非酒精性脂肪性肝病(non-alcoholic fatty liver disease, NAFLD) 是与胰岛素抵抗和遗传易感性密切相关的获得性代谢应激性肝损伤, 其疾病谱包括非酒精性单纯性脂肪肝、非酒精性脂肪性肝炎(non-alcoholic steatohepatitis, NASH)、肝硬化和肝细胞癌[1]。NAFLD在全球范围内的发病率为25%左右, 随种族和地理位置不同有所差异[2]。一项纳入了392项研究结果的最新流行病学报告显示, NAFLD在中国的发病率达到29.2%, 已成为我国慢性肝病的重要原因, 不但影响了大众健康, 还给医疗系统带来沉重负担[3]。
目前尚无治疗NAFLD的有效药物, 国内外NAFLD指南[4-6]推荐的控制措施主要为改变生活习惯, 如健康饮食、加强运动锻炼和减轻体重等, 但是患者对限制热量摄入、增加能量消耗等生活方式改变的依从性不高。目前指南中推荐的药物主要用于改善NAFLD相关的代谢性疾病, 对NAFLD早期如单纯性脂肪肝有一定作用, 但是对晚期NASH等疗效有限, 不能有效改善肝脏纤维化, 并且部分药物存在引起患者体重增加、肝功能损伤等风险[7]。因此, 开发能减缓、阻止或逆转NAFLD且不良反应轻的药物十分必要。近年来, 天然来源化合物用于治疗NAFLD的研究不断增加, 已成为研究者关注的热点。本文将对天然来源化合物抗NAFLD研究进行综述, 并对存在的问题及改进的策略进行阐述, 以期为临床治疗NAFLD提供新的思路。
1 天然来源化合物治疗NAFLD的研究现状NAFLD发病机制复杂, 确切机制尚未明确, 不同患者发病机制及疾病进程多样, 单一靶点药物未达到满意药效, 不同靶点药物联合用药或开发多靶点药物有望成为解决NAFLD的有效途径[8]。天然来源化合物结构丰富、生物活性多样, 能以多靶点、多作用机制有效改善复杂性疾病, 是开发新型NAFLD药物的宝贵资源。许多天然来源化合物具有降糖、降脂、抗炎、保肝等作用, 将其应用于NAFLD防治的研究逐渐增多。这些化合物主要是从植物或传统中草药分离或衍生获得, 大致可分为黄酮类、生物碱、萜类、醌类、苯丙素类和多酚等[9], 现阶段研究较多的化合物有: 水飞蓟宾(1)、姜黄素(2)、双环醇(3)、白藜芦醇(4)、甘草酸(5) 和小檗碱(6) 等, 结构见图 1。了解它们对NAFLD的调节机制、药理作用和安全性有助于对其进一步开发利用, 下文进行逐一介绍。
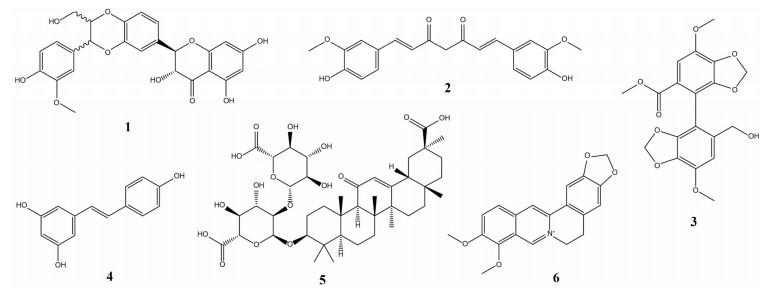
|
Figure 1 The chemical structures of six natural-derived compounds on non-alcoholic fatty liver disease (NAFLD) |
水飞蓟宾(silybin) 是菊科植物水飞蓟中提取的水飞蓟素(silymarin) 的主要活性成分, 属黄酮木质素类化合物, 由非对映异构体水飞蓟宾A及水飞蓟宾B以摩尔比1∶1组成。水飞蓟宾目前主要用于中毒性肝损害、慢性肝炎和肝硬化的支持治疗。在NAFLD动物模型中, 水飞蓟宾能显著减轻动物体重, 降低血清总胆固醇(total cholesterol, TC) 水平和肝脏甘油三酯(triglycerides, TG) 水平, 改善胰岛素敏感性[10, 11], 从而改善NAFLD, 其作用机制可能与抗脂质过氧化、激活法尼醇X受体(farnesoid X receptor, FXR) 信号传导改善脂代谢、上调沉默信号调节因子2同系物1 (silent information regulation-2 homolog 1, SIRT1)、减少NOD样受体家族3 (NOD-like receptor family pyrin domain containing 3, NLRP3) 炎症小体生成等相关[12]。一项随机双盲对照临床试验[13]显示, 水飞蓟宾可以改善NAFLD患者丙氨酸转氨酶(alanine transaminase, ALT) 和天冬氨酸转氨酶(aspartate transaminase, AST) 水平。另一项试验中, Navarro等[14]将78例无肝硬化NASH患者随机分为对照组、低剂量水飞蓟宾组和高剂量水飞蓟宾组, 给药48周后结果显示, 与对照组相比, 给药组患者肝脂肪变和小叶性炎症缓解, 但组织学改善程度无统计学意义。临床研究表明, 长期使用水飞蓟宾可抗炎、抗纤维化, 无明显不良反应, 对NAFLD治疗具有开发利用价值, 但其治疗持续时间和剂量标准化等问题尚待解决。
1.2 姜黄素姜黄素(curcumin) 是从植物姜黄块茎分离得到的一种天然酚类物质, 具有抗炎、抗氧化活性。实验证实, 姜黄素能通过激活单磷酸腺苷活化蛋白激酶(adenosine monophosphate activated protein kinase, AMPK), 增加过氧化物酶体增殖物激活受体α (peroxisome proliferators-activated receptor α, PPARα) 信号通路, 减少肝脏TG积累, 减轻炎症, 缓解脂肪变性[12, 15]。此外, 有研究发现姜黄素可能通过介导改善肠屏障完整性、恢复肠道菌群结构, 从而减少高脂饮食(high fat diet, HFD) 大鼠的肝脏脂肪沉积[16]。Saadati等[17]研究发现, NAFLD患者在改变生活方式基础上每天口服姜黄素1 500 mg, 12周后肝纤维化明显缓解, 肝酶水平好转, 但与单纯改变生活方式对照组无明显差异。另一项对NAFLD患者的随机对照试验(randomized controlled trial, RCT) meta分析结果显示, 姜黄素能有效降低患者血清低密度脂蛋白胆固醇、TG、TC、稳态模型胰岛素抵抗指数(homeostasis model assessment-insulin resistance, HOMA-IR)、AST水平及体重, 且患者耐受性良好[18]。目前, 确证姜黄素的NAFLD治疗机制和疗效, 开发成适合临床使用的制剂成为研究热点。
