2. 浙江大学药学院, 浙江 杭州 310058
2. College of Pharmaceutical Sciences, Zhejiang University, Hangzhou 310058, China
癌症已成为21世纪威胁人类健康最主要的疾病之一[1]。传统的癌症治疗方法主要包括手术治疗、化疗和放疗。其中, 化疗是最常用的癌症治疗策略之一。然而, 化疗药物往往存在水溶性差、肿瘤靶向能力低和严重的不良反应等缺点, 极大地限制了其临床应用[2]。在过去几十年的研究中, 纳米技术在不同疾病的诊断和治疗领域展现出巨大的潜力[3, 4]。纳米粒具有高载药量、理化性质可调节、灵活修饰和多模式疗法有机结合等优势, 使其适合包载抗肿瘤药物, 从而提升药物溶解度、稳定性和改善药动学等[5]。然而对于肿瘤治疗, 理想的纳米药物还应具有延长药物血液循环和肿瘤组织靶向等特性, 以达到更好的抗肿瘤疗效。因此, 基于功能化修饰的靶向药物递送系统(targeted drug delivery systems, TDDS) 逐渐受到研究者的青睐, 其使得安全高效和经济可控的药物递送成为可能[6, 7]。然而, 大多数纳米粒作为外来物质会被机体内的免疫系统识别和清除, 纳米粒通过表面修饰可延长其在血液中的循环时间并实现特定的靶向, 从而在提高疗效的同时减少不良反应[8, 9]。如纳米粒的聚乙二醇(polyethylene glycol, PEG) 包被曾被验证可减少网状内皮系统对其的快速消除。但近年来的一些研究发现, 重复使用PEG化纳米粒可能诱导免疫反应, 反而会加速纳米粒的清除[10]。此外, 纳米粒所需的靶向能力尤其依赖于其表面改性, 导致纳米粒制备的复杂化[11]。因此, 迫切需要寻求一种更安全、有效和制备便捷的纳米递药系统, 增强抗肿瘤的靶向治疗。
细胞曾被用作载体来递送小分子或基因等, 有文献[12-14]报道认为其能显著提高药物的血液循环时间、靶向效率和基因的转染效率。Gao课题组[15]在2010年提出了将干细胞作为靶向递送载体用于肿瘤的基因治疗, 并基于病毒和非病毒基因转染体系构建了携载有自杀基因的干细胞靶向载体。在黑色素瘤肺转移的小鼠模型中, 重组间充质干细胞(mesenchymal stem cell, MSC) 通过旁观者效应被递送至肺组织进而显著抑制黑色素转移瘤的生长[16, 17]。进一步研究发现, 氧化铁纳米粒可促进MSC中Cx43过表达, 携载自杀基因可有效促进脑胶质瘤小鼠的生存率[18]。此外, MSC还可携载抗肿瘤药物, 如含紫杉醇(paclitaxel, PTX) 的纳米粒用于脑胶质瘤的靶向治疗[19]。然而, 活细胞本身从低温保存到活性恢复的过程中, 需要提供营养物质和氧气, 且在静脉注射时存在一定的微血管阻塞和致瘤性[20, 21]。同时, 基于活细胞的药物递送载体仍存在一些不足, 包括药物可能会被细胞中的溶酶体消化、药物的释放难以控制、毒性药物会影响细胞自身的活性及在运输过程中药物可能会泄漏或胞吐等[22]。为了解决活细胞载药存在的问题, 一种自上而下的细胞膜纳米技术逐渐受到研究者的青睐[23, 24]。2011年, Zhang课题组[25]首次报道了细胞膜包被技术, 将红细胞膜壳和聚(乳酸-乙醇酸共聚物) [poly(lactic-co-glycolic acid), PLGA] 核通过共挤出工艺制成具有核-壳结构的细胞膜包被纳米粒(cell membrane-coated nanoparticles, CMC@NPs), 实现了纳米技术和仿生技术的有机结合, 促进药物的体内递送。在这一体系中, CMC@NPs可以伪装成自体细胞, 从而逃避免疫系统清除并延长在血液中的循环时间, 这对肿瘤靶向的高渗透和长滞留效应(enhanced permeability and retention effect, EPR) 是非常必要的。同时, 天然细胞膜的复杂成分和多配体整合优势可以嫁接至CMC@NPs中, 使得CMC@NPs具有独特的生物学功能。此外, 不同类型的细胞膜可以赋予CMC@NPs多种功能, 实现其多样化的体内生物学行为[23, 26]。如红细胞膜包被的纳米粒(red blood cell membrane-coated nanoparticles, RBCM@NPs) 可改善递药系统的生物相容性, 延长体内循环, 改善药代动力学行为[27-29]; 肿瘤细胞膜包被的纳米粒(cancer cell membrane-coated nanoparticles, CCM@NPs) 具有同源靶向能力, 增强药物在肿瘤组织的聚集[30-32]; 免疫细胞膜包被的纳米粒可规避体内免疫系统的清除, 大大延长了药物的体循环[33, 34]; 此外, 干细胞膜也具有向肿瘤部位和炎症部位归巢能力, 也被用于构建多种治疗载体[35-37]。除了细胞膜的多功能外, 纳米粒内核可进行不同设计和用于不同药物的装载, 实现肿瘤的化疗、光热疗法(photothermal, PTT)、光动力疗法(photodynamic therapy, PDT) 以及肿瘤成像等功能(图 1)[38-42]。因此, 作为一种有前景的肿瘤靶向载体, CMC@NPs的合理化设计逐渐成为研究热点。本文总结了CMC@NPs的构建与表征方法以及膜仿生技术在肿瘤靶向治疗上的应用, 并进一步探讨了膜仿生技术在临床应用中的挑战和机遇。
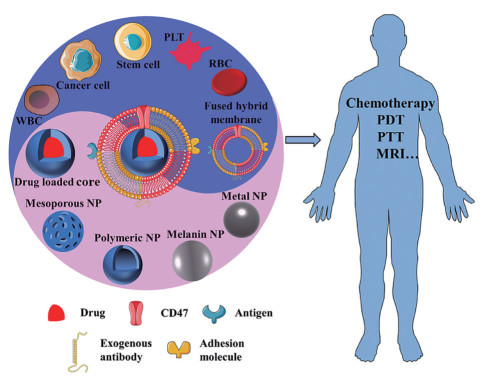
|
Figure 1 Various cell membrane-coated nanoparticles (CMC@NPs) developed for cancer treatment. Cell membranes were extracted and leveraged to wrap around different types of nanoparticles for cancer theranostic. (Adapted from Ref. 42 with permission. Copyright © 2020 Elsevier). PLT: Platelet; RBC: Red blood cell; WBC: White blood cell; CD47: Cluster of differentiation 47; PDT: Photodynamic therapy; PTT: Photothermal therapy; MRI: Magnetic resonance imaging; NP: Nanoparticle |
