2. 军事科学院军事医学研究院辐射医学研究所, 北京 100850;
3. 蚌埠医学院药学院, 安徽 蚌埠 233030
2. Beijing Institute of Radiation Medicine, Beijing 100850, China;
3. College of Pharmacy, Bengbu Medical College, Bengbu 233030, China
血脑屏障(blood brain barrier, BBB) 是由脑毛细血管内皮细胞、基膜、周细胞和足端星形胶质细胞组成, 用以保护脑区免受血液循环中有害物质(如神经毒性血浆成分、血细胞和病原体) 侵袭的动态物理屏障。BBB在保护大脑的同时也阻断了治疗药物进入中枢神经系统(central nervous system, CNS) 发挥作用, 如小分子药物、蛋白质、多肽和基因等皆因BBB阻断而不能进入大脑发挥作用[1]。因此, 高效、安全的脑靶向给药系统研究十分重要, 而生物相容性、可降解性及低免疫原性是脑靶向递送系统跨越BBB实现药物高效递送的基本要求。
外泌体是细胞衍生的纳米级胞外囊泡(extracellular vesicles, EV), 由细胞分泌的脂质双分子层膜围绕形成的囊状结构, 一般直径介于30~5 000 nm之间。EV主要分为3类, 即外泌体、微泡和凋亡小体, 三者的大小、来源和生理功能均不同。其中外泌体作为来源于内吞途径的纳米级EV研究较多。
外泌体既可通过蛋白质、脂质、DNA和mRNA介导细胞间通讯, 又可作为小分子药物或核酸药物的载体将治疗分子递送至特定靶部位中实现靶向递送。因此, 外泌体作为一种天然药物递送系统, 正被广泛运用于脑靶向递送, 其具体优点包括: ①由于其纳米级尺寸, 外泌体可实现BBB高效渗透; ②外泌体为机体内源性产物, 可显著降低毒性和免疫原性风险; ③外泌体介导的药物递送绕过了P-糖蛋白药物泵出系统(对外来物质及代谢废物具有清除作用), 降低耐药性; ④天然外泌体具有一定治疗作用, 通过阐明其作用机制可发现新型药物; ⑤不同病理条件会促进BBB对外泌体的摄取; ⑥通过改造外泌体可实现高靶向性、高载药性和高穿透性。
本综述将从外泌体组成、来源、分泌过程、提取方法、脑靶向机制、给药途径和在脑部疾病中应用等方面全面阐明外泌体在脑部疾病中的重要作用。
1 外泌体简介 1.1 外泌体组成外泌体主要由脂质、蛋白质和核酸三部分构成(图 1)。外层为磷脂双分子层发挥保护和载药功能; 磷脂双分子层的脂肪酸尾部使整个磷脂分子呈现横向振动, 保证了外泌体结构的灵活性; 而磷脂双分子层表面的胆固醇及表层黏附蛋白增强了外泌体的刚性, 保证了整个结构的稳定性。
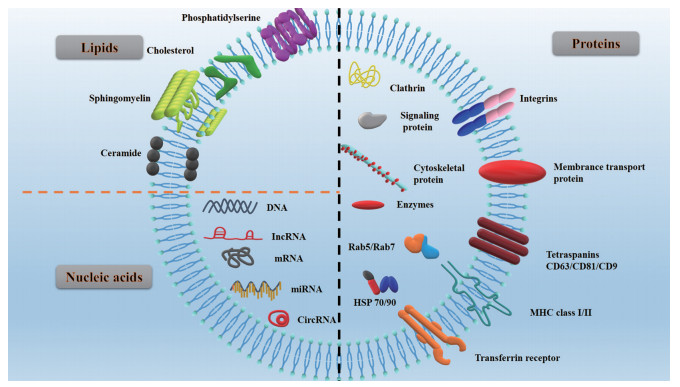
|
Figure 1 Schematic structure of exosomes. HSP: Heat shock protein; MHC: Major histocompatibility complex |
外泌体中的核酸成分, 如DNA (双链DNA、单链DNA、线粒体DNA) 和RNA (信使RNA、微小核糖核酸、小核RNA、非编码RNA、小细胞质RNA), 可改变靶细胞中遗传信息的表达进而实现疾病治疗的目的。外泌体中脂质成分主要包括磷脂酰丝氨酸、胆固醇、鞘磷脂和神经酰胺等。目前已有技术利用外泌体中脂质变化实现疾病诊断及治疗[2]。外泌体中蛋白可黏附于磷脂双分子层表面也可存在于内部囊腔中, 具体可分为信号蛋白、酶、细胞骨架蛋白、伴侣蛋白(HSP 90、HSP 70) 和多泡体(multivesicular bodies, MVB) 制造蛋白, 其功能是调控外泌体生成、介导细胞间通讯和调节机体生理病理过程。
1.2 外泌体来源几乎所有类型的体液和组织中均可发现外泌体的存在, 包括血液、尿液、母乳、羊水/腹水、唾液和脂肪组织[3-8]。根据分泌来源不同, 外泌体生理功能不同[9], 如来自活化抗原呈递细胞(巨噬细胞、T淋巴细胞) 的外泌体具有免疫调节及抗肿瘤作用[10, 11]; 母乳中分离的外泌体可改变T细胞平衡, 增强机体免疫系统[11, 12]; 来源于骨髓、脂肪组织和脐带血的间充质干细胞产生的外泌体对机体脏器具有修复再生功能[13-15]。
1.3 外泌体的分泌过程首先, 胞吞囊泡通过胞吞作用出现在质膜的脂筏结构域中, 于胞内形成早期核内体(early endosomes)。接着在高尔基复合体作用下, 早期核内体变成了晚期核内体(late endosomes); 同时, 腔内小泡(intraluminal vesicles, ILVs) 聚集于内核体腔内。早期内核体中存在的分子可回收到质膜中, 也可整合到ILV中; 同时mRNA或蛋白质等可装载到ILV中。由于早期内核体向内出芽及细胞质隔离, 这些小泡在晚期内核体中不断积累最终形成MVB, 之后MVB分别与溶酶体或质膜融合。在MVB与溶酶体结合过程中ILV被降解; 而与质膜融合后最终实现外泌体的释放[16-18] (图 2)。
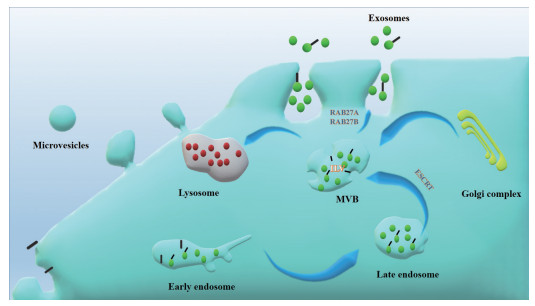
|
Figure 2 Schematic diagram of exosomes generation. The early endosomes gradually mature into late endosomes, and the late endosomes subsequently transform into MVB. MVB bind to lysosomes for degradation, fuse with plasma membrane to secrete exosomes. ILV: Intraluminal vesicle; MVB: Multivesicular bodies; ESCRT: Endosomal sorting complex required for transport |
外泌体分离纯化一直是外泌体研究中高度关注的问题, 获取高度纯化的外泌体对后续研究具有重要意义。目前常用的外泌体提取方法包括超速离心法、免疫磁珠法和试剂盒提取等。
超速离心又称差速离心, 是外泌体提取最常用的方法。主要分为三个阶段: 第一阶段低速离心除去细胞和凋亡碎片; 第二阶段增大离心速度除去大型囊泡; 第三阶段超速离心以沉淀外泌体。超速离心法操作简便, 但外泌体纯度可能存在一定差异性, 且多次离心也可能对外泌体结构造成损害[19]。
免疫磁珠法[20]主要机制是由于外泌体表面具有特异性标记物(如CD63、CD9蛋白), 采用抗标记物抗体的磁珠与外泌体囊泡孵育后发生结合, 从而将外泌体吸附分离出来。该方法特异性高, 但效率低且容易影响外泌体活性, 不利于下游实验的进行。
试剂盒提取法是利用商业化的外泌体提取试剂盒提取分离外泌体。分离方法包括尺寸排阻色谱的分离纯化、免疫亲和色谱和化合物沉淀法等。试剂盒法分离效率及纯化效果随着技术发展逐渐提高, 具有良好应用前景[21]。
此外, 还有借助超速离心与蔗糖密度梯度相结合实现外泌体与非囊泡颗粒分离的密度梯度离心法、基于聚合物的沉淀技术[22]及超滤膜过滤法等分离纯化外泌体的方法。
2 外泌体脑靶向机制尽管脂质体、胶束和纳米粒等纳米制剂借助其纳米级尺寸可在一定程度上实现BBB跨越用于脑靶向递送, 但不容忽视的血液毒性及机体内蓄积等问题尚未解决。如: 聚乙二醇化聚乳酸-羟基乙酸纳米粒在多次给药后会诱导抗体产生, 血液循环半衰期也明显缩短[23, 24]。作为生物内源性的外泌体以其低毒性、低免疫原性、高生物相容性和高稳定性的优点, 有效克服了纳米制剂的不足, 能实现无毒、高效跨越BBB。
2.1 天然外泌体穿越BBB外泌体根据其所含成分的不同, 结合不同受体细胞的生理状态可实现天然靶向性, 如肿瘤外泌体由于整合素的作用具有特定器官趋向性[25]。借助外泌体表面配体与脑内皮细胞受体特异性结合实现BBB穿透是目前天然外泌体跨越BBB的主要作用机制。巨噬细胞(macrophages, Mφ) 外泌体借助具有整合素淋巴细胞功能的相关抗原-1和BBB细胞间黏附分子-1之间的相互作用而跨越BBB[26]。血液外泌体借助转铁蛋白受体也表现出天然脑靶向功能[27]。分化簇46 (cluster of differentiation 46) 则是BBB细胞捕获脑转移癌细胞外泌体的主要受体[28]。
病理条件也会促进BBB细胞对外泌体的选择性摄取。炎症因子ICAM-1上调加强了BBB细胞对Mφ外泌体的摄取; 药代动力学数据显示, Mφ外泌体在炎性脑中蓄积量为健康脑含量的5.8倍[26]。
外泌体也可借助外泌体表面特殊黏附蛋白通过脑微血管内皮细胞内吞作用而实现BBB跨越[29], 如四跨膜蛋白(tetraspanins)、CD11b、CD18受体及整合素、主要组织相容性复合体(major histocompatibility complex, MHC)[30]等。上述外泌体表面黏附蛋白也在一定程度上介导神经元与胶质细胞之间的信息交流(图 3)。
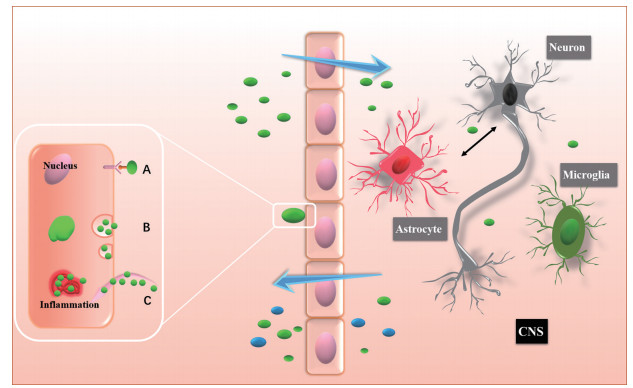
|
Figure 3 Schematic diagram of exosomes entering and leaving brain. Exosomes can enter nerve cells through (A) ligand-receptor specific binding and (B) endocytosis. Inflammation of the nervous system will also promote the absorption of exosomes (C). After the exosomes enter central nervous system (CNS), in addition to specific targets, the cyclic communication of exosomes will also be realized among neurons, astrocytes, and microglia, which jointly mediate the treatment of CNS diseases |