1.3 双环醇双环醇(bicyclol) 是由传统中药北五味子衍生物改造而成的合成药物, 主要用于改善慢性乙肝等慢性肝炎临床症状和肝功能, 属于保肝护肝药。药理研究表明双环醇可以清除自由基, 保护线粒体功能, 诱导自噬和凋亡, 防治脂质过氧化损伤并具有一定抗病毒作用[19]。在四氯化碳致急性肝损伤小鼠模型中, 双环醇通过降低血清ALT、白细胞介素1β (interleukin-1β, IL-1β)、肿瘤坏死因子-α (tumor necrosis factor-α, TNF-α) 等炎症相关细胞因子水平, 起到缓解肝损伤作用[20]。在四环素诱导脂肪肝小鼠模型中, 双环醇降低肝脏TG水平, 减轻肝组织病理变化, 可能机制为通过内质网应激相关途径减少细胞凋亡[21]。此外, 王亚男等[22]研究发现, 双环醇可能通过改善全身和肝脏的胰岛素抵抗, 降低2型糖尿病KKAy小鼠的空腹血糖水平。2014年一项纳入伴有空腹血糖受损NAFLD患者的RCT显示, 与维生素E治疗组对照, 双环醇联合二甲双胍治疗24周, 能显著降低NAFLD患者血清ALT水平, NAFLD活动评分(NAFLD activity scores, NAS) 显著降低, 病理学检查结果显示, 肝组织炎症、细胞气球样变减少[23]。双环醇在大量肝脏疾病治疗中使用安全, 未报告严重不良事件, 综合临床试验结果的干预总有效率明显高于对照组[24], 可能是NAFLD的潜在治疗药物。
1.4 白藜芦醇白藜芦醇(resveratrol) 为天然多酚类化合物, 有保护心脏、抗炎和抗氧化作用。白藜芦醇可能通过调节多信号通路起到缓解NAFLD作用, 如激活AMPK, 抑制胆固醇调节元件结合蛋白-1c (sterol regulatory element-binding protein-1c, SREBP-1c) 减少脂质生成[25]; 上调SIRT1, 抑制NLRP3炎症小体激活, 降低核因子κB (nuclear factor-κB, NF-κB) 活性, 预防炎症反应减少肝脏脂肪生成等[26]。白藜芦醇口服吸收差, 但其在肠道中能被微生物代谢, 研究表明白藜芦醇与其肠道代谢产物可能通过调节肠道菌群组成, 增加益生菌乳酸杆菌和双歧杆菌丰度, 抑制有害菌如粪肠球菌生长, 下调乙酰辅酶A羧化酶(acetyl CoA carboxylase, ACC) 等脂质生成相关基因表达, 从而降低内脏脂肪含量, 发挥抗NAFLD作用[27, 28]。但白藜芦醇在临床治疗中的效果尚有争议。一项持续3个月纳入60例NAFLD患者的RCT试验显示, 在改变生活方式基础上, 患者每天补充600 mg白藜芦醇, 治疗后患者血清中ALT、AST、TC和TNF-α水平显著降低[29]。而一项包含302例患者的系统评价提示, 目前证据尚不支持白藜芦醇在NAFLD治疗中的有效性, 主要由于其无法改善肝纤维化程度[30]。由此可见, 白藜芦醇的疗效还需要进一步的机制探索以及大规模、长期的临床试验验证。
1.5 甘草酸类甘草酸(glycyrrhizic acid) 制剂是当前用于抗炎保肝治疗的一线药物之一, 主要有甘草酸单铵、甘草酸二铵和异甘草酸镁等。甘草酸为五环三萜类化合物, 其能减少炎症介导的FXR受体抑制, 降低血清胆汁酸水平, 从而恢复胆汁酸稳态[31]; 降低肝脏ACC和脂肪酸合成酶(fatty acid synthase, FAS) 水平, 减少脂肪生成; 诱导PPARα表达和脂蛋白脂酶的生成, 促进脂质代谢; 抑制肝星状细胞的活化, 减轻肝纤维化程度[32]。Li等[33]给予HFD诱导NAFLD小鼠甘草酸二铵后, 结果显示肠道菌群丰富度提高, 产短链脂肪酸相关菌水平上升, 紧密连接蛋白表达增加, 进而肠道屏障功能增强。在HFD诱导小鼠NAFLD模型中[34], 甘草酸可以增加脂肪酸的β氧化, 抑制葡萄糖6磷酸酶活性, 减少糖异生, 还可调节胰岛素受体底物的磷酸化, 增加胰岛素敏感性, 进而改善HFD诱导的肥胖。一项纳入国内5个随机临床试验的系统评价结果提示, 甘草酸制剂对NAFLD安全有效, 患者用药后肝酶水平和影像学表现改善, 但鉴于纳入的临床试验方法学质量均较低, 其治疗NAFLD的临床疗效还无法明确证实[35]。