CMC@NPs的构建方法主要有自上而下和微流控电穿孔两种方式, 而自上而下方式是目前制备CMC@NPs最常用的方法[24, 35]。首先是细胞膜的提取, 通常是将大量纯化的细胞进行低渗、超声或反复冻融处理以获取细胞膜碎片。对于细胞团块或组织样本, 一般采用机械研磨的方式。细胞膜碎片洗涤后通过多孔聚碳酸酯膜挤出, 获得细胞膜衍生的囊泡。接着, 将细胞膜衍生的囊泡与所制备的纳米粒通过挤出仪机械反复挤压数次, 以进一步获得膜仿生核-壳纳米粒。这种基于机械挤压的构建方式耗时耗力, 无法实现批量制备。现阶段, 有一些研究者采用更为简单的超声法制备CMC@NPs[43-45], 然而超声法制备的膜包被层往往不太均匀, 且局部高温可能会造成膜蛋白的变性。近年来, 微流控电穿孔技术因可实现大批量制备而备受关注。微流芯片系统由五部分组成: 两个入口、Y形合并通道、S形混合通道、电穿孔区和出口。纳米粒和膜囊泡通过入口注入该系统, 并通过Y形合并通道和S形混合通道合并和混合。在电穿孔区, 两个电极之间的电脉冲可以促进纳米粒进入膜囊泡, 并通过出口得到CMC@NPs[46, 47]。如Liu等[46]利用微流控电穿孔技术高效地将外泌体膜和肿瘤细胞膜用于PLGA纳米粒的包裹, 在体内展现出良好的免疫规避和同源靶向功能, 见图 2和表 1[26, 35, 38, 40, 43-54]。
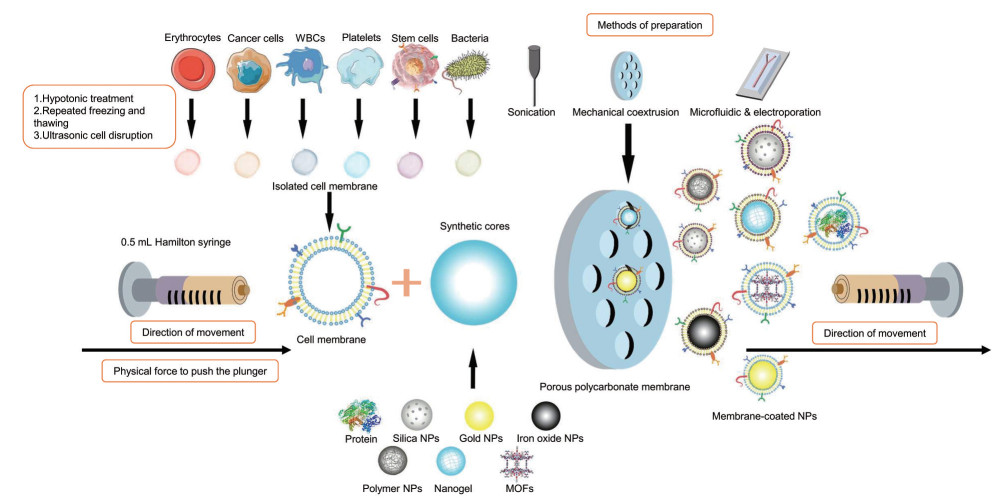
|
Figure 2 Physical method of cell membrane coating technology. Extracted cell membranes and synthetic nanoparticles were coextruded through a porous polycarbonate membrane. (Adapted from Ref. 35 with permission. Copyright © 2019 Elsevier). MOFs: Metal-organic frameworks |
| Table 1 Strategies for preparing CMC@NPs and their characteristics |
CMC@NPs的物理化学结构和表面蛋白对其功能发挥起着至关重要的作用。在CMC@NPs成功制备后, 通常要在体外验证细胞膜的化学结构、形态、理化特性及表面蛋白的完整性等因素, 以确保膜包被工艺设计的合理性和有效性。其中, 透射电子显微镜(transmission electron microscopy, TEM) 和扫描电子显微镜(scanning electron microscope, SEM) 可较好地观察膜仿生纳米形貌和纳米粒的核-壳结构。此外, 动态光散射(dynamic light scattering, DLS) 和zeta电位也可分别用于表征细胞膜包被的纳米粒的粒径和电位。一般地, 在膜包被后纳米粒的水合粒径和负电性均有所增加, 这些性质的转变可能会进一步影响其在生物体内的分布。此外, 采用十二烷基硫酸钠-聚丙烯酰胺凝胶电泳检测分离的细胞膜、未包被的纳米粒和膜包被纳米粒的蛋白条带, 验证膜表面蛋白的完整性。同时, 利用Western blot分析细胞膜上的特征蛋白。近年来, 荧光染料也被用于验证CMC@NPs的成功制备。Fan等[55]利用荧光染料标记红细胞膜, 通过激光扫描共聚焦显微镜实现对膜包被纳米粒的可视化分析。此外, 紫外-可见光吸收光谱也可用于膜仿生纳米粒的表征。Ren等[56]报道了一种红细胞膜包被的磁性纳米粒, 其在400 nm处新增的吸收峰与红细胞膜囊泡的特征吸收峰一致, 说明膜包被过程对膜的理化特征没有显著性影响。
2 细胞膜仿生纳米递药平台的分类红细胞是血液中最丰富的细胞成分, 在人体中的保留时间长达120天。由于成熟的红细胞没有细胞核和复杂的细胞器, 细胞膜提取和纯化较方便; 同时, 红细胞膜表面的“自我标记”膜蛋白CD47作用于抑制性受体SIRP-α, 可以减少网状内皮系统对RBCM@NPs的清除[48]。RBCM@NPs是第一个被报道的细胞膜仿生系统[25], 也是目前生物医学领域最广泛使用的天然载体之一。此外, Chai等[27]报道了靶向肽修饰的红细胞膜包被纳米晶, 所构建的RGD-RBC-NCs具有高载药量、长期稳定性和良好的生物相容性, 在皮下肿瘤和原位神经胶质瘤模型中显示出良好的肿瘤蓄积和优异的抗肿瘤疗效。近年来, 除了红细胞膜外, 血小板膜、免疫细胞膜、肿瘤细胞膜、干细胞膜及杂交膜等均被用于包被具有特定治疗功能的纳米粒, 并进行不同疾病的治疗。Chen等[57]制备了由肿瘤细胞膜壳和PLGA核组成的膜仿生纳米体系用以装载吲哚菁绿(indocyanine green, ICG), 产生了有效的肿瘤多模态成像和PTT疗效。近年来, 随着免疫疗法在肿瘤治疗领域的不断发展, 越来越多的免疫细胞作为仿生载体被用来递送药物。中性粒细胞膜仿生递药系统已被开发用于治疗复发性脑胶质瘤[58], 并能在一定程度上抑制滑膜炎症, 缓解关节炎的损害[43]。此外, 结合不同类型细胞膜功能的杂化膜有望进一步提升纳米递药系统的抗肿瘤疗效。例如, Bu等[59]提出了一种血小板-肿瘤干细胞杂化膜包被的氧化铁纳米粒, 用于增强头颈鳞状细胞癌的PTT治疗。血小板膜因表面含有“别吃我”信号而表现出免疫逃避能力, 同时肿瘤干细胞膜因特定表面黏附分子而具有同源靶向能力。因此, 杂化膜在免疫逃避、肿瘤主动靶向、磁共振成像和PTT治疗方面比单一膜包被的纳米粒展现出更优越的特性。
3 细胞膜仿生纳米技术用于肿瘤靶向治疗 3.1 红细胞膜仿生纳米递药系统红细胞是血液中最丰富的细胞类型之一, 用于运输氧气和养分[60]。自1667年进行第一次输血以来, 红细胞在急诊和住院治疗中发挥着重要的作用[61]。红细胞膜上表达的各种免疫调节标志物可被视为自身成分而避免免疫系统的清除。因此, RBCM@NPs能够整合天然红细胞的独特优点, 大大提升了药物的体内循环时间, 进而促进药物的肿瘤靶向和治疗[62, 63]。
RBCM@NPs最早提出是采用自上而下的方法将红细胞膜和PLGA通过重复挤出制备得到RBCM@NPs, 这种方法保护了细胞膜的完整性和功能性, 在小鼠模型中显著延长了体内半衰期。Zhang研究团队[64]将RBCM@NPs用于多柔比星(doxorubicin, DOX) 的装载, 与相同量的游离DOX相比, 这种负载DOX的RBCM@NPs显示出了更强的肿瘤抑制作用和更小的全身毒性。近年来, 仿生技术和光疗结合为肿瘤治疗带来了新的突破。Pei等[49]构建了红细胞膜仿生纳米粒RBC [M(TPC-PTX)] 用于协同化疗和PDT, 实现了活性氧(reactive oxygen species, ROS) 响应型PTX二聚体(PTX2-TK) 和光敏剂5, 10, 15, 20-四苯基二氯(5, 10, 15, 20-tetraphenylchlorin, TPC) 的共载。如图 3所示, TPC在光照下产生ROS, 不仅用于PDT, 还可触发PTX的释放。体内结果表明, 红细胞膜的伪装显著延长了纳米粒的血液循环并改善了药物在肿瘤部位的聚集。此外, 红细胞膜已扩展用于多种纳米材料的包被, 包括具有出色的光热性能但生物相容性较差的Fe3O4 NPs[46]和Au NPs[65]。由于纳米粒的EPR作用受限于肿瘤部位的血管生成[66], RBCM@NPs的肿瘤靶向效率相对较低。目前, 大多数具有主动靶向的DDS都是经过化学修饰以赋予其特定的靶向性, 但可能会损害红细胞膜的完整性[27]。为了克服这一缺点, 有研究开发了脂质插入技术以实现膜的功能化。叶酸配体首先与二硬脂酰磷酸乙醇胺脂质结合, 而后配体-脂质缀合物被插入到红细胞膜中, 配体功能化的红细胞膜实现了针对模型肿瘤细胞的主动靶向能力[27, 53]。