通过化学修饰或基因修饰, 增加外泌体在脑部病变部位的靶向性及局部浓度, 从而降低毒性和不良反应, 最大限度提高治疗效果(图 4)。
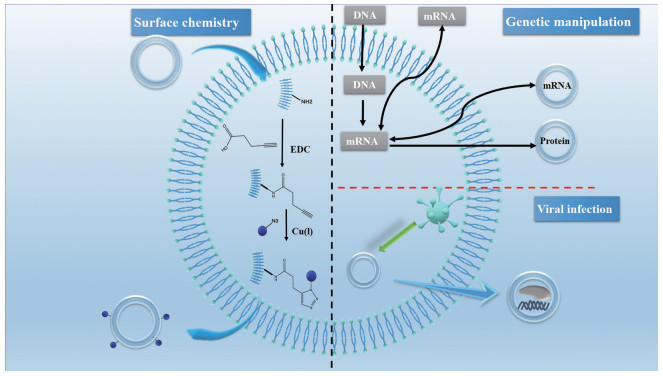
|
Figure 4 Schematic diagram of surface engineered exosomes modification. Chemical modification can be performed directly on the membrane. For example, carbodiimide can be used to modify natural amines to present azide groups for click chemistry reactions. Genetic engineering exosomes can be used to introduce coding and non-coding oligonucleotides into cells, which can be packaged into exosomes to promote gene expression or regulate transcription in recipient cells. At the same time, genetically modified proteins can also be incorporated into exosomes. Virus infection can obtain the exosomes carrying the target genes or drugs |
化学修饰是借助脂质结合蛋白、膜结合蛋白或脂质-脂质相互作用的化学反应将肽、蛋白质、脂质、适配体、聚合物等与外泌体结合实现脑靶向递送, 即在外泌体表面连接靶向分子使其特异性靶向脑部。该靶向修饰主要依赖于靶向配体与生物蛋白的偶联作用, 但需注意在化学修饰过程中可能会出现表面蛋白失活的现象[31, 32]。
通过磺酰叠氮环加成反应, 将偶联胶质瘤靶向肽的姜黄素外泌体经静脉注射后成功跨越BBB实现了肿瘤的靶向递送[33]。同样载姜黄素外泌体借助c (RGDyK) 肽实现高效跨越动脉闭塞小鼠模型的BBB, 发挥抑制炎症及促肿瘤细胞凋亡作用[34]。借助外泌体脂质双层膜上磷脂与ApoA-I模拟肽之间的分子识别, 可实现甲氨蝶呤的脑部肿瘤靶向递送[35]。
化学修饰外泌体除靶向递送药物外还可直接用于递送活性外泌体。如来源于间充质干细胞的外泌体经狂犬病毒糖蛋白肽修饰后, 可实现皮质及海马区的精准靶向结合, 进而通过调节炎症反应修复阿尔茨海默症(Alzheimer disease's, AD) 的记忆缺陷[36]。
2.2.2 基因修饰基因修饰是通过引导蛋白或多肽的基因序列与特定的外泌体膜蛋白基因序列相融合以实现外泌体脑靶向递送的一种方法。首先, 配体或归巢肽与外泌体表面表达的跨膜蛋白融合。随后, 被编码融合蛋白的质粒转染供体细胞, 供体细胞随之会分泌表面带有靶向配体的外泌体。基因修饰仅对多肽和蛋白表达有效, 同时也仅限于可编码基因的表达, 而且此方法还需要构建质粒并在受体细胞中过表达此蛋白。除此方法外, 可将外泌体中特定基因转移到肿瘤中建立一个针对药物的治疗靶点, 从而实现肿瘤与药物靶向对接[32]。
将编码Lamp2b质粒转染的树突状细胞作为供体细胞, 随后供体细胞分泌含Lamp2b蛋白的外泌体, 借助外泌体膜上Lamp2b与神经元特异性RVG肽的强烈融合, 最终外泌体成功跨越BBB将siRNA靶向递送至大脑[37]。
病毒可在外泌体经内吞体途径离开细胞时感染外泌体[38]。对此过程进行干预, 可实现利用病毒改造外泌体来携带目的基因或药物。如外泌体参与朊病毒蛋白的N端修饰, 可选择性地将不同朊病毒蛋白递送到神经元细胞中[39]。
3 外泌体脑部给药途径外泌体给药途径分为全身给药和局部给药。在全身给药途径中, 静脉注射最常见, 其次为腹腔注射和鼻腔给药。局部给药途径相对较少使用, 主要为颅内注射给药(图 5)。
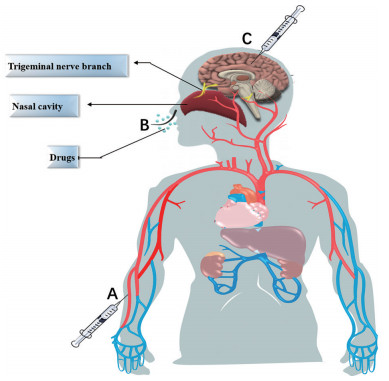
|
Figure 5 Brain-targeted administration routes of exosomes. A: For intravenous injection, drugs mainly reach the brain through the transportation of the human blood circulation system. Drugs can be distributed in whole body, but the content in the local brain area is low; B: Nasal administration mainly involves the absorption of drugs through the nasal mucosa, which are taken up by the end of olfactory neurons and transferred to the olfactory bulb and then to the brain; C: Intracranial injection is to directly inject the drug into brains. Intraperitoneal injection is to inject drugs outside the serous membrane of gastrointestinal tract and inside the peritoneum to achieve efficient absorption |
静脉注射是外泌体全身给药最常用的途径。外泌体经静脉注射进入血液循环主要问题在于肝脏的清除作用及较短的血浆半衰期[40]。研究表明, 粒径100 nm纳米粒静脉注射后肝脏清除作用最小, 并可维持血液循环时间最长[41], 因此通过差速离心法分离直径100 nm的外泌体是目前常用筛选方法。此外, 外泌体的肝脏清除源于巨噬细胞的吞噬摄取。通过降低巨噬细胞活性进而降低肝脏清除, 可显著延长外泌体血浆半衰期[42]。静脉注射外泌体可用于多种药物递送, 如通过静脉注射外泌体可将siRNA靶向递送至小鼠大脑[37]; M2小胶质细胞衍生的外泌体经静脉注射后可保护小鼠大脑免受缺血再灌注损伤[43]; 过氧化氢酶外泌体静脉注射后降低了小鼠神经炎症及神经毒性的发生[44]。
鼻腔给药基于较明确的鼻-脑通路, 经三叉神经或嗅神经通路可实现无创性脑靶向递送, 但经鼻腔给药递送药物量非常低, 要求药物本身活性高。而外泌体因载药量高可弥补此不足, 再通过外泌体本身跨BBB优势与鼻腔给药优势相结合, 成为现阶段极具潜力的脑靶向递送载体[45]。外泌体载抗炎小分子姜黄素后可通过鼻腔给药治疗小鼠脑部炎症。姜黄素直接鼻腔给药递送至大脑药量较少, 外泌体载药后可显著提高其溶解性、稳定性和生物利用度[46]。载姜黄素胚胎干细胞外泌体经鼻给药后, 成功恢复了缺血再灌注损伤模型的神经血管细胞[47]。癫痫小鼠经鼻给予人骨髓来源的间充质干细胞外泌体可减少小鼠神经元丢失及预防炎症, 维持正常神经状态, 并维持小鼠认知和记忆功能[48]。
腹腔注射可避免外泌体直接进入血液循环, 降低了肝和脾清除作用。如小鼠腹腔注射姜黄素外泌体可显著改善AD小鼠的认知功能[49]。
与全身给药相比, 颅内注射外泌体可显著提高其在局部的作用时间及浓度, 还可防止外泌体进入体循环进而避免肝和脾清除作用, 如颅内注射间充质干细胞外泌体可显著改善小鼠认知障碍[50]。
4 外泌体用于脑部疾病诊断与治疗外泌体凭借其天然跨BBB的特点将药物递送至脑区发挥靶向作用。此外, 从血液(血清和血浆)、脑脊液、尿液和其他生物液体中分离出来的外泌体中含有的蛋白质、遗传物质及脂类等还可作为生物标记物, 用于脑部疾病诊断、治疗及神经修复等(图 6)。
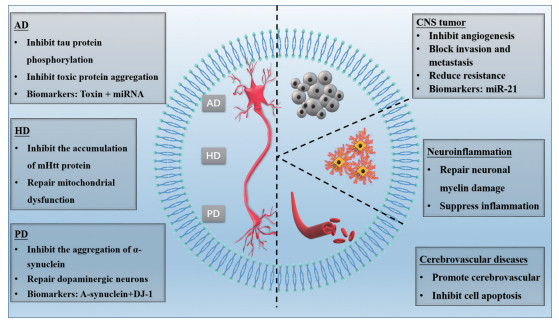
|
Figure 6 The application of exosomes in the treatment of brain diseases. AD: Alzheimer's disease; HD: Huntington's disease; PD: Parkinson's disease |
神经退行性疾病(neurodegenerative diseases), 如帕金森病(Parkinson's disease, PD)、AD、肌萎缩侧索硬化症(Amyotrophic lateral sclerosis, ALS)、亨廷顿病(Huntington's disease, HD), 发病机制类似, 即不同蛋白质在致病过程中错误折叠并沉积在特定区域而导致。随后这些错误折叠的蛋白质“扩散”到特定大脑区域, 说明疾病过程涉及到这些蛋白质的细胞间运动[51, 52]。外泌体在此过程中除了传递代谢废物外, 也在细胞间呈递异常物质, 从而参与神经元修复、变性及凋亡等环节。
4.1.1 ADAD是以认知功能障碍和行为损伤为主要表征的神经系统退行性疾病。之前认为其主要机制为tau蛋白神经原纤维缠结及β淀粉样蛋白的聚集, 近来质疑较多。而外泌体在AD发病过程中扮演重要角色, 既可介导Aβ在神经元之间的扩散, 又可实现tau蛋白传播; 同时外泌体还可释放炎症因子在细胞间扩散, 继而引发神经炎症, 进一步导致Aβ累积及tau蛋白过度磷酸化而最终导致AD[53-55]。