1.6 小檗碱小檗碱(berberine) 是从中药黄连中分离得到的一种季铵类生物碱, 临床主要用于抗肠道感染。近期多项研究发现, 小檗碱能通过抑制NF-κB通路的活化, 增加肝脏细胞低密度脂蛋白受体(low density lipoprotein receptor, LDLR) 表达, 从而降低血浆胆固醇水平[36]; 还可通过激活AMPK通路, 减少脂质生成, 促进脂肪酸氧化和机体能量消耗[37]; 另外, 小檗碱还能以蛋白激酶D依赖方式上调肝和肌肉细胞胰岛素受体基因表达水平, 通过多靶点作用改善糖尿病、血脂异常等代谢性疾病[38, 39]。有研究发现给予高脂小鼠小檗碱后, 可改善实验动物肠道菌群结构, 减轻代谢性内毒血症, 降低肠道促炎细胞因子和趋化因子表达, 增加肠上皮细胞紧密连接蛋白表达, 恢复肠道完整性, 提示小檗碱对肠道微生态的调节作用[40]。另一项动物实验显示, 小檗碱可通过调节SREBP-1c通路抑制内质网应激, 从而降低蛋氨酸胆碱缺乏饮食诱导的NAFLD小鼠肝脏炎症和纤维化[41]。在临床试验中, 小檗碱能降低NAFLD患者血糖血脂水平并升高脂联素水平, 改善胰岛素抵抗[42]; 2型糖尿病伴NAFLD患者服用小檗碱12周后, 血清ALT、AST、TC、TG和血流变指标水平明显降低, 肝脏病变得到有效改善[43]。此外, 复旦大学附属中山医院高鑫研究组[44]的一项NAFLD临床试验表明, 在减轻体重和改善血脂方面, 小檗碱干预组与吡格列酮组相比更为有效; 进一步血清脂质组学分析发现, 小檗碱能调节多种脂质, 血清中神经酰胺和神经酰胺-1-磷酸盐水平显著降低[45]。综上所述, 小檗碱能够通过多靶点综合治疗NAFLD, 未来在更大范围的临床研究将有利于更好地阐述药物的作用。
2 存在问题及解决手段天然来源化合物在NAFLD治疗中具有多靶点调控、药理作用广泛、毒副作用小、安全耐受性好、经济方便等优势, 但存在胃肠道吸收差、口服生物利用度低等问题, 极大限制了上述化合物的发展及临床应用。为提高生物利用度、增加药效、降低用药剂量、减少毒副作用, 研究者开展了大量工作, 在制剂改良方面取得很大进展。新型药物递送系统的研发, 有助于改善天然来源化合物的多种性能, 如提高稳定性, 增加药物溶解及吸收, 改变半衰期, 延长作用时间, 将药物靶向特定部位等。目前研究较成熟可用于天然来源化合物的递送系统主要有包合物、固体分散体和纳米制剂, 下文进行详细阐述。
2.1 包合技术和固体分散技术包合物主要通过络合作用, 将药物分子包合或嵌入载体(环糊精是最常用的载体材料) 的筒状结构内形成超微粒分散物。形成包合物后, 药物高度分散且润湿性增加, 有利于药物的溶出, 提高生物利用度。Battu等[46]采用羟丙基-β-环糊精包合盐酸小檗碱, 制备得到的包合物稳定性好, 药物溶解度提高。固体分散体是指将药物以分子、无定形、微晶态等高度分散状态均匀分散在载体中形成的一种以固体形式存在的分散系统, 可促进药物的溶出。Meng等[47]用溶剂挥发法制备得到聚乙二醇(polyethylene glycol, PEG) 6000小檗碱固体分散体, 实验发现小檗碱体内生物利用度增加5倍, 能更有效改善糖尿病大鼠的糖脂代谢状态。
2.2 纳米技术随着纳米技术的不断进步, 天然化合物纳米制剂引起越来越多关注, 如制备脂质体、聚合物胶束、纳米粒、纳米乳等。Maradana等[48]将姜黄素制备成脂质体, 使其对炎性树突状细胞具有靶向性, 可减轻饮食诱导的NASH小鼠肝脏炎症、纤维化和胰岛素抵抗。Yang等[49]制备了载甘草酸二铵的乳糖修饰PEG接枝壳聚糖复合物胶束, 得到的载药胶束物理稳定性好, 大鼠药代动力学研究发现血中甘草酸二铵的清除速率延缓, 且具有肝靶向性, 有利于肝脏疾病的治疗。另一项研究采用具有肝靶向性的半乳糖修饰氧化淀粉-溶菌酶包载白藜芦醇, 制备的纳米制剂能降低NAFLD小鼠肝脏TG水平, 有效恢复小鼠肝脏胰岛素敏感性[50]。Xue等[51]制备了小檗碱固体脂质纳米粒, 可有效减少db/db小鼠肝脂肪变性, 降低血清ALT水平, 下调肝脏中FAS基因表达, 有效缓解脂质堆积。针对小檗碱肠道菌群调节作用, 笔者所在课题组制备了pH/肠道菌群双刺激响应型小檗碱壳聚糖果胶纳米粒, 可增加肠道菌富集部位小檗碱的释放, 实验表明这种新型小檗碱递送系统可有效改善HFD引起的动物血脂和血糖升高, 减少肝脂肪堆积[52]。Chen等[53]开发的自乳化水飞蓟宾纳米药物递送系统可有效减少肥胖大鼠肝脏胶原沉积和肝纤维化程度。但是目前天然来源化合物纳米载体治疗NAFLD的研究开展仍不广泛, 生产步骤多、成本高昂和安全性等均为制约其发展的因素。
3 展望NAFLD指除酒精和其他明确因素所致, 以肝细胞内脂质过度沉积为主要特征的临床病理综合征, 已成为最常见慢性肝病。现代社会高糖高脂饮食及久坐不动的生活方式导致NAFLD发病人群不断扩大, 目前尚无有效的治疗药物, 同时NAFLD会增加2型糖尿病、心血管疾病和慢性肾脏疾病等多种疾病风险, 严重威胁着公众生命健康。NAFLD发病机制复杂涉及多种因素, 天然来源化合物具有多靶点、多机制调节的特点, 可以通过调控疾病的多个环节提高疗效, 降低毒副作用, 是治疗复杂性疾病的理想药物候选物, 对改善NAFLD具有独特优势。生物利用度低和有些化合物单药疗效不显著是现阶段天然来源化合物临床应用的限制因素, 选择合适的递送系统改善药物生物利用度, 增加疗效成为研究趋势。此外, 选用适当的动物模型模拟发病机制, 明确治疗机制, 完善临床疗效评价指标并阐明临床疗效和人群适用性, 对药物的开发及NAFLD的有效防治至关重要。
作者贡献: 余孝游负责文献调研和文章撰写; 王璐璐、蒋建东确定文章思路的提出及文章审阅。
利益冲突: 本文作者均声明无利益冲突。