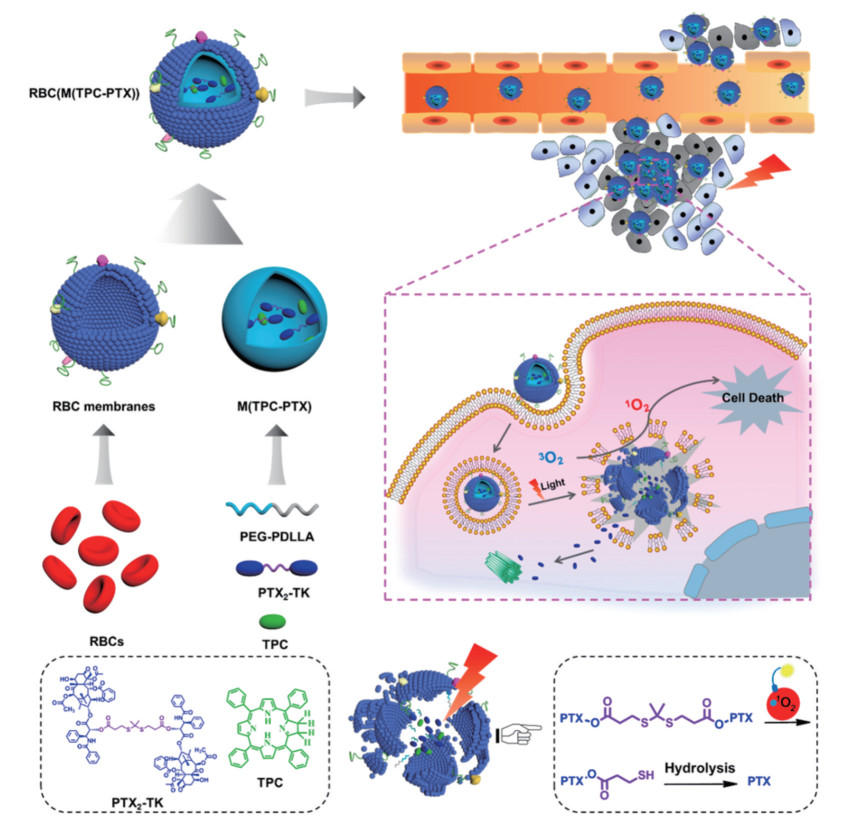
|
Figure 3 Schematic illustration of RBC membrane-coated dimeric prodrug NPs [RBC(M(TPC-PTX))] for light triggered on-demand drug release and combined photodynamic/chemotherapy. (Adapted from Ref. 49 with permission. Copyright © 2018 American Chemical Society). TPC: 5, 10, 15, 20-Tetraphenylchlorin; PTX: Paclitaxel |
除了红细胞外, 肿瘤细胞也可用作仿生纳米递药系统的膜来源。肿瘤细胞是具有无限增殖能力, 可通过体外方法有效培养和生产的细胞[67]。肿瘤细胞膜具有许多独特性, 包括免疫逃逸、抗细胞死亡和延长循环时间等[50]。同时, 肿瘤细胞膜继承了源细胞同源靶向和抗原库的功能, 已应用于肿瘤靶向治疗和免疫治疗领域[68]。在早期研究中, 肿瘤细胞膜包被的PLGA纳米粒与RBCM@NPs和单纯纳米粒相比, 同源肿瘤细胞对其摄取量分别增加了40倍和20倍。加入佐剂单磷酰脂质A后, 树突状细胞显著上调成熟标志物CD40、CD80和CD86。之后, 使用CCM@NPs成为抗肿瘤治疗和免疫治疗领域的一个新兴话题[30]。在肿瘤治疗中, 肿瘤细胞膜包被在不同纳米粒上, 并进一步与不同的治疗方法结合, 以达到特定的靶向功能。例如, 采用PLGA包载PTX化疗药, 而后进行4T1细胞膜包被。与单纯纳米粒相比, 所制备的仿生纳米粒的PTX肿瘤靶向效率和半衰期显著增加, 在原位移植肿瘤模型和晚期转移瘤模型中表现出良好的抗肿瘤和抗转移功效(图 4)[39]。
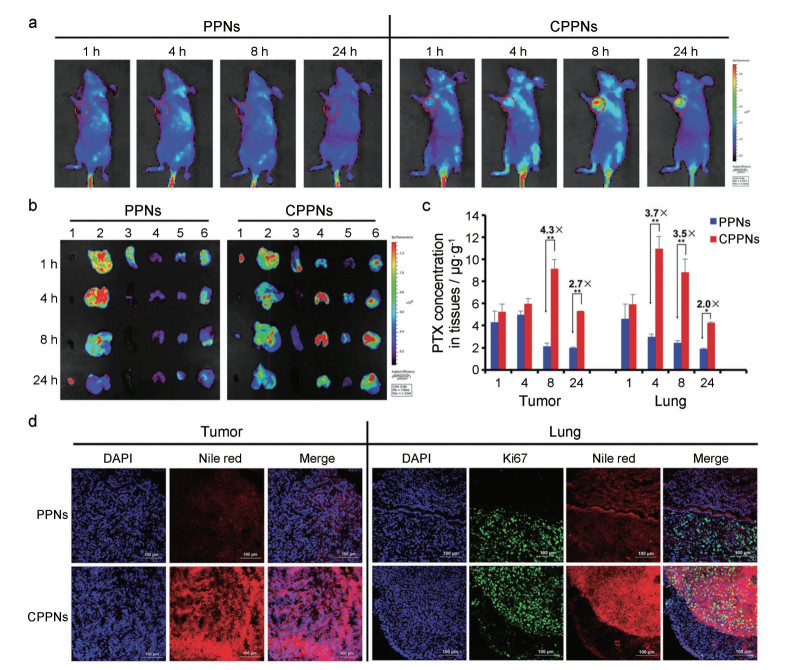
|
Figure 4 A: In vivo time-dependent biodistribution of PPNs and CPPNs in 4T1-breast-tumor-bearing mice detected by fluorescence imaging. B: Ex vivo fluorescence images of major organs. Lanes 1-6: heart, liver, spleen, lung, kidney, and tumor, respectively. C: Quantitative analysis of tumor and lung accumulation of PTX in PPNs or CPPNs. n = 3, x ± s. D: Representative fluorescence images of tumor and lung tissues at 8 h after intravenous administration, respectively. Scale bar = 100 μm. (Adapted from Ref. 39 with permission. Copyright © 2016 John Wiley and Sons). PPN: PTX-loaded polymeric nanoparticles; CPPN: Cancer-cell-membrane coated PPN |
然而, 纳米粒表面的完整细胞膜可能会阻止药物的释放。其中, 光热加速可实现药物的快速释放。有研究报道将光敏剂ICG和DOX纳米粒共载在肿瘤细胞膜内。体外实验证明, 激光照射后, 72 h内药物释放增加了4倍, 且在体内实现了对肿瘤的协同治疗(图 5)[69]。与此类似, 以携载DOX的热敏感金纳米笼作为内核, 4T1肿瘤细胞膜作为外壳的膜仿生纳米粒(CDAuNs), 在激光照射下, CDAuNs中约75%的DOX在前8 h内突释。而在没有激光照射的情况下, DOX释放只有38%。同时, 化疗/PTT疗法的协同疗法展现出对肿瘤生长和转移的高度抑制[70]。

|
Figure 5 A: Development of cracked cancer cell membranes (CCCMs) and NIR-responsive carrier-free nanosystems (DICNPs). B: Schematic illustration of in vivo blood circulation of DICNPs and their preferential tumor targeting and enhanced chemo-photothermal therapy upon NIR irradiation. (Adapted from Ref. 69 with permission. Copyright © 2018 Elsevier). NIR: Near infrared |
近年来, PTT疗法的发展推动了脑胶质瘤的荧光成像和光疗。然而, 目前仍缺乏可穿过血脑屏障(blood brain barrier, BBB) 的合适靶向剂。Jia等[71]通过将神经胶质瘤细胞的膜蛋白嵌入纳米粒中, 构建了负载ICG的仿生脂质纳米粒(BLIPO-ICG) 用于肿瘤的荧光成像、边缘检测和小鼠原位神经胶质瘤的光疗。BLIPO-ICG纳米粒展现出优异的同型靶向和免疫逃逸能力。在近红外激光照射(1 W·cm2, 5 min) 后, BLIPO-ICG NPs的PTT效应有效抑制了胶质瘤细胞的增殖, 肿瘤生长抑制率为94.2%, 且对正常脑组织没有表现出明显的损伤。以上结果表明, 仿生蛋白脂质纳米粒作为一种有前途的光疗纳米平台, 可用于脑胶质瘤的特异性成像和治疗。此外, 为了解决小分子金属化合物体内循环时间短和急性毒性高等问题, Zhang等[72]首次使用了肿瘤细胞膜递送金属离子。在这一仿生体系中, 带负电的细胞膜可与带正电的金属离子有效结合而无需添加其他材料。其中, MRu@M在荷瘤小鼠上实现了良好的PTT和光声成像。
3.3 干细胞膜仿生纳米递药系统干细胞是一类具有自我复制能力的多潜能细胞, 根据来源可分为胚胎干细胞和成体干细胞[35]。其中, MSC是一类易于提取和体外扩增的具有多向分化潜能的干细胞, 可分化为成骨细胞、脂肪细胞、成纤维细胞、软骨细胞和神经细胞等多种细胞[73, 74]。MSC具有低免疫原性和肿瘤趋向性, 近年来被广泛用作药物和基因的递送载体[75, 76], 其靶向机制主要受肿瘤区域释放的细胞因子和MSC上相应受体的调节[77]。因而, MSC已被广泛用于治疗各种肿瘤, 包括卵巢癌、胰腺癌、黑色素瘤、胶质瘤和淋巴瘤[17, 78-80]。然而, 较大尺寸的MSC很难穿透BBB, 且直接注射的MSC在体循环中稳定性差, 存在一定的安全隐患。而使用MSC膜包被纳米粒不仅保留了干细胞膜的功能特性, 还可保持纳米粒的小尺寸和负载特性。因此, MSC膜包被的纳米粒(mesenchymal stem cell-coated nanoparticles, MSCM@NPs) 已被用作一种可将药物靶向递送至肿瘤部位的新策略[81]。