由于外泌体可运载AD相关毒蛋白, 且这些毒蛋白在疾病初期阶段即可被检测, 所以可采用正电子发射断层扫描和脑脊液分析来研究淀粉样蛋白和tau蛋白用于AD诊断[49]。如AD患者脑脊液中Aβ1-42及Aβ1-42与P-tau蛋白的联合检测均可用于AD早期诊断[56, 57]。由于在AD病理状态下, 外泌体中含有疾病特异性或失调的miRNAs, 所以外泌体中除毒蛋白外, 某些特定miRNA也可作为早期AD诊断标准。通过对比AD患者和健康对照者血浆中miRNA的差异表达发现AD患者血浆中miR-342-3p明显下调, 因此miR-342-3p有可能作为AD早期诊断的生物标志物[58]。
除AD诊断, 外泌体还可用于AD治疗。外泌体可通过介导神经细胞通讯, 保护神经元免受氧化应激刺激[59]。来源于间充质干细胞的外泌体可用于神经功能的修复, 具有神经保护作用[60]。来源于人脂肪间充质干细胞外泌体抑制了神经元细胞凋亡, 促进了神经再生和修复, 具有神经保护作用[61]。中性肽酶neprilysin参与Aβ降解, 在间充质干细胞外泌体中含量远远高于神经细胞[62-64]。同时在大鼠原代细胞外泌体中也分离出参与Aβ降解的胱抑素C[65, 66]。以上表明外泌体可作为AD的潜在治疗药物。
除自身作为药物治疗外, 外泌体还可作为小分子药物、miRNA和siRNA等核酸片段的递送载体用以治疗AD。如外源性siRNA通过电穿孔载于RVG修饰靶向外泌体中, 可显著降低AD小鼠脑组织中tau蛋白表达, 降低Aβ沉积[37]。已知当糖原合成酶激酶-3β (glycogen synthase kinase-3β, GSK-3β) 被激活时, 其残基去磷酸化, 可诱导tau蛋白磷酸化。蛋白激酶B (protein kinase B, PKB) 是GSK-3β潜在的上游调节因子, 激活的PKB通过促进GSK-3β磷酸化可抑制GSK-3β激活。姜黄素外泌体可促进PKB磷酸化, 进一步抑制GSK-3β激活, 降低tau蛋白磷酸化, 最终达到治疗AD的效果。小鼠腹腔注射姜黄素外泌体后, PKB/GSK-3β通路抑制tau蛋白磷酸化是姜黄素外泌体改善AD认知功能的主要机制[49] (图 7)。
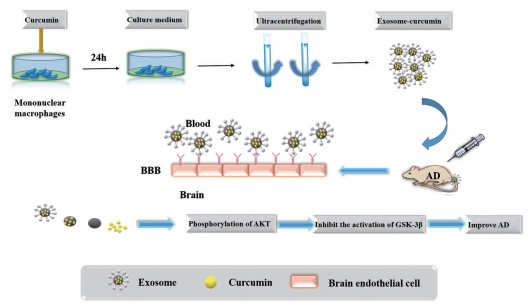
|
Figure 7 Curcumin exosomes for treatment of AD. Curcumin-loaded exosomes were purified by ultracentrifugation, and AD rats were injected with curcumin exosomes. Due to the interaction between exosomes and brain epithelial cells, curcumin crossed blood brain barrier (BBB) and inhibited AD induced tau phosphorylation, and finally improved the cognitive function of mice. PKB: Protein kinase B; GSK-3β: Glycogen synthase kinase-3β |
以静止性震颤、运动迟缓、肌肉强直和姿势不稳的特征性运动症状为主要临床表现的神经系统退行性疾病, 主要病理性特征为α突触蛋白沉积导致的路易小体及黑质致密区多巴胺能神经元的缺失及变性。多巴胺能神经元的变性导致兴奋性乙酰胆碱和抑制性多巴胺神经递质失衡最终诱发运动失衡[67]。外泌体可用于PD诊断和治疗。
目前PD诊断多基于运动症状的观察, 误诊率较高。尤其对于中早期PD, 亟需新的生物标记物。外泌体携带的PD相关蛋白或许可成为一种新选择。采用抗体识别技术分离CNS外泌体, 发现CNS外泌体可外输至血液, 同时PD患者血浆中CNS来源外泌体的α突触核蛋白水平明显高于正常值, 与PD患病程度密切相关, 所以可把该突触核蛋白作为PD高灵敏度、高特异性的生物标记物[68]。尿液外泌体中DJ-1和LRRK2均为PD基因产物, 分别与PD患者年龄及认知障碍呈现显著依赖性关系, 也可作为PD生物标记物[69, 70]。
外泌体同样可作为PD治疗药物的载体。巨噬细胞经编码过氧化氢酶的质粒转染后可分泌载过氧化氢酶质粒DNA、mRNA及活性过氧化氢酶的外泌体。该外泌体跨越BBB将过氧化氢酶质粒DNA、mRNA等遗传物质转移至相邻神经元炎症区域, 随后在靶细胞中指导合成过氧化氢酶, 成功地抑制了PD炎症反应, 发挥了神经保护作用[71]。转基因小鼠注射RVG肽修饰的载siRNA外泌体后, 其体内PD相关mRNA和蛋白水平均降低, PD症状有效缓解[72]。
4.1.3 ALS是上运动神经元和下运动神经元的选择性丧失, 最终导致随意肌控制力进行性丧失的一种神经退行性疾病[73]。Cu/Zn超氧化物歧化酶基因突变、兴奋性氨基酸毒性作用、线粒体功能障碍、免疫炎性反应和星形胶质细胞功能异常等均可能导致ALS运动神经元死亡。但具体作用机制仍不明确, 目前既没有确切治疗方法, 也没有可靠的生物标记物。
外泌体参与星形胶质细胞与神经元细胞之间突变蛋白的递送最终诱发细胞凋亡, 同时外泌体递送miR-124可驱动小胶质细胞加重ALS炎症反应[74, 75]。因此, 深入研究ALS中外泌体形成、转运及扩散异常蛋白的具体分子机制, 探索潜在的高特异性诊断及预后生物标志物具有重要意义。
4.1.4 HD又称慢性或进行性舞蹈病, 是一种由编码亨廷顿蛋白(mHtt) 的基因CAG异常扩张引起的常染色体显性遗传病。HD以认知障碍、神经精神症状和不自主的运动症状为主要临床表征。基因异常产物mHtt累积会通过引发转录失调、激活内在凋亡途径、线粒体功能障碍及改变蛋白质相互作用导致细胞死亡, 其中线粒体功能障碍被认为是主要致病原因[76]。外泌体可通过调节转录及抑制mHtt蛋白累积实现HD的治疗。
小鼠初级皮质神经元有效内化了载靶向亨廷顿蛋白基因的外源性核糖核酸的外泌体, 并促进mHtt基因的沉默, 实现了HD治疗[77]。HD转录功能障碍可能由抑制元件1沉默转录因子(repressor element 1 silencing transcription factor, REST) 引发, 进而加重HD表现。从过表达miR-24细胞系中孵育含miRNA外泌体, 将其注射至转基因HD小鼠的双侧纹状体中, 成功降低了REST表达, 可用于治疗HD[78]。
向HD小鼠纹状体注射星形胶质细胞外泌体, 降低了mHtt密度; 且小鼠原代星形胶质细胞释放的外泌体中没有检测到mHtt蛋白, 表明星形胶质细胞衍生的外泌体具有潜在HD治疗作用[79]。源于脂肪组织干细胞的外泌体经静脉注射后可显著抑制mHtt累积, 发挥缓解细胞凋亡及修复线粒体障碍的作用, 最终实现缓解HD进展的目的[76]。
4.2 脑部肿瘤由于胶质瘤细胞的高度浸润和侵袭现象, 脑胶质瘤现已成为最具侵袭性和致命性的CNS肿瘤。化疗是脑胶质瘤常见治疗方法, 但化疗药物很难跨越BBB实现靶向递送[80, 81]。虽然纳米粒一定程度上克服了上述不足, 但血液循环时间短、BBB和肿瘤屏障穿透性低等问题仍不可避免[82]。外泌体以其低免疫原性、低毒性、高生物相容性和高载药量成为目前治疗脑胶质瘤的研究热点。
外泌体在胶质瘤的生长和扩散中发挥了重要作用。缺氧的神经胶质瘤干细胞所分泌的Linc01060外泌体能激活神经胶质瘤细胞中的原癌基因信号通路, 从而促进神经胶质瘤发展[83]。胶质瘤外泌体还可通过载miR-296、miR-29a和miR-30e等调控因子调控内皮细胞应答从而介导血管生成, 所以这些调控因子均可作为脑胶质瘤潜在治疗靶点。外泌体也参与肿瘤的迁移和侵袭过程, 同时外泌体还可通过将胞内药物转移至胞外及调控耐药蛋白的表达而诱导肿瘤细胞产生耐药性[84]。
目前, 外泌体应用于脑胶质瘤的研究包括诊断和治疗两方面。富含miRNAs的外泌体参与肿瘤微环境中细胞通讯, 并在肿瘤生物学中发挥重要作用。miRNA的易获取性和稳定性使其成为脑胶质瘤的理想生物标记物[85, 86]。LncRNAs和CircRNAs都具有作为脑胶质瘤诊断预后生物标记物的潜力[87]。
超声具有非侵入性、可逆性和瞬时开放BBB的特性, 借助超声构建了一个靶向胶质瘤的高效药物递送系统。小鼠尾静脉注射共孵育的R-Exos-Dox和B-Exos-Dox (来源于巨噬细胞和血清的载多柔比星外泌体), 通过小鼠脑部超声刺激局部药物释放并发挥位点特异性治疗作用。结果表明, 超声能显著促进外泌体到达肿瘤部位, 高效发挥治疗作用[88]。载STAT-3蛋白抑制剂的外泌体通过鼻腔给药跨越BBB进入脑区, 继而被小胶质细胞选择性吸收, 抑制炎性细胞因子如IL-1β和IL-6β表达, 最终达到抑制肿瘤生长的目的[89]。
4.3 脑血管疾病脑血管疾病是指发生在脑部血管、因颅内血液循环障碍而造成脑组织损伤的一类疾病。缺血性脑卒中是心脑血管疾病中致死和致残的主要疾病之一, 也是导致死亡和神经功能障碍的主要原因。激活组织纤溶酶原是目前唯一有效方法, 但由于其治疗窗狭窄, 只有5%患者获益。安全有效地将药物递送到靶部位是治疗缺血性脑卒中的关键。外泌体以其低免疫原性、高稳定性、高递送效率和跨BBB能力被应用于缺血性脑卒中的治疗中。
载miRNAs外泌体具有修复缺血性脑卒中的能力。载miRNA-210外泌体可促进缺血性脑区血管生成[90]。膜上修饰RVG肽的外泌体可特异性靶向神经元细胞, 将环状RNA递送到脑卒中缺血区域, 促进了运动能力恢复, 且这种方法可在脑卒中24 h内发挥疗效, 具有应用前景[91]。