| [1] |
Gerges SH, Wahdan SA, Elsherbiny DA, et al. Non-alcoholic fatty liver disease: an overview of risk factors, pathophysiological mechanisms, diagnostic procedures, and therapeutic interventions[J]. Life Sci, 2021, 271: 119220. DOI:10.1016/j.lfs.2021.119220 |
| [2] |
Nasr P, Iredahl F, Dahlstrom N, et al. Evaluating the prevalence and severity of NAFLD in primary care: the EPSONIP study protocol[J]. BMC Gastroenterol, 2021, 21: 180. DOI:10.1186/s12876-021-01763-z |
| [3] |
Zhou F, Zhou J, Wang W, et al. Unexpected rapid increase in the burden of NAFLD in China from 2008 to 2018:a systematic review and meta-analysis[J]. Hepatology, 2019, 70: 1119-1133. DOI:10.1002/hep.30702 |
| [4] |
European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease[J]. Diabetologia, 2016, 59: 1121-1140. DOI:10.1007/s00125-016-3902-y |
| [5] |
Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases[J]. Hepatology, 2018, 67: 328-357. DOI:10.1002/hep.29367 |
| [6] |
National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association; Fatty Liver Disease Expert Committee, Chinese Medical Doctor Association. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: a 2018 update[J]. Chin J Hepatol (中华肝脏病杂志), 2018, 26: 195-203. |
| [7] |
Kung J, Henry RR. Thiazolidinedione safety[J]. Expert Opin Drug Saf, 2012, 11: 565-579. DOI:10.1517/14740338.2012.691963 |
| [8] |
Vuppalanchi R, Noureddin M, Alkhouri N, et al. Therapeutic pipeline in nonalcoholic steatohepatitis[J]. Nat Rev Gastroenterol Hepatol, 2021, 18: 373-392. DOI:10.1038/s41575-020-00408-y |
| [9] |
Zhou H, Ma C, Wang C, et al. Research progress in use of traditional Chinese medicine monomer for treatment of non-alcoholic fatty liver disease[J]. Eur J Pharmacol, 2021, 898: 173976. DOI:10.1016/j.ejphar.2021.173976 |
| [10] |
Sun R, Xu D, Wei Q, et al. Silybin ameliorates hepatic lipid accumulation and modulates global metabolism in an NAFLD mouse model[J]. Biomed Pharmacother, 2020, 123: 109721. DOI:10.1016/j.biopha.2019.109721 |
| [11] |
Yang L, Liu Q, Zhang H, et al. Silibinin improves nonalcoholic fatty liver by regulating the expression of miR122:an in vitro and in vivo study[J]. Mol Med Rep, 2021, 23: 335. DOI:10.3892/mmr.2021.11974 |
| [12] |
