) /STAT1/NF-κB级联反应来改善LPS诱导的氧化应激和神经炎症(图 8)。
|
Figure 8 The signaling pathway diagram of baicalein against neuroinflammation. "  |
作者贡献: 杨吾燕和闫姣姣负责进行实验和数据分析, 完成文章撰写; 高丽和秦雪梅负责实验思路的指导; 所有作者均对本文有所贡献。
利益冲突: 无利益冲突。
| [1] |
You MM, Chen YF, Pan YM, et al. Royal Jelly attenuates LPS-induced inflammation in BV-2 microglial cells through modulating NF-κB and p38/JNK signaling pathways[J]. Mediators Inflamm, 2018, 2018: 7834381. |
| [2] |
Ransohoff RM. How neuroinflammation contributes to neurodegeneration[J]. Science, 2016, 353: 777-783. DOI:10.1126/science.aag2590 |
| [3] |
Chen J, Yin W, Tu Y, et al. L-F001, a novel multifunctional ROCK inhibitor, suppresses neuroinflammation in vitro and in vivo: involvement of NF-κB inhibition and Nrf2 pathway activation[J]. Eur J Pharmacol, 2017, 806: 1-9. DOI:10.1016/j.ejphar.2017.03.025 |
| [4] |
Loane DJ, Kumar A. Microglia in the TBI brain: the good, the bad, and the dysregulated[J]. Exp Neurol, 2016, 275 Pt 3: 316-327. |
| [5] |
Yao C, Liu X, Zhou Z, et al. Melatonin attenuates expression of cyclooxygenase-2 (COX-2) in activated microglia induced by lipopolysaccharide (LPS)[J]. J Toxicol Environ Health A, 2019, 82: 437-446. DOI:10.1080/15287394.2019.1615019 |
| [6] |
Dinda B, Dinda S, DasSharma S, et al. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders[J]. Eur J Med Chem, 2017, 131: 68-80. DOI:10.1016/j.ejmech.2017.03.004 |
| [7] |
Duan DD, Wang KX, Zhou YZ, et al. Baicalein exerts beneficial effects in D-galactose-induced aging rats through attenuation of inflammation and metabolic dysfunction[J]. Rejuvenation Res, 2017, 20: 506-516. DOI:10.1089/rej.2017.1919 |
| [8] |
Gao L, Li J, Zhou Y, et al. Effects of baicalein on cortical proinflammatory cytokines and the intestinal microbiome in senescence accelerated mouse prone 8[J]. ACS Chem Neurosci, 2018, 9: 1714-1724. DOI:10.1021/acschemneuro.8b00074 |
| [9] |
Yan JJ, Gao L, Qin XM, et al. Baicalein attenuates the neuro-inflammation in LPS-activated BV-2 microglial cells through suppression of pro-inflammatory cytokines, COX2/NF-κB expressions and regulation of metabolic abnormality[J]. Int Immunopharmacol, 2020, 79: 1060902. |
| [10] |
Song H, Lu Y, Qu Z, et al. Effects of aged garlic extract and FruArg on gene expression and signaling pathways in lipopolysaccharide-activated microglial cells[J]. Sci Rep, 2016, 6: 35323. DOI:10.1038/srep35323 |
| [11] |
Juknat A, Pietr M, Kozela E, et al. Microarray and pathway analysis reveal distinct mechanisms underlying cannabinoid-mediated modulation of LPS-induced activation of BV-2 micro-glial cells[J]. PLoS One, 2013, 8: e61462. DOI:10.1371/journal.pone.0061462 |
| [12] |
Zhou H, Qu Z, Mossine VV, et al. Proteomic analysis of the effects of aged garlic extract and its FruArg component on lipopolysaccharide-induced neuroinflammatory response in microglial cells[J]. PLoS One, 2014, 9: e113531. DOI:10.1371/journal.pone.0113531 |
| [13] |
Lee SB, Lee WS, Shin JS, et al. Xanthotoxin suppresses LPS-induced expression of iNOS, COX-2, TNF-α, and IL-6 via AP-1, NF-κB, and JAK-STAT inactivation in RAW264.7 macrophages[J]. Int Immunopharmacol, 2017, 49: 21-29. DOI:10.1016/j.intimp.2017.05.021 |
| [14] |
Yauger YJ, Bermudez S, Moritz KE, et al. Iron accentuated reactive oxygen species release by NADPH oxidase in activated microglia contributes to oxidative stress in vitro[J]. J Neuroinflammation, 2019, 16: 41. DOI:10.1186/s12974-019-1430-7 |
| [15] |
Rendra E, Riabov V, Mossel DM, et al. Reactive oxygen species (ROS) in macrophage activation and function in diabetes[J]. Immunobiology, 2019, 224: 242-253. DOI:10.1016/j.imbio.2018.11.010 |
| [16] |
Qi Z, Yin F, Lu L, et al. Baicalein reduces lipopolysaccharide-induced inflammation via suppressing JAK/STATs activation and ROS production[J]. Inflamm Res, 2013, 62: 845-855. DOI:10.1007/s00011-013-0639-7 |