利用MSC良好的肿瘤靶向性, 本课题组前期设计了MSC膜包被介孔二氧化硅纳米粒(MSN@M), 其有效地保持了MSC的主动隐身和自我定位药物递送能力, 在荷瘤鼠中表现出良好的肿瘤靶向和组织穿透能力, 且负载DOX后能显著抑制肿瘤的生长[37]。此外, Gao等[82]设计了装载DOX的新型MSC膜包被的纳米凝胶(SCMGs)。与非膜包被纳米粒相比, 膜包被纳米粒在长期储存中表现出更高的稳定性。相较pH 5.0的酸性环境, 明胶-DOX和SCMGs-DOX在pH 7.4中的药物释放明显减少, 表明该膜包被体系可用于pH敏感的药物递送。体内研究发现, 膜包被的纳米粒能更有效地靶向肿瘤, SCMG-DOX治疗后, 所有肿瘤的生长均得到有效抑制。这项研究揭示了MSCM@NPs具有改善肿瘤归巢性和更优越的抗肿瘤特性。之后, Gao等[83]进一步开发了MSC膜包被的介孔二氧化硅纳米粒(MSN) 用于肿瘤的PDT治疗, 其采用传统的共挤出方法将MSC膜包被到MSN上。新的纳米平台保留了MSC的肿瘤靶向特性, 显著增强了肿瘤抑制功效, 为肿瘤的PDT和PTT治疗提供了新的思路。此外, Yang等[36]报道了一种载有DOX的MSC膜包被的PLGA纳米粒。实验通过超声处理将PLGA纳米粒包在膜上, 结果表明, 肿瘤细胞对膜包被纳米粒的摄取效率是膜未包被纳米粒的3倍, 有效提高了药物对肿瘤细胞的杀伤效率(图 6)。此外, 将脂质体膜与干细胞膜融合所构建的融合膜作为一种新型的平台技术, 展现出药物控释、良好的稳定性、体内长循环和靶向递送等优势[54]。
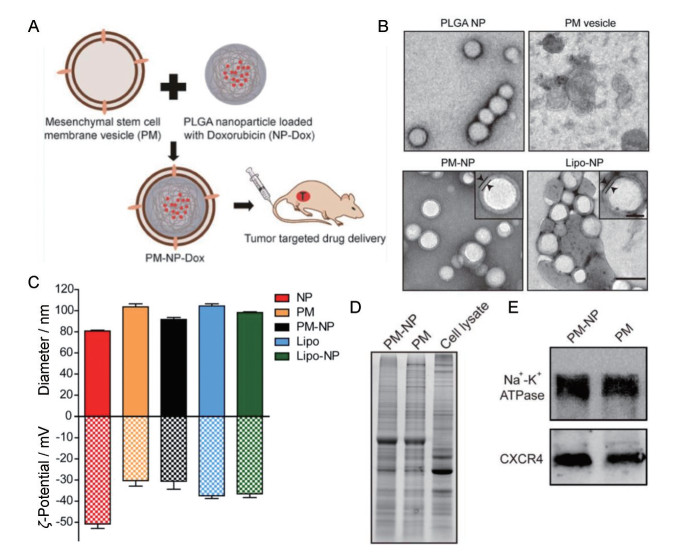
|
Figure 6 A: Schematic illustration of MSC membrane-coated PLGA nanoparticles (PLGA NPs) for tumor targeted drug delivery. B: TEM images of different NPs. Scale bar = 100 nm. Magnified images in PM-NP and lipo-NP are inserted. Scale bar = 40 nm. C: Average hydrodynamic diameters and surface charges of different NPs were examined by DLS. n = 3, x ± s. D: Expression profile of proteins in different formulations of MSCs was analyzed by SDS-PAGE. E: Expression levels of two membrane proteins in PM-NP and PM were analyzed by Western blot. (Adapted from Ref. 36 with permission. Copyright © 2018 American Chemical Society) |
白细胞是最大的血细胞, 直径从7 μm (小淋巴细胞) 到20 μm (单核细胞) 不等。白细胞包括单核细胞/巨噬细胞、淋巴细胞和粒细胞等。白细胞可识别炎症并在病变区域积聚[84]。一旦白细胞进入炎症组织, 会受到过度表达的趋化因子的影响并激活。有研究表明, 慢性炎症是癌症的主要临床病理特征之一[85]。在肿瘤进展过程中发生的炎症可使免疫细胞向肿瘤部位迁移。研究人员通过制备用白细胞膜包被的纳米粒证明了这种趋化性。选择性阻断白细胞膜上的淋巴细胞功能相关抗原-1或趋化因子受体(CXCR1和CXCR2) 可显著抑制肿瘤对白细胞的募集[86]。大量趋化因子在肿瘤组织中过度表达, 并且白细胞膜上存在大量的配体-受体相互作用, 这些特征使得白细胞膜包被纳米粒有望用于肿瘤的靶向递送[87]。
研究发现, 浸润在肿瘤基质中的单核细胞可转化为M2巨噬细胞, 为肿瘤存活和血管生成创造了合适的微环境[88]。尽管如此, 巨噬细胞在肿瘤中具有较高的含量和迁移率, 这为仿生纳米粒深入渗透到传统化疗药物难以到达的肿瘤内无血管和缺氧区域提供了潜在的可能性。受这些特性的启发, 一项研究设计了巨噬细胞膜包被的介孔二氧化硅纳米粒(MPCM-MSNCs)。巨噬细胞膜上的α4整合素和肿瘤细胞膜上的VCAM-1之间发生相互作用, 使之主动、非破坏性和选择性地结合到肿瘤上。研究发现, 几乎所有的MSNCs在与巨噬细胞共孵育24 h后被吞噬, 而超过30%的MPCM-MSNCs仍然是游离的。细胞摄取的降低导致MSNC功效的增加[89]。此外, 巨噬细胞膜包被的金纳米壳(MPCM-AuNSs) 也被构建用于肿瘤的PTT治疗。4T1荷瘤小鼠的体内荧光成像证实了MPCM-AuNSs在给药48 h后仍蓄积在肿瘤组织, 且产生了良好的抑瘤效果[34]。
T淋巴细胞具有良好的肿瘤靶向性, T淋巴细胞膜能够保护纳米粒免受血液中的抗体识别和血清蛋白吸附, 当通过细胞毒性T淋巴细胞膜递送纳米药物时, 载有PTX的纳米粒具有优异的肿瘤特异性识别功能[90]。Kang等[51]通过超声辅助重排方法设计了中性粒细胞膜包被的纳米粒(NMNPs) 用于包载蛋白酶抑制剂卡非佐米。NMNPs可以选择性地捕获血液循环中的肿瘤细胞并阻止转移灶的形成, 从而产生良好的抗肿瘤转移作用。此外, 自然杀伤细胞膜包被的纳米粒可用于抑制原位瘤和远端瘤的生长(图 7)[91]。
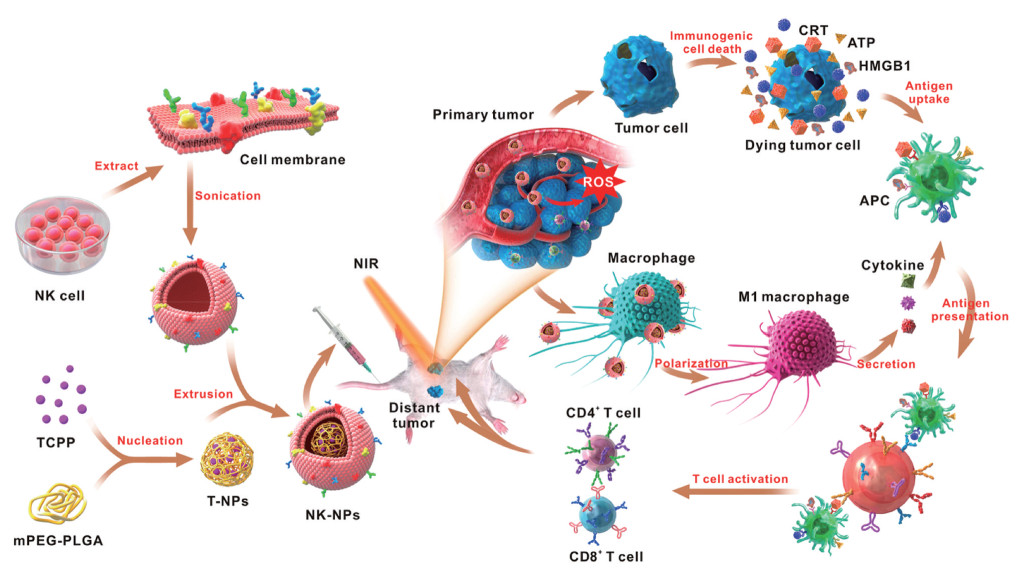
|
Figure 7 Schematic illustration of NK cell-membranes-coated nanoparticles for PDT-induced cell-membrane immunotherapy. (Adapted from Ref. 91 with permission. Copyright © 2018 American Chemical Society). CRT: Calreticulin; ATP: Adenosine triphosphate; HMGB1: High-mobility group box 1 protein; APC: Antigen-presenting cell |