值得注意的是, 低浓度外泌体具有神经修复作用, 但高浓度外泌体可能具有神经毒性[92], 且存在脱靶效应和半衰期短等缺点, 可通过表面功能化实现外泌体跨BBB的定位功能。多肽c (RGDyK) 在脑血管内皮细胞中与整合素αγβ3具有高亲和力, 特别是在缺血后可利用其实现靶向递送。通过点击化学反应将其偶联到外泌体表面, 制备得到的姜黄素cRGD-Exo经静脉注射后靶向于脑缺血小鼠的病变区域, 进入神经元、小胶质和星形胶质细胞, 成功抑制了脑缺血病变区域的炎症反应和细胞凋亡[34]。
颅内动脉粥样硬化是脑卒中的常见病因, 复发率很高。通过研究外泌体miRNA在颅内动脉粥样硬化复发性缺血案例中的作用, 发现了10种与血管生成因子显著相关的miRNA, 并证明其具有抗血管生成及抑制血管生成因子上调的功能[93], 这表明或可通过干预miRNA来实现颅内动脉粥样硬化以至脑卒中的治疗。
4.4 神经炎症神经炎症是指因神经发炎而引起感觉障碍、运动障碍和神经功能障碍的一种疾病。外泌体同样也可用于神经炎症治疗。
活化的星形胶质细胞小胶质细胞释放促炎细胞因子, 在一定程度上促进了神经元死亡。模型组大鼠空间学习及感觉功能恢复与病变区域星形胶质细胞密度呈负相关, 而外泌体组大鼠比模型组星形胶质细胞密度更低。这表明大鼠在给予间充质干细胞外泌体后, 星形胶质细胞激活被显著抑制, 起到了神经修复及抗神经炎症作用[94]。
炎症因子ICAM-1上调还可加强BBB细胞对Mφ外泌体的摄取量, 此时外泌体可携带脑源性神经营养因子跨BBB递送至炎症区域, 促进神经细胞生存[26]。载姜黄素外泌体可诱导小胶质细胞凋亡并延缓实验性自身免疫性脑脊髓炎的进展[95]。神经炎症的进一步加重会使神经细胞去髓鞘化, 进而诱发更严重的认知障碍。而间充质干细胞分泌的外泌体可修复由脂多糖诱发的炎症神经损伤, 通过修复神经元髓鞘损伤最终改善机体认知功能[96]。
4.5 药物依赖性药物成瘾是一种由药物对大脑长期影响而引起的慢性复发性疾病。阿片受体μ (MOR) 是阿片类镇痛药的主要作用靶点, 与阿片类药物的主要成瘾性显著相关。载MOR siRNA的外泌体可通过表面修饰靶向肽RVG将siRNA递送至CNS, 通过下调MOR表达水平强烈抑制吗啡依赖性, 用于吗啡成瘾的治疗[97]。
5 结论与展望外泌体作为最小的细胞外囊泡, 由于其自身低毒性、低免疫原性、高稳定性、高递送效率、高生物相容性及跨BBB功能, 被广泛应用于脑靶向递送系统。外泌体既可通过自身递送参与神经细胞间信息交流以实现机体免疫调节和受损组织细胞的再生, 又可通过内吞或供受体特异性结合实现药物脑靶向递送。还可通过化学或基因修饰及病毒转染对外泌体进行工程化改造, 使其具有高效脑靶向功能, 如RVG肽是目前最常用的外泌体脑靶向的化学修饰归巢肽; 天然或工程化外泌体被广泛用于CNS疾病的诊断、治疗和预后中, 可通过外泌体对神经细胞的修复和保护作用或其对肿瘤细胞的毒性作用实现神经系统疾病的治疗。根据神经系统疾病种类及进程的不同, 大脑会释放含有不同生物标记因子的外泌体, 通过检测这些外泌体中特异性因子, 成功实现了神经系统疾病的诊断。
目前已知外泌体主要通过MVB途径生成, 内吞体分选复合体和Rab蛋白分别干预其小分子载药及膜融合过程[98]。但促使MVB消亡、转化外泌体的机制及其如何与溶酶体结合等分子机制还需进一步阐明[18], 不同生理病理状态、不同种类的外泌体是否由一种或多种机制进行调控也需进一步探究。
工程化外泌体在CNS疾病中展现出巨大潜力, 但临床大规模生产仍然是亟待解决的问题[99]。目前基于细胞分离的外泌体产量较低, 再加上安全性问题, 开发更安全、更丰富的外泌体来源(牛奶、水果) 具有重要意义[100]。
外泌体稳定性在脑部递送中十分重要。表面修饰的外泌体有可能通过非特异性内吞实现内化, 但如果内吞后外泌体表面配体失活, 将无法与配体特异性结合, 也就无法实现药物靶向递送。因此如何实现配体功能化更新也非常重要[101]。
外泌体在CNS疾病诊断与治疗中展现出良好应用前景。当然, 外泌体对其他疾病也有治疗作用, 如肿瘤疫苗研发等。外泌体本身也具有一定药理作用, 如促进凝血、增强内皮细胞连接和诱导自然杀伤细胞激活等。随着对外泌体的深入研究, 外泌体将与检验、精准医学和再生医学等领域不断结合, 具有广阔应用前景。外泌体的未来发展有赖于深入的分子生物学机制、高效的临床批量生产效率及更多更稳定的工程化外泌体, 为实际应用奠定基础。
作者贡献: 李祺负责查阅文献和撰写; 王秀负责总结归纳; 杜丽娜负责归纳和修改。
利益冲突: 本文不存在任何与本稿件相关的利益冲突。
| [1] |
Pardridge WM. The blood-brain barrier: bottleneck in brain drug development[J]. NeuroRx, 2005, 2: 3-14. DOI:10.1602/neurorx.2.1.3 |
| [2] |
Wang W, Zhu N, Yan T, et al. The crosstalk: exosomes and lipid metabolism[J]. Cell Commun Signal, 2020, 18: 119. DOI:10.1186/s12964-020-00581-2 |
| [3] |
Caby MP, Lankar D, Vincendeau-Scherrer C, et al. Exosomal-like vesicles are present in human blood plasma[J]. Int Immunol, 2005, 17: 879-887. DOI:10.1093/intimm/dxh267 |
| [4] |
Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine[J]. Proc Natl Acad Sci U S A, 2004, 101: 13368-13373. DOI:10.1073/pnas.0403453101 |
| [5] |
Admyre C, Johansson SM, Qazi KR, et al. Exosomes with immune modulatory features are present in human breast milk[J]. J Immunol, 2007, 179: 1969-1978. DOI:10.4049/jimmunol.179.3.1969 |
| [6] |
Keller S, Ridinger J, Rupp AK, et al. Body fluid derived exosomes as a novel template for clinical diagnostics[J]. J Transl Med, 2011, 9: 86. DOI:10.1186/1479-5876-9-86 |
| [7] |
Ogawa Y, Miura Y, Harazono A, et al. Proteomic analysis of two types of exosomes in human whole saliva[J]. Biol Pharm Bull, 2011, 34: 13-23. DOI:10.1248/bpb.34.13 |
| [8] |
Zhang Y, Yu M, Tian W. Physiological and pathological impact of exosomes of adipose tissue[J]. Cell Prolif, 2016, 49: 3-13. DOI:10.1111/cpr.12233 |
| [9] |
Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery[J]. J Control Release, 2015, 219: 396-405. DOI:10.1016/j.jconrel.2015.07.030 |
| [10] |
Schorey JS, Cheng Y, Singh PP, et al. Exosomes and other extracellular vesicles in host-pathogen interactions[J]. EMBO Rep, 2015, 16: 24. DOI:10.15252/embr.201439363 |
| [11] |
Kaur S, Singh SP, Elkahloun AG, et al. CD47-dependent immunomodulatory and angiogenic activities of extracellular vesicles produced by T cells[J]. Matrix Biol, 2014, 37: 49-59. DOI:10.1016/j.matbio.2014.05.007 |
| [12] |
Zonneveld MI, Brisson AR, van Herwijnen MJ, et al. Recovery of extracellular vesicles from human breast milk is influenced by sample collection and vesicle isolation procedures[J]. J Extracell Vesicles, 2014, 3: 24215. DOI:10.3402/jev.v3.24215 |
| [13] |
Ilmer M, Vykoukal J, Recio Boiles A, et al. Two sides of the same coin: stem cells in cancer and regenerative medicine[J]. FASEB J, 2014, 28: 2748-2761. DOI:10.1096/fj.13-244640 |
| [14] |