| [17] |
Zheng C, Qu YX, Wang B, et al. COX-2/PGE2 facilitates fracture healing by activating the Wnt/β-catenin signaling pathway[J]. Eur Rev Med Pharmacol Sci, 2019, 23: 9721-9728. |
| [18] |
Tse JKY. Gut microbiota, nitric oxide, and microglia as prerequisites for neurodegenerative disorders[J]. ACS Chem Neurosci, 2017, 8: 1438-1447. DOI:10.1021/acschemneuro.7b00176 |
| [19] |
Zoete V, Schuepbach T, Bovigny C, et al. Attracting cavities for docking. Replacing the rough energy landscape of the protein by a smooth attracting landscape[J]. J Comput Chem, 2016, 37: 437-447. DOI:10.1002/jcc.24249 |
| [20] |
Sayers EW, Beck J, Bolton EE, et al. Database resources of the national center for biotechnology information[J]. Nucleic Acids Res, 2021, 49: D10-D17. DOI:10.1093/nar/gkaa892 |
| [21] |
David G, Grosdidier A, Wirth M, et al. SwissTargetPrediction: a web server for target prediction of bioactive small molecules[J]. Nucleic Acids Res, 2014, 42: 32-38. |
| [22] |
Keiser MJ, Roth BL, Armbruster BN, et al. Relating protein pharmacology by ligand chemistry[J]. Nat Biotechnol, 2007, 25: 197-206. DOI:10.1038/nbt1284 |
| [23] |
Szklarczyk D, Franceschini A, Wyder S, et al. STRING v10: protein-protein interaction networks, integrated over the tree of life[J]. Nucleic Acids Res, 2015, 43: D447-D452. DOI:10.1093/nar/gku1003 |
| [24] |
Lopes CT, Franz M, Kazi F, et al. Cytoscape web: an interactive web-based network browser[J]. Bioinformatics, 2010, 26: 2347-2348. DOI:10.1093/bioinformatics/btq430 |
| [25] |
Assenov Y, Ramírez F, Schelhorn SE, et al. Computing topological parameters of biological networks[J]. Bioinformatics, 2008, 24: 282-284. DOI:10.1093/bioinformatics/btm554 |
| [26] |
Bader GD, Hogue CW. An automated method for finding mole-cular complexes in large protein interaction networks[J]. BMC Bioinformatics, 2003, 4: 2. DOI:10.1186/1471-2105-4-2 |
| [27] |
Sterling T, Irwin JJ. ZINC 15--ligand discovery for everyone[J]. J Chem Inf Model, 2015, 55: 2324-2337. DOI:10.1021/acs.jcim.5b00559 |
| [28] |
Berman HM, Westbrook J, Feng Z, et al. The protein data bank[J]. Nucleic Acids Res, 2000, 28: 235-242. DOI:10.1093/nar/28.1.235 |
| [29] |
Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading[J]. J Comput Chem, 2010, 31: 455-461. |
| [30] |
Nathan A, Nils W, Jens M. bcl: : Cluster: a method for clustering biological molecules coupled with visualization in the Pymol molecular graphics system[J]. IEEE Int Conf Comput Adv Bio Med Sci, 2011, 2011: 13-18. |
| [31] |
Sherman BT, Hosack DA, Yang J, et al. DAVID: database for annotation, visualization, and integrated discovery[J]. Genome Biol, 2003, 4: P3. DOI:10.1186/gb-2003-4-5-p3 |
| [32] |
Irwin JJ, Shoichet BK, Mysinger MM, et al. Automated docking screens: a feasibility study[J]. J Med Chem, 2009, 52: 5712-5720. DOI:10.1021/jm9006966 |
| [33] |
Morris GM, Huey R, Olson AJ. Using AutoDock for ligand-receptor docking[J]. Curr Protoc Bioinformatics, 2008, Chapter 8: Unit 8.14. |
| [34] |
Li S, Fan TP, Zhang WD, et al. Network pharmacology in traditional Chinese medicine[J]. Evid Based Complement Alternat Med, 2014, 2014: 138460. |
| [35] |
Farkhondeh T, Pourbagher-Shahri AM, Ashrafizadeh M, et al. Green tea catechins inhibit microglial activation which prevents the development of neurological disorders[J]. Neural Regen Res, 2020, 15: 1792-1798. DOI:10.4103/1673-5374.280300 |
| [36] |
Orihuela R, McPherson CA, Harry GJ. Microglial M1/M2 polari-zation and metabolic states[J]. Br J Pharmacol, 2016, 173: 649-665. DOI:10.1111/bph.13139 |
| [37] |
Zhang J, Zheng Y, Luo Y, et al. Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/TLR4/NF-κB pathways in BV2 cells[J]. Mol Immunol, 2019, 116: 29-37. DOI:10.1016/j.molimm.2019.09.020 |