近年来, 随着膜仿生技术的不断发展, 越来越多的研究关注到将两种不同来源的细胞膜融合制备杂化膜仿生纳米粒(hybrid membrane-coated nanoparticles, HM@NPs) 以实现不同生物膜功能的有机结合[26, 42]。而选择细胞膜的标准主要取决于不同细胞的独特特征和疾病治疗的需求。例如, Zhang课题组[52]报道了一种红细胞-血小板混合膜包被纳米粒, 具有来自两种细胞的表面膜蛋白标记物, 由此产生的双膜包被纳米粒在小鼠模型中表现出良好的长循环和分布。与单膜红细胞包被纳米粒和血小板包被纳米粒相比, 杂化膜包被纳米粒表现出两种单膜包被纳米粒的交叉特征。因而, 杂化膜包括红细胞-肿瘤细胞杂化膜包被纳米粒[45, 92]、血小板-白细胞杂化膜包被纳米粒[93]和肿瘤干细胞-血小板杂化膜包被纳米粒[53], 在肿瘤的靶向治疗上备受青睐。
例如, Wang等[94]设计了基于红细胞-肿瘤细胞杂化膜包被的pH敏感胶束, 胶束由负载有集落刺激因子-1受体(CSF-1R) 抑制剂BLZ-945的右旋糖酐接枝的聚组氨酸共聚物构建而成。该纳米胶束(DH@ECm) 的粒径约190 nm, 静脉给药后可靶向肿瘤相关巨噬细胞, 具有免疫伪装和肿瘤靶向能力。在肿瘤酸性微环境中, 胶束显示出膜逃逸效应, 以促进与肿瘤相关巨噬细胞的识别和相互作用。体内实验表明, DH@ECm还可逆转肿瘤免疫系统并升高CD8+ T细胞的数量, 肿瘤抑制率高达64.5%。同样, Wang等[45]融合了红细胞和黑色素瘤肿瘤细胞的膜材料以构建杂化仿生膜(RBC-B16), 并将杂化膜包被在载有DOX的硫化铜纳米粒(DCuS@[RBC-B16] NPs), 用于黑色素瘤的联合治疗。DCuS@[RBC-B16] NPs显示了两个源细胞的固有特性。与单纯的CuS NPs相比, DCuS@[RBC-B16] NPs在体外对源细胞系表现出高度特异性的自我识别, 并显著延长了体循环时间。因此, DCuS@[RBC-B16] NPs平台表现出优异的协同PTT/化学疗法, 对黑色素瘤肿瘤生长抑制率近100% (图 8)。总之, 融合多种细胞类型的膜包被仿生纳米粒将有助于不同肿瘤的个性化治疗。表 2[25, 27, 30, 34, 36, 37, 40, 44, 45, 47, 48, 50-54, 56, 57, 64, 65, 69, 70, 82, 83, 89-94]总结了不同细胞膜构建的纳米粒在肿瘤靶向治疗上的研究。
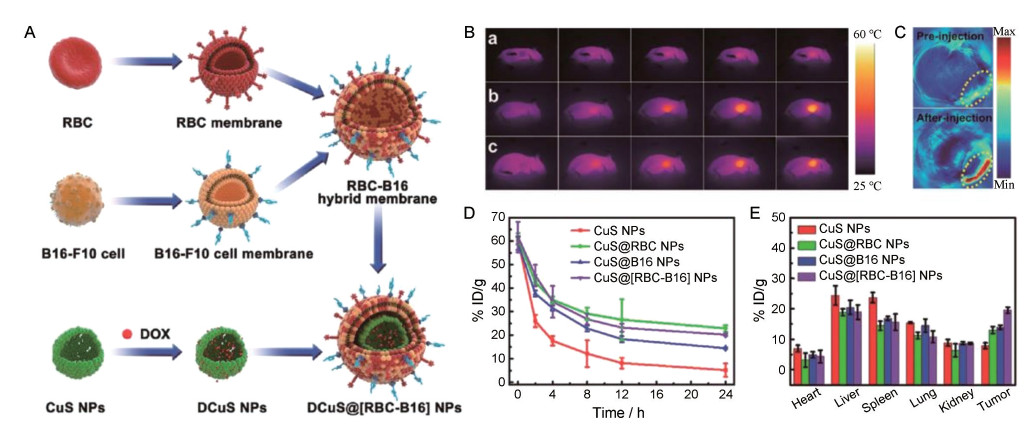
|
Figure 8 A: Schematic method for the preparation of RBC-B16 hybrid membrane. The resulting hybrid membrane is prepared to camouflage DOX-loaded hollow copper sulfide nanoparticles to produce DCuS@[RBC-B16] NPs. B: In vivo infrared thermography after administration with (a) NS, (b) CuS@[RBC-B16], and (c) DCuS@[RBC-B16]. C: Photoacoustic imaging of the B16-F10-tumor-bearing mice preinjection or postinjection of CuS@[RBC-B16]. D, E: In vivo blood retention (D) and biodistribution (E) of different NPs at predetermined point after injection. (Adapted from Ref. 45 with permission. Copyright © 2018 American Chemical Society) |
| Table 2 Classification of CMC@NPs. PLGA: Poly(lactic-co-glycolic acid); PEG-PDLLA: Methoxypoly(ethylene glycol)-block-poly(D, L-lactide); PVP: Polyvinyl pyrrolidone; MSN: Mesoporous silica nanoparticles |
本文重点介绍了CMC@NPs及其在肿瘤靶向治疗中的应用。这一新型仿生平台进一步改进了用于肿瘤靶向治疗的纳米DDS。来自不同源细胞的膜可以将多种功能有机整合到同一纳米粒上, 而无需复杂的合成技术。纳米粒的细胞膜包被技术创新性地缩小了合成纳米材料与生物系统之间的差距, 进一步从生物学的角度促进了肿瘤靶向治疗方法的发展。一方面, 合成的纳米粒核心可以支持和稳定细胞膜壳的形态结构, 同时允许包载一种或多种类型的小分子或生物大分子治疗药物; 另一方面, 通过细胞膜包被可避免纳米药物被免疫系统清除, 克服生物屏障, 并以足够的治疗剂量优先定位在肿瘤组织中。此外, 通过选择脂质插入方法构建的膜包被技术, 可实现细胞的特异性靶向, 而不涉及可能破坏膜表面蛋白的化学反应[95]。根据疾病属性, 将不同纳米材料和不同细胞膜结合在一起的仿生系统具有很高的灵活性和特异性。因此, 不仅能用于药物递送、肿瘤靶向治疗, 在解毒和免疫调节等其他领域也具有广阔的应用前景[96]。除了细胞膜外, 越来越多的新型膜也被用于仿生纳米制剂的构建, 如大肠杆菌膜制备的囊泡作为过氧化氢酶载体有效改善了肿瘤乏氧, 增强了肿瘤的放疗[97]。此外, M2巨噬细胞来源的外泌体膜被用于仿生纳米粒的制备, 实现了药物在肺部的有效蓄积, 为过敏性哮喘提供了新的治疗策略[98]。
然而, 基于细胞膜的DDS在国内外仍处于起步阶段[24]。首先, CMC@NPs的生产技术相对不成熟。目前制备方法很多, 路线繁琐、标准不一致和重现性较差, 难以实现规模化制备; 其次, 表征方法有限, 膜包被的成功仅通过粒度检测和形态观察来验证。蛋白质印迹分析只能证明CMC@NPs的表面组成与源细胞膜的表面组成是否相似, 但未能验证膜包被后膜是否被部分破坏。此外, CMC@NPs在保存过程中, 如何解决膜蛋白的变性, 以避免对内源性抗原产生潜在的免疫反应等。总体而言, 由于目前尚未有合适的方法可以保证CMC@ NPs制备的可控性、使用过程中的安全性和有效性, 这一创新性的技术仍不能满足临床应用的要求。展望未来, 尽管从实验室到临床仍有许多问题需要解决, 但CMC@NPs的天然优势和应用潜力仍不可否认, 细胞膜仿生系统为纳米药物设计和应用提供了新的思维模式。
作者贡献: 黄领领负责文献检索、整理及论文初稿的撰写; 吴宏辉负责论文的修改; 许东航和高建青负责论文选题、指导与修改。
利益冲突: 所有作者声明不存在任何利益冲突。
| [1] |
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71: 209-249. DOI:10.3322/caac.21660 |
| [2] |
Mehrling T. Chemotherapy is getting 'smarter'[J]. Future Oncol, 2015, 11: 549-552. DOI:10.2217/fon.14.248 |
| [3] |