Emanueli C, Shearn AI, Angelini GD, et al. Exosomes and exosomal miRNAs in cardiovascular protection and repair[J]. Vascul Pharmacol, 2015, 71: 24-30. DOI:10.1016/j.vph.2015.02.008 |
| [15] |
Huang L, Ma W, Ma Y, et al. Exosomes in mesenchymal stem cells, a new therapeutic strategy for cardiovascular diseases?[J]. Int J Biol Sci, 2015, 11: 238-245. DOI:10.7150/ijbs.10725 |
| [16] |
Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release[J]. Cell Mol Life Sci, 2018, 75: 193-208. DOI:10.1007/s00018-017-2595-9 |
| [17] |
He C, Zheng S, Luo Y, et al. Exosome theranostics: biology and translational medicine[J]. Theranostics, 2018, 8: 237-255. DOI:10.7150/thno.21945 |
| [18] |
Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes[J]. Curr Opin Cell Biol, 2014, 29: 116-125. DOI:10.1016/j.ceb.2014.05.004 |
| [19] |
Wu X, Showiheen SAA, Sun AR, et al. Exosomes extraction and identification[J]. Methods Mol Biol, 2019, 2054: 81-91. |
| [20] |
Oksvold MP, Neurauter A, Pedersen KW. Magnetic bead-based isolation of exosomes[J]. Methods Mol Biol, 2015, 1218: 465-481. |
| [21] |
Zhang Y, Bi J, Huang J, et al. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications[J]. Int J Nanomedicine, 2020, 15: 6917-6934. DOI:10.2147/IJN.S264498 |
| [22] |
Yang D, Zhang W, Zhang H, et al. Progress, opportunity, and perspective on exosome isolation-efforts for efficient exosome-based theranostics[J]. Theranostics, 2020, 10: 3684-3707. DOI:10.7150/thno.41580 |
| [23] |
Bertrand N, Grenier P, Mahmoudi M, et al. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics[J]. Nat Commun, 2017, 8: 777. DOI:10.1038/s41467-017-00600-w |
| [24] |
Zhang P, Sun F, Liu S, et al. Anti-PEG antibodies in the clinic: current issues and beyond PEGylation[J]. J Control Release, 2016, 244: 184-193. DOI:10.1016/j.jconrel.2016.06.040 |
| [25] |
Hoshino A, Costa-Silva B, Shen TL, et al. Tumour exosome integrins determine organotropic metastasis[J]. Nature, 2015, 527: 329-335. DOI:10.1038/nature15756 |
| [26] |
Yuan D, Zhao Y, Banks WA, et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain[J]. Biomaterials, 2017, 142: 1-12. DOI:10.1016/j.biomaterials.2017.07.011 |
| [27] |
Qu M, Lin Q, Huang L, et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson's disease[J]. J Control Release, 2018, 287: 156-166. DOI:10.1016/j.jconrel.2018.08.035 |
| [28] |
Kuroda H, Tachikawa M, Yagi Y, et al. Cluster of differentiation 46 is the major receptor in human blood-brain barrier endothelial cells for uptake of exosomes derived from brain-metastatic melanoma cells (sk-mel-28)[J]. Mol Pharm, 2019, 16: 292-304. DOI:10.1021/acs.molpharmaceut.8b00985 |
| [29] |
Chen CC, Liu L, Ma F, et al. Elucidation of exosome migration across the blood-brain barrier model in vitro[J]. Cell Mol Bioeng, 2016, 9: 509-529. DOI:10.1007/s12195-016-0458-3 |
| [30] |
Ye SJ, Hu KL. Research progress of exosomes as drug delivery vehicles in the treatment of brain diseases[J]. 药学学报, 2020, 55: 1540-1548. |
| [31] |
Liang Y, Duan L, Lu J, et al. Engineering exosomes for targeted drug delivery[J]. Theranostics, 2021, 11: 3183-3195. DOI:10.7150/thno.52570 |
| [32] |
Wang X, Zhang H, Yang H, et al. Cell-derived exosomes as promising carriers for drug delivery and targeted therapy[J]. Curr Cancer Drug Targets, 2018, 18: 347-354. DOI:10.2174/1568009617666170710120311 |
| [33] |
Jia G, Han Y, An Y, et al. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo[J]. Biomaterials, 2018, 178: 302-316. DOI:10.1016/j.biomaterials.2018.06.029 |
| [34] |
Tian T, Zhang HX, He CP, et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy[J]. Biomaterials, 2018, 150: 137-149. DOI:10.1016/j.biomaterials.2017.10.012 |
| [35] |
Ye Z, Zhang T, He W, et al. Methotrexate-loaded extracellular vesicles functionalized with therapeutic and targeted peptides for the treatment of glioblastoma multiforme[J]. ACS Appl Mater Interfaces, 2018, 10: 12341-12350. DOI:10.1021/acsami.7b18135 |
| [36] |
Cui GH, Guo HD, Li H, et al. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer's disease[J]. Immun Ageing, 2019, 16: 10. DOI:10.1186/s12979-019-0150-2 |
| [37] |
Alvarez-Erviti L, Seow Y, Yin H, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes[J]. Nat Biotechnol, 2011, 29: 341-345. DOI:10.1038/nbt.1807 |