Yan T, Yan N, Wang P, et al. Herbal drug discovery for the treatment of nonalcoholic fatty liver disease[J]. Acta Pharm Sin B, 2020, 10: 3-18. DOI:10.1016/j.apsb.2019.11.017 |
| [13] |
Anushiravani A, Haddadi N, Pourfarmanbar M, et al. Treatment options for nonalcoholic fatty liver disease: a double-blinded randomized placebo-controlled trial[J]. Eur J Gastroenterol Hepatol, 2019, 31: 613-617. DOI:10.1097/MEG.0000000000001369 |
| [14] |
Navarro VJ, Belle SH, D'amato M, et al. Silymarin in non-cirrhotics with non-alcoholic steatohepatitis: a randomized, double-blind, placebo controlled trial[J]. PLoS One, 2019, 14: e0221683. DOI:10.1371/journal.pone.0221683 |
| [15] |
Sun Y, Peng ML. Recent advances in curcumin and its derivatives for treatment of liver diseases[J]. Acta Pharm Sin (药学学报), 2014, 49: 1483-1490. |
| [16] |
Feng W, Wang H, Zhang P, et al. Modulation of gut microbiota contributes to curcumin-mediated attenuation of hepatic steatosis in rats[J]. Biochim Biophys Acta Gen Subj, 2017, 1861: 1801-1812. DOI:10.1016/j.bbagen.2017.03.017 |
| [17] |
Saadati S, Sadeghi A, Mansour A, et al. Curcumin and inflammation in non-alcoholic fatty liver disease: a randomized, placebo controlled clinical trial[J]. BMC Gastroenterol, 2019, 19: 133. DOI:10.1186/s12876-019-1055-4 |
| [18] |
Wei Z, Liu N, Tantai X, et al. The effects of curcumin on the metabolic parameters of non-alcoholic fatty liver disease: a meta-analysis of randomized controlled trials[J]. Hepatol Int, 2019, 13: 302-313. DOI:10.1007/s12072-018-9910-x |
| [19] |
Zhao T, Mao L, Yu Z, et al. Therapeutic potential of bicyclol in liver diseases: lessons from a synthetic drug based on herbal derivative in traditional Chinese medicine[J]. Int Immunopharmacol, 2021, 91: 107308. DOI:10.1016/j.intimp.2020.107308 |
| [20] |
Zhao TM, Wang Y, Deng Y, et al. Bicyclol attenuates acute liver injury by activating autophagy, anti-oxidative and anti-inflammatory capabilities in mice[J]. Front Pharmacol, 2020, 11: 463. DOI:10.3389/fphar.2020.00463 |
| [21] |
Yao XM, Li Y, Li HW, et al. Bicyclol attenuates tetracycline-induced fatty liver associated with inhibition of hepatic ER stress and apoptosis in mice[J]. Can J Physiol Pharmacol, 2016, 94: 1-8. DOI:10.1139/cjpp-2015-0074 |
| [22] |
Wang YN, Zhang XL, Yin Z, et al. Experimental study of bicyclol for type 2 diabetic treatment using KKAy mice[J]. Acta Pharm Sin (药学学报), 2019, 54: 1041-1047. |