Shi JJ, Kantoff PW, Wooster R, et al. Cancer nanomedicine: progress, challenges and opportunities[J]. Nat Rev Cancer, 2017, 17: 20-37. DOI:10.1038/nrc.2016.108 |
| [4] |
Wolfram J, Ferrari M. Clinical cancer nanomedicine[J]. Nano Today, 2019, 25: 85-98. DOI:10.1016/j.nantod.2019.02.005 |
| [5] |
Bocci G, Kerbel RS. Pharmacokinetics of metronomic chemotherapy: a neglected but crucial aspect[J]. Nat Rev Clin Oncol, 2016, 13: 659-673. DOI:10.1038/nrclinonc.2016.64 |
| [6] |
Xu Y, Han XX, Li YY, et al. Sulforaphane mediates glutathione depletion via polymeric nanoparticles to restore cisplatin chemosensitivity[J]. ACS Nano, 2019, 13: 13445-13455. DOI:10.1021/acsnano.9b07032 |
| [7] |
Zhang FW, Ni QQ, Jacobson O, et al. Polymeric nanoparticles with glutathione-sensitive heterodimeric multifunctional prodrug for in vivo drug monitoring and synergistic cancer therapy[J]. Angew Chem Int Ed Engl, 2018, 57: 7066-7070. DOI:10.1002/anie.201801984 |
| [8] |
Hu Y, Liu T, Li JX, et al. Selenium nanoparticles as new strategy to potentiate γδ T cell anti-tumor cytotoxicity through upregulation of tubulin-α acetylation[J]. Biomaterials, 2019, 222: 119397. DOI:10.1016/j.biomaterials.2019.119397 |
| [9] |
Zhang N, Song J, Liu Y, et al. Photothermal therapy mediated by phase-transformation nanoparticles facilitates delivery of anti-PD1 antibody and synergizes with antitumor immunotherapy for melanoma[J]. J Control Release, 2019, 306: 15-28. DOI:10.1016/j.jconrel.2019.05.036 |
| [10] |
Lubich C, Allacher P, de la Rosa M, et al. The mystery of antibodies against polyethylene glycol (PEG)-what do we know?[J]. Pharm Res, 2016, 33: 2239-2249. DOI:10.1007/s11095-016-1961-x |
| [11] |
Pannuzzo M, Esposito S, Wu LP, et al. Overcoming nanoparticle-mediated complement activation by surface PEG pairing[J]. Nano Lett, 2020, 20: 4312-4321. DOI:10.1021/acs.nanolett.0c01011 |
| [12] |
Han M, Lv Q, Tang XJ, et al. Overcoming drug resistance of MCF-7/ADR cells by altering intracellular distribution of doxorubicin via MVP knockdown with a novel siRNA polyamidoamine-hyaluronic acid complex[J]. J Control Release, 2012, 163: 136-144. DOI:10.1016/j.jconrel.2012.08.020 |
| [13] |
Pang L, Zhang C, Qin J, et al. A novel strategy to achieve effective drug delivery: exploit cells as carrier combined with nanoparticles[J]. Drug Deliv, 2017, 24: 83-91. DOI:10.1080/10717544.2016.1230903 |
| [14] |
Li LM, Han M, Jiang XC, et al. Peptide-tethered hydrogel scaffold promotes recovery from spinal cord transection via synergism with mesenchymal stem cells[J]. ACS Appl Mater Inter, 2017, 9: 3330-3342. DOI:10.1021/acsami.6b12829 |
| [15] |
Hu YL, Fu YH, Tabata Y, et al. Mesenchymal stem cells: a promising targeted-delivery vehicle in cancer gene therapy[J]. J Control Release, 2010, 147: 154-162. DOI:10.1016/j.jconrel.2010.05.015 |
| [16] |
Zhang TY, Huang B, Yuan ZY, et al. Gene recombinant bone marrow mesenchymal stem cells as a tumor-targeted suicide gene delivery vehicle in pulmonary metastasis therapy using non-viral transfection[J]. Nanomed Nanotechnol, 2014, 10: 257-267. DOI:10.1016/j.nano.2013.06.003 |