| [38] |
Koppers-Lalic D, Hogenboom MM, Middeldorp JM, et al. Virus-modified exosomes for targeted RNA delivery; a new approach in nanomedicine[J]. Adv Drug Deliv Rev, 2013, 65: 348-356. DOI:10.1016/j.addr.2012.07.006 |
| [39] |
Vella LJ, Sharples RA, Lawson VA, et al. Packaging of prions into exosomes is associated with a novel pathway of PrP processing[J]. J Pathol, 2007, 211: 582-590. DOI:10.1002/path.2145 |
| [40] |
Zhu YJ, Wang Y, Tang JL, et al. Progress in the metabolism of exosomes in vivo[J]. Chin J Emerg Med (中华急诊医学杂志), 2019, 5: 652-654. |
| [41] |
Schiffelers RM, Bakker-Woudenberg IA, Storm G. Localization of sterically stabilized liposomes in experimental rat Klebsiella pneumoniae pneumonia: dependence on circulation kinetics and presence of poly (ethylene) glycol coating[J]. Biochim Biophys Acta, 2000, 1468: 253-261. DOI:10.1016/S0005-2736(00)00265-0 |
| [42] |
Imai T, Takahashi Y, Nishikawa M, et al. Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice[J]. J Extracell Vesicles, 2015, 4: 26238. DOI:10.3402/jev.v4.26238 |
| [43] |
Song Y, Li Z, He T, et al. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124[J]. Theranostics, 2019, 9: 2910-2923. DOI:10.7150/thno.30879 |
| [44] |
Kojima R, Bojar D, Rizzi G, et al. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson's disease treatment[J]. Nat Commun, 2018, 9: 1305. DOI:10.1038/s41467-018-03733-8 |
| [45] |
Fan Y, Chen M, Zhang J, et al. Updated progress of nanocarrier-based intranasal drug delivery systems for treatment of brain diseases[J]. Crit Rev Ther Drug Carrier Syst, 2018, 35: 433-467. DOI:10.1615/CritRevTherDrugCarrierSyst.2018024697 |
| [46] |
Sun D, Zhuang X, Xiang X, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes[J]. Mol Ther, 2010, 18: 1606-1614. DOI:10.1038/mt.2010.105 |
| [47] |
Kalani A, Chaturvedi P, Kamat PK, et al. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury[J]. Int J Biochem Cell Biol, 2016, 79: 360-369. DOI:10.1016/j.biocel.2016.09.002 |
| [48] |
Long Q, Upadhya D, Hattiangady B, et al. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus[J]. Proc Natl Acad Sci U S A, 2017, 114: e3536-e3545. |
| [49] |
Wang H, Sui H, Zheng Y, et al. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the tau protein through the AKT/GSK-3β pathway[J]. Nanoscale, 2019, 11: 7481-7496. DOI:10.1039/C9NR01255A |
| [50] |
Zhao W, Zhang H, Yan J, et al. An experimental study on the treatment of diabetes-induced cognitive disorder mice model with exosomes deriving from mesenchymal stem cells (MSCS)[J]. Pak J Pharm Sci, 2019, 32: 1965-1970. |
| [51] |
Braak H, Rüb U, Gai WP, et al. Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen[J]. J Neural Transm (Vienna), 2003, 110: 517-536. DOI:10.1007/s00702-002-0808-2 |
| [52] |
Hill AF. Extracellular vesicles and neurodegenerative diseases[J]. J Neurosci, 2019, 39: 9269-9273. DOI:10.1523/JNEUROSCI.0147-18.2019 |
| [53] |
Sardar Sinha M, Ansell-Schultz A, Civitelli L, et al. Alzheimer's disease pathology propagation by exosomes containing toxic amyloid-beta oligomers[J]. Acta Neuropathol, 2018, 136: 41-56. DOI:10.1007/s00401-018-1868-1 |
| [54] |
Clavaguera F, Bolmont T, Crowther RA, et al. Transmission and spreading of tauopathy in transgenic mouse brain[J]. Nat Cell Biol, 2009, 11: 909-913. DOI:10.1038/ncb1901 |
| [55] |
Cai ZY, Xiao M, Quazi SH, et al. Exosomes: a novel therapeutic target for Alzheimer's disease?[J]. Neural Regen Res, 2018, 13: 930-935. DOI:10.4103/1673-5374.232490 |
| [56] |
Lewczuk P, Kornhuber J, Vanmechelen E, et al. Amyloid beta peptides in plasma in early diagnosis of Alzheimer's disease: a multicenter study with multiplexing[J]. Exp Neurol, 2010, 223: 366-370. DOI:10.1016/j.expneurol.2009.07.024 |
| [57] |
Schoonenboom NS, Pijnenburg YA, Mulder C, et al. Amyloid beta (1-42) and phosphorylated tau in CSF as markers for early-onset Alzheimer disease[J]. Neurology, 2004, 62: 1580-1584. DOI:10.1212/01.WNL.0000123249.58898.E0 |
| [58] |
Lugli G, Cohen AM, Bennett DA, et al. Plasma exosomal miRNAs in persons with and without Alzheimer disease: altered expression and prospects for biomarkers[J]. PLoS One, 2015, 10: e0139233. DOI:10.1371/journal.pone.0139233 |
| [59] |