| [23] |
Han Y, Shi JP, Ma AL, et al. Randomized, vitamin E-controlled trial of bicyclol plus metformin in non-alcoholic fatty liver disease patients with impaired fasting glucose[J]. Clin Drug Investig, 2014, 34: 1-7. DOI:10.1007/s40261-013-0136-3 |
| [24] |
Li H, Liu NN, Peng ZG. Effect of bicyclol on blood biomarkers of NAFLD: a systematic review and meta-analysis[J]. BMJ Open, 2020, 10: e039700. DOI:10.1136/bmjopen-2020-039700 |
| [25] |
Tian Y, Ma J, Wang W, et al. Resveratrol supplement inhibited the NF-κB inflammation pathway through activating AMPKα-SIRT1 pathway in mice with fatty liver[J]. Mol Cell Biochem, 2016, 422: 75-84. DOI:10.1007/s11010-016-2807-x |
| [26] |
Charytoniuk T, Drygalski K, Konstantynowicz-Nowicka K, et al. Alternative treatment methods attenuate the development of NAFLD: a review of resveratrol molecular mechanisms and clinical trials[J]. Nutrition, 2017, 34: 108-117. DOI:10.1016/j.nut.2016.09.001 |
| [27] |
Chaplin A, Carpene C, Mercader J. Resveratrol, metabolic syndrome, and gut microbiota[J]. Nutrients, 2018, 10: 1651. DOI:10.3390/nu10111651 |
| [28] |
Wang P, Wang J, Li D, et al. Targeting the gut microbiota with resveratrol: a demonstration of novel evidence for the management of hepatic steatosis[J]. J Nutr Biochem, 2020, 81: 108363. DOI:10.1016/j.jnutbio.2020.108363 |
| [29] |
Chen S, Zhao X, Ran L, et al. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: a randomized controlled trial[J]. Dig Liver Dis, 2015, 47: 226-232. DOI:10.1016/j.dld.2014.11.015 |
| [30] |
Jakubczyk K, Skonieczna-Zydecka K, Kaldunska J, et al. Effects of resveratrol supplementation in patients with non-alcoholic fatty liver disease-a meta-analysis[J]. Nutrients, 2020, 12: 2435. DOI:10.3390/nu12082435 |
| [31] |
Yan T, Wang H, Cao L, et al. Glycyrrhizin alleviates nonalcoholic steatohepatitis via modulating bile acids and meta-inflammation[J]. Drug Metab Dispos, 2018, 46: 1310-1319. DOI:10.1124/dmd.118.082008 |
| [32] |
Wang C, Duan X, Sun X, et al. Protective effects of glycyrrhizic acid from edible botanical glycyrrhiza glabra against non-alcoholic steatohepatitis in mice[J]. Food Funct, 2016, 7: 3716-3723. DOI:10.1039/C6FO00773B |
| [33] |