| [17] |
Zhang TY, Huang B, Wu HB, et al. Synergistic effects of co-administration of suicide gene expressing mesenchymal stem cells and prodrug-encapsulated liposome on aggressive lung melanoma metastases in mice[J]. J Control Release, 2015, 209: 260-271. DOI:10.1016/j.jconrel.2015.05.007 |
| [18] |
Li A, Zhang TY, Huang T, et al. Iron oxide nanoparticles promote Cx43-overexpression of mesenchymal stem cells for efficient suicide gene therapy during glioma treatment[J]. Theranostics, 2021, 11: 8254-8269. DOI:10.7150/thno.60160 |
| [19] |
Wang XL, Gao JQ, Ouyang XM, et al. Mesenchymal stem cells loaded with paclitaxel-poly(lactic-co-glycolic acid) nanoparticles for glioma-targeting therapy[J]. Int J Nanomedicine, 2018, 13: 5231-5248. DOI:10.2147/IJN.S167142 |
| [20] |
Tan SW, Wu TT, Zhang D, et al. Cell or cell membrane-based drug delivery systems[J]. Theranostics, 2015, 5: 836-881. |
| [21] |
Mohr A, Zwacka R. The future of mesenchymal stem cell-based therapeutic approaches for cancer-from cells to ghosts[J]. Cancer Lett, 2018, 414: 239-249. DOI:10.1016/j.canlet.2017.11.025 |
| [22] |
Jiang X, Röcker C, Hafner, M, et al. Endo- and exocytosis of zwitterionic quantum dot nanoparticles by live HeLa cells[J]. ACS Nano, 2010, 4: 6787-6797. DOI:10.1021/nn101277w |
| [23] |
Fang RH, Kroll AV, Gao WW, et al. Cell membrane coating nanotechnology[J]. Adv Mater, 2018, 30: 1706759. DOI:10.1002/adma.201706759 |
| [24] |
Zhen X, Cheng PH, Pu KY. Recent advances in cell membrane-camouflaged nanoparticles for cancer phototherapy[J]. Small, 2019, 15: 1804105. DOI:10.1002/smll.201804105 |
| [25] |
Hu CMJ, Zhang L, Aryal S, et al. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform[J]. Proc Natl Acad Sci U S A, 2011, 108: 10980-10985. DOI:10.1073/pnas.1106634108 |
| [26] |
Raza F, Zafar H, Zhang SL, et al. Recent advances in cell membrane‐derived biomimetic nanotechnology for cancer immunotherapy[J]. Adv Healthc Mater, 2021, 10: 2002081. DOI:10.1002/adhm.202002081 |
| [27] |
Chai ZL, Ran DN, Lu LW, et al. Ligand-modified cell membrane enables the targeted delivery of drug nanocrystals to glioma[J]. ACS Nano, 2019, 13: 5591-5601. DOI:10.1021/acsnano.9b00661 |
| [28] |
Su JH, Sun HP, Meng QS, et al. Long circulation red‐blood‐cell‐mimetic nanoparticles with peptide‐enhanced tumor penetration for simultaneously inhibiting growth and lung metastasis of breast cancer[J]. Adv Funct Mater, 2016, 26: 1243-1252. DOI:10.1002/adfm.201504780 |
| [29] |
Ren H, Liu JQ, Li YQ, et al. Oxygen self-enriched nanoparticles functionalized with erythrocyte membranes for long circulation and enhanced phototherapy[J]. Acta Biomater, 2017, 59: 269-282. DOI:10.1016/j.actbio.2017.06.035 |
| [30] |
Fang RH, Hu CMJ, Luk BT, et al. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery[J]. Nano Lett, 2014, 14: 2181-2188. DOI:10.1021/nl500618u |
| [31] |
Jiang Y, Krishnan N, Zhou JR, et al. Engineered cell‐membrane‐coated nanoparticles directly present tumor antigens to promote anticancer immunity[J]. Adv Mater, 2020, 32: 2001808. DOI:10.1002/adma.202001808 |