Wang S, Cesca F, Loers G, et al. Synapsin I is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia-derived exosomes[J]. J Neurosci, 2011, 31: 7275-7290. DOI:10.1523/JNEUROSCI.6476-10.2011 |
| [60] |
Xin H, Li Y, Chopp M. Exosomes/miRNAs as mediating cell-based therapy of stroke[J]. Front Cell Neurosci, 2014, 8: 377. |
| [61] |
Hao P, Liang Z, Piao H, et al. Conditioned medium of human adipose-derived mesenchymal stem cells mediates protection in neurons following glutamate excitotoxicity by regulating energy metabolism and GAP-43 expression[J]. Metab Brain Dis, 2014, 29: 193-205. DOI:10.1007/s11011-014-9490-y |
| [62] |
Katsuda T, Tsuchiya R, Kosaka N, et al. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes[J]. Sci Rep, 2013, 3: 1197. DOI:10.1038/srep01197 |
| [63] |
Iwata N, Tsubuki S, Takaki Y, et al. Metabolic regulation of brain abeta by neprilysin[J]. Science, 2001, 292: 1550-1552. DOI:10.1126/science.1059946 |
| [64] |
Yasojima K, Akiyama H, McGeer EG, et al. Reduced neprilysin in high plaque areas of Alzheimer brain: a possible relationship to deficient degradation of beta-amyloid peptide[J]. Neurosci Lett, 2001, 297: 97-100. DOI:10.1016/S0304-3940(00)01675-X |
| [65] |
Mi W, Pawlik M, Sastre M, et al. Cystatin C inhibits amyloid-beta deposition in Alzheimer's disease mouse models[J]. Nat Genet, 2007, 39: 1440-1442. DOI:10.1038/ng.2007.29 |
| [66] |
Sastre M, Calero M, Pawlik M, et al. Binding of cystatin C to Alzheimer's amyloid beta inhibits in vitro amyloid fibril formation[J]. Neurobiol Aging, 2004, 25: 1033-1043. DOI:10.1016/j.neurobiolaging.2003.11.006 |
| [67] |
Wu X, Zheng T, Zhang B. Exosomes in Parkinson's disease[J]. Neurosci Bull, 2017, 33: 331-338. DOI:10.1007/s12264-016-0092-z |
| [68] |
Shi M, Liu C, Cook TJ, et al. Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson's disease[J]. Acta Neuropathol, 2014, 128: 639-650. DOI:10.1007/s00401-014-1314-y |
| [69] |
Ho DH, Yi S, Seo H, et al. Increased DJ-1 in urine exosome of korean males with Parkinson's disease[J]. Biomed Res Int, 2014, 2014: 704678. |
| [70] |
Fraser KB, Rawlins AB, Clark RG, et al. Ser (P) -1292 LRRK2 in urinary exosomes is elevated in idiopathic Parkinson's disease[J]. Mov Disord, 2016, 31: 1543-1550. DOI:10.1002/mds.26686 |
| [71] |
Haney MJ, Zhao Y, Harrison EB, et al. Specific transfection of inflamed brain by macrophages: a new therapeutic strategy for neurodegenerative diseases[J]. PLoS One, 2013, 8: e61852. DOI:10.1371/journal.pone.0061852 |
| [72] |
Cooper JM, Wiklander PB, Nordin JZ, et al. Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice[J]. Mov Disord, 2014, 29: 1476-1485. DOI:10.1002/mds.25978 |
| [73] |
van Es MA, Hardiman O, Chio A, et al. Amyotrophic lateral sclerosis[J]. Lancet, 2017, 390: 2084-2098. DOI:10.1016/S0140-6736(17)31287-4 |
| [74] |
Basso M, Pozzi S, Tortarolo M, et al. Mutant copper-zinc superoxide dismutase (sod1) induces protein secretion pathway alterations and exosome release in astrocytes: implications for disease spreading and motor neuron pathology in amyotrophic lateral sclerosis[J]. J Biol Chem, 2013, 288: 15699-15711. DOI:10.1074/jbc.M112.425066 |
| [75] |
Pinto S, Cunha C, Barbosa M, et al. Exosomes from NSC-34 cells transfected with hSOD1-G93A are enriched in miR-124 and drive alterations in microglia phenotype[J]. Front Neurosci, 2017, 11: 273. DOI:10.3389/fnins.2017.00273 |
| [76] |
Lee M, Liu T, Im W, et al. Exosomes from adipose-derived stem cells ameliorate phenotype of Huntington's disease in vitro model[J]. Eur J Neurosci, 2016, 44: 2114-2119. DOI:10.1111/ejn.13275 |
| [77] |
Didiot MC, Hall LM, Coles AH, et al. Exosome-mediated delivery of hydrophobically modified siRNA for Huntingtin mRNA silencing[J]. Mol Ther, 2016, 24: 1836-1847. DOI:10.1038/mt.2016.126 |
| [78] |
Lee ST, Im W, Ban JJ, et al. Exosome-based delivery of miR-124 in a Huntington's disease model[J]. J Mov Disord, 2017, 10: 45-52. DOI:10.14802/jmd.16054 |
| [79] |
Hong Y, Zhao T, Li XJ, et al. Mutant huntingtin inhibits αB-crystallin expression and impairs exosome secretion from astrocytes[J]. J Neurosci, 2017, 37: 9550-9563. DOI:10.1523/JNEUROSCI.1418-17.2017 |
| [80] |
Pinel S, Thomas N, Boura C, et al. Approaches to physical stimulation of metallic nanoparticles for glioblastoma treatment[J]. Adv Drug Deliv Rev, 2019, 138: 344-357. DOI:10.1016/j.addr.2018.10.013 |