Li Y, Liu T, Yan C, et al. Diammonium glycyrrhizinate protects against nonalcoholic fatty liver disease in mice through modulation of gut microbiota and restoration of intestinal barrier[J]. Mol Pharm, 2018, 15: 3860-3870. DOI:10.1021/acs.molpharmaceut.8b00347 |
| [34] |
Sun X, Duan X, Wang C, et al. Protective effects of glycyrrhizic acid against non-alcoholic fatty liver disease in mice[J]. Eur J Pharmacol, 2017, 806: 75-82. DOI:10.1016/j.ejphar.2017.04.021 |
| [35] |
Sun X, Zhang L, Wei W, et al. Systematic evaluation and meta analysis on nonalcoholic steatohepatitis treated with glycyrrhizin[J]. Chin J Surg Integr Tradit West Med (世界中西医结合杂志), 2015, 10: 265-271. |
| [36] |
Zuo X, Luo JQ, Jiang XH, et al. The anti-atherosclerotic effect and mechanism study of berberine in hyperlipidemic ApoE-/- mice[J]. Acta Pharm Sin (药学学报), 2019, 54: 104-110. |
| [37] |
Ma XL, Jiang W, Fan WM, et al. Berberine ameliorates dexamethasone-induced metabolic disorder in C57 mice[J]. Acta Pham Sin (药学学报), 2020, 55: 2636-2641. |
| [38] |
Yao J, Kong W, Jiang J. Learning from berberine: treating chronic diseases through multiple targets[J]. Sci China Life Sci, 2015, 58: 854-859. DOI:10.1007/s11427-013-4568-z |
| [39] |
Wang Y, Jiang JD. A new research mode of drug PK-PD mediated by the gut microbiota: insights into the pharmacokinetics of berberine[J]. Acta Pharm Sin (药学学报), 2018, 53: 659-666. |
| [40] |
Zhu L, Zhang D, Zhu H, et al. Berberine treatment increases Akkermansia in the gut and improves high-fat diet-induced atherosclerosis in Apoe-/- mice[J]. Atherosclerosis, 2018, 268: 117-126. DOI:10.1016/j.atherosclerosis.2017.11.023 |
| [41] |
Zhang Z, Li B, Meng X, et al. Berberine prevents progression from hepatic steatosis to steatohepatitis and fibrosis by reducing endoplasmic reticulum stress[J]. Sci Rep, 2016, 6: 20848. DOI:10.1038/srep20848 |
| [42] |
Bai RM, Zheng BB, Zhang RD, et al. Effects of berberine on insulin resistance and serum adiponectin of nonalcoholic fatty liver patients[J]. Pract Geriatr (实用老年医学), 2011, 25: 423-426. |
| [43] |
Xie XM, Meng XJ, Zhou XJ, et al. The effency of berberine in newly diagnosed type 2 diabetes mellitus with nonalcoholic fatty liver disease patients and the influence of blood rheology[J]. China J Chin Mater Med (中国中药杂志), 2011, 36: 3032-3035. |
| [44] |