| [38] |
Chhor V, Le Charpentier T, Lebon S, et al. Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro[J]. Brain Behav Immun, 2013, 32: 70-85. DOI:10.1016/j.bbi.2013.02.005 |
| [39] |
Nogawa S, Forster C, Zhang F, et al. Interaction between indu-cible nitric oxide synthase and cyclooxygenase-2 after cerebral ischemia[J]. Proc Natl Acad Sci U S A, 1998, 95: 10966-10971. DOI:10.1073/pnas.95.18.10966 |
| [40] |
Brown GC, Bal-Price A. Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria[J]. Mol Neurobiol, 2003, 27: 325-355. DOI:10.1385/MN:27:3:325 |
| [41] |
Kawano T, Anrather J, Zhou P, et al. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity[J]. Nat Med, 2006, 12: 225-229. DOI:10.1038/nm1362 |
| [42] |
Mattson MP, Camandola S. NF-kappaB in neuronal plasticity and neurodegenerative disorders[J]. J Clin Invest, 2001, 107: 247-254. DOI:10.1172/JCI11916 |
| [43] |
Kim YJ, Hwang SY, Oh ES, et al. IL-1β, an immediate early protein secreted by activated microglia, induces iNOS/NO in C6 astrocytoma cells through p38 MAPK and NF-κB pathways[J]. J Neurosci Res, 2006, 84: 1037-1046. DOI:10.1002/jnr.21011 |
| [44] |
Baeuerle PA, Baltimore D. NF-kappa B: ten years after[J]. Cell, 1996, 87: 13-20. DOI:10.1016/S0092-8674(00)81318-5 |
| [45] |
Ohmori Y, Schreiber RD, Hamilton TA. Synergy between interferon-gamma and tumor necrosis factor-alpha in transcriptional activation is mediated by cooperation between signal transducer and activator of transcription 1 and nuclear factor kappa B[J]. J Biol Chem, 1997, 272: 14899-14907. DOI:10.1074/jbc.272.23.14899 |
| [46] |
Yang XJ, Seto E. HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention[J]. Oncogene, 2007, 26: 5310-5318. DOI:10.1038/sj.onc.1210599 |
| [47] |
Lo JY, Kamarudin MN, Hamdi OA, et al. Curcumenol isolated from Curcuma zedoaria suppresses Akt-mediated NF-κB activation and p38 MAPK signaling pathway in LPS-stimulated BV-2 microglial cells[J]. Food Funct, 2015, 6: 3550-3559. DOI:10.1039/C5FO00607D |
| [48] |
Zhong LM, Zong Y, Sun L, et al. Resveratrol inhibits inflammatory responses via the mammalian target of rapamycin signaling pathway in cultured LPS-stimulated microglial cells[J]. PLoS One, 2012, 7: e32195. DOI:10.1371/journal.pone.0032195 |
| [49] |
Luu K, Greenhill CJ, Majoros A, et al. STAT1 plays a role in TLR signal transduction and inflammatory responses[J]. Immunol Cell Biol, 2014, 92: 761-769. DOI:10.1038/icb.2014.51 |
| [50] |
Lee HH, Shin JS, Lee WS, et al. Biflorin, isolated from the flower buds of Syzygium aromaticum L., suppresses LPS-induced inflam-matory mediators via STAT1 inactivation in macrophages and protects mice from endotoxin shock[J]. J Nat Prod, 2016, 79: 711-720. DOI:10.1021/acs.jnatprod.5b00609 |
| [51] |
Li JQ, Zhou YZ, Gao L, et al. Integration of transcriptomics and network analysis deciphers the mechanisms of baicalein in improving learning and memory impairment in senescence-accelerated mouse prone 8 (SAMP8)[J]. Eur J Pharmacol, 2019, 865: 172789. DOI:10.1016/j.ejphar.2019.172789 |
| [52] |
Yang CS, Kim JJ, Lee SJ, et al. TLR3-triggered reactive oxygen species contribute to inflammatory responses by activating signal transducer and activator of transcription-1[J]. J Immunol, 2013, 190: 6368-6377. DOI:10.4049/jimmunol.1202574 |
| [53] |
Sareila O, Kelkka T, Pizzolla A, et al. NOX2 complex-derived ROS as immune regulators[J]. Antioxid Redox Signal, 2011, 15: 2197-2208. DOI:10.1089/ars.2010.3635 |
| [54] |
El-Benna J, Dang PM, Gougerot-Pocidalo MA. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane[J]. Semin Immunopathol, 2008, 30: 279-289. DOI:10.1007/s00281-008-0118-3 |
 2022, Vol. 57
2022, Vol. 57