| [81] |
Tang W, Fan W, Lau J, et al. Emerging blood-brain-barrier-crossing nanotechnology for brain cancer theranostics[J]. Chem Soc Rev, 2019, 48: 2967-3014. DOI:10.1039/C8CS00805A |
| [82] |
Feng J, Lepetre-Mouelhi S, Gautier A, et al. A new painkiller nanomedicine to bypass the blood-brain barrier and the use of morphine[J]. Sci Adv, 2019, 5: eaau5148. DOI:10.1126/sciadv.aau5148 |
| [83] |
Li J, Liao T, Liu H, et al. Hypoxic glioma stem cell-derived exosomes containing linc01060 promote progression of glioma by regulating the MZF1/c-Myc/HIF1α axis[J]. Cancer Res, 2021, 81: 114-128. DOI:10.1158/1538-7445.AM2021-114 |
| [84] |
Zhao C. The role of exosomes in the progression of glioma[J]. Electron J Clin Med Lit (临床医药文献电子杂志), 2019, 6: 197-198. |
| [85] |
Godlewski J, Krichevsky AM, Johnson MD, et al. Belonging to a network-micrornas, extracellular vesicles, and the glioblastoma microenvironment[J]. Neuro Oncol, 2015, 17: 652-662. DOI:10.1093/neuonc/nou292 |
| [86] |
Saadatpour L, Fadaee E, Fadaei S, et al. Glioblastoma: exosome and microrna as novel diagnosis biomarkers[J]. Cancer Gene Ther, 2016, 23: 415-418. DOI:10.1038/cgt.2016.48 |
| [87] |
Cheng J, Meng J, Zhu L, et al. Exosomal noncoding RNAs in glioma: biological functions and potential clinical applications[J]. Mol Cancer, 2020, 19: 66. DOI:10.1186/s12943-020-01189-3 |
| [88] |
Bai L, Liu Y, Guo K, et al. Ultrasound facilitates naturally equipped exosomes derived from macrophages and blood serum for orthotopic glioma treatment[J]. ACS Appl Mater Interfaces, 2019, 11: 14576-14587. DOI:10.1021/acsami.9b00893 |
| [89] |
Zhuang X, Xiang X, Grizzle W, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain[J]. Mol Ther, 2011, 19: 1769-1779. DOI:10.1038/mt.2011.164 |
| [90] |
Zhang H, Wu J, Wu J, et al. Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice[J]. J Nanobiotechnol, 2019, 17: 29. DOI:10.1186/s12951-019-0461-7 |
| [91] |
Yang L, Han B, Zhang Z, et al. Extracellular vesicle-mediated delivery of circular RNA SCMH1 promotes functional recovery in rodent and nonhuman primate ischemic stroke models[J]. Circulation, 2020, 142: 556-574. DOI:10.1161/CIRCULATIONAHA.120.045765 |
| [92] |
Venugopal C, Shamir C, Senthilkumar S, et al. Dosage and passage dependent neuroprotective effects of exosomes derived from rat bone marrow mesenchymal stem cells: an in vitro analysis[J]. Curr Gene Ther, 2017, 17: 379-390. |
| [93] |
Jiang H, Toscano JF, Song SS, et al. Differential expression of circulating exosomal micrornas in refractory intracranial atherosclerosis associated with antiangiogenesis[J]. Sci Rep, 2019, 9: 19429. DOI:10.1038/s41598-019-54542-y |
| [94] |
Zhang Y, Chopp M, Meng Y, et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury[J]. J Neurosurg, 2015, 122: 856-867. DOI:10.3171/2014.11.JNS14770 |
| [95] |
Zheng M, Huang M, Ma X, et al. Harnessing exosomes for the development of brain drug delivery systems[J]. Bioconjug Chem, 2019, 30: 994-1005. DOI:10.1021/acs.bioconjchem.9b00085 |
| [96] |
Drommelschmidt K, Serdar M, Bendix I, et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate inflammation-induced preterm brain injury[J]. Brain Behav Immun, 2017, 60: 220-232. DOI:10.1016/j.bbi.2016.11.011 |
| [97] |
Liu Y, Li D, Liu Z, et al. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse[J]. Sci Rep, 2015, 5: 17543. DOI:10.1038/srep17543 |
| [98] |
Catalano M, O'Driscoll L. Inhibiting extracellular vesicles formation and release: a review of EV inhibitors[J]. J Extracell Vesicles, 2020, 9: 1703244. DOI:10.1080/20013078.2019.1703244 |
| [99] |
van der Meel R, Fens MH, Vader P, et al. Extracellular vesicles as drug delivery systems: lessons from the liposome field[J]. J Control Release, 2014, 195: 72-85. DOI:10.1016/j.jconrel.2014.07.049 |
| [100] |
Antimisiaris SG, Mourtas S, Marazioti A. Exosomes and exosome-inspired vesicles for targeted drug delivery[J]. Pharmaceutics, 2018, 10: 218. DOI:10.3390/pharmaceutics10040218 |
| [101] |
Armstrong JP, Holme MN, Stevens MM. Re-engineering extracellular vesicles as smart nanoscale therapeutics[J]. ACS Nano, 2017, 11: 69-83. DOI:10.1021/acsnano.6b07607 |
 2022, Vol. 57
2022, Vol. 57