Yan HM, Xia MF, Wang Y, et al. Efficacy of berberine in patients with non-alcoholic fatty liver disease[J]. PLoS One, 2015, 10: e0134172. DOI:10.1371/journal.pone.0134172 |
| [45] |
Chang X, Wang Z, Zhang J, et al. Lipid profiling of the therapeutic effects of berberine in patients with nonalcoholic fatty liver disease[J]. J Transl Med, 2016, 14: 266. DOI:10.1186/s12967-016-0982-x |
| [46] |
Battu SK, Repka MA, Maddineni S, et al. Physicochemical characterization of berberine chloride: a perspective in the development of a solution dosage form for oral delivery[J]. AAPS PharmSciTech, 2010, 11: 1466-1475. DOI:10.1208/s12249-010-9520-y |
| [47] |
Meng ZJ, Zhang M, Wei SN, et al. Amorphous solid dispersion of berberine with absorption enhancer demonstrates a remarkable hypoglycemic effect via improving its bioavailability[J]. Int J Pharm, 2014, 467: 50-59. DOI:10.1016/j.ijpharm.2014.03.017 |
| [48] |
Maradana MR, Yekollu SK, Zeng B, et al. Immunomodulatory liposomes targeting liver macrophages arrest progression of nonalcoholic steatohepatitis[J]. Metabolism, 2018, 78: 80-94. DOI:10.1016/j.metabol.2017.09.002 |
| [49] |
Yang KW, Li XR, Yang ZL, et al. Novel polyion complex micelles for liver-targeted delivery of diammonium glycyrrhizinate: in vitro and in vivo characterization[J]. J Biomed Mater Res Part A, 2009, 88: 140-148. |
| [50] |
Teng W, Zhao L, Yang S, et al. The hepatic-targeted, resveratrol loaded nanoparticles for relief of high fat diet-induced nonalcoholic fatty liver disease[J]. J Control Release, 2019, 307: 139-149. DOI:10.1016/j.jconrel.2019.06.023 |
| [51] |
Xue M, Zhang L, Yang MX, et al. Berberine-loaded solid lipid nanoparticles are concentrated in the liver and ameliorate hepato-steatosis in db/db mice[J]. Int J Nanomedicine, 2015, 10: 5049-5057. |
| [52] |
Guo HH, Ma C, Zheng WS, et al. Dual-stimuli-responsive gut microbiota-targeting berberine-CS/PT-NPs improved metabolic status in obese hamsters[J]. Adv Funct Mater, 2019, 29: 16. |
| [53] |
Chen CH, Chen CJ, Elzoghby AO, et al. Self-assembly and directed assembly of lipid nanocarriers for prevention of liver fibrosis in obese rats: a comparison with the therapy of bariatric surgery[J]. Nanomedicine (Lond), 2018, 13: 1551-1566. DOI:10.2217/nnm-2018-0001 |
 2022, Vol. 57
2022, Vol. 57