| [32] |
Zhang JJ, Lin Y, Zhou H, et al. Cell membrane‐camouflaged NIR II fluorescent Ag2Te quantum dots‐based nanobioprobes for enhanced in vivo homotypic tumor imaging[J]. Adv Healthc Mater, 2019, 8: 1900341. DOI:10.1002/adhm.201900341 |
| [33] |
Zhang Y, Cai KM, Li C, et al. Macrophage-membrane-coated nanoparticles for tumor-targeted chemotherapy[J]. Nano Lett, 2018, 18: 1908-1915. DOI:10.1021/acs.nanolett.7b05263 |
| [34] |
Xuan MJ, Shao JX, Dai LL, et al. Macrophage cell membrane camouflaged Au nanoshells for in vivo prolonged circulation life and enhanced cancer photothermal therapy[J]. ACS Appl Mater Inter, 2016, 8: 9610-9618. DOI:10.1021/acsami.6b00853 |
| [35] |
Wu HH, Zhou Y, Tabata Y, et al. Mesenchymal stem cell-based drug delivery strategy: from cells to biomimetic[J]. J Control Release, 2019, 294: 102-113. DOI:10.1016/j.jconrel.2018.12.019 |
| [36] |
Yang N, Ding YP, Zhang YL, et al. Surface functionalization of polymeric nanoparticles with umbilical cord-derived mesenchymal stem cell membrane for tumor-targeted therapy[J]. ACS Appl Mater Inter, 2018, 10: 22963-22973. DOI:10.1021/acsami.8b05363 |
| [37] |
Li YS, Wu HH, Jiang XC, et al. Active stealth and self-positioning biomimetic vesicles achieved effective antitumor therapy[J]. J Control Release, 2021, 335: 515-526. DOI:10.1016/j.jconrel.2021.05.031 |
| [38] |
Rao L, Bu LL, Ma L, et al. Platelet-facilitated photothermal therapy of head and neck squamous cell carcinoma[J]. Angew Chem Int Ed Engl, 2018, 57: 986-991. DOI:10.1002/anie.201709457 |
| [39] |
Sun HP, Su JH, Meng QS, et al. Cancer‐cell‐biomimetic nanoparticles for targeted therapy of homotypic tumors[J]. Adv Mater, 2016, 28: 9581-9588. DOI:10.1002/adma.201602173 |
| [40] |
Qin J, Luo ZM, Men YZ, et al. Red blood cell membrane-camouflaged melanin nanoparticles for enhanced photothermal therapy[J]. Biomaterials, 2017, 143: 29-45. DOI:10.1016/j.biomaterials.2017.07.027 |
| [41] |
Li JC, Zhen X, Lyu Y, et al. Cell membrane coated semiconducting polymer nanoparticles for enhanced multimodal cancer phototheranostics[J]. ACS Nano, 2018, 12: 8520-8530. DOI:10.1021/acsnano.8b04066 |
| [42] |
Chen HY, Deng J, Wang Y, et al. Hybrid cell membrane-coated nanoparticles: a multifunctional biomimetic platform for cancer diagnosis and therapy[J]. Acta Biomater, 2020, 112: 1-13. DOI:10.1016/j.actbio.2020.05.028 |
| [43] |
Zhang QZ, Dehaini D, Zhang Y, et al. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis[J]. Nat Nanotechnol, 2018, 13: 1182-1190. DOI:10.1038/s41565-018-0254-4 |
| [44] |
Yang JX, Teng YL, Fu Y, et al. Chlorins e6 loaded silica nanoparticles coated with gastric cancer cell membrane for tumor specific photodynamic therapy of gastric cancer[J]. Int J Nanomedicine, 2019, 14: 5061-5071. DOI:10.2147/IJN.S202910 |
| [45] |
Wang DD, Dong HF, Li M, et al. Erythrocyte-cancer hybrid membrane camouflaged hollow copper sulfide nanoparticles for prolonged circulation life and homotypic-targeting photothermal/chemotherapy of melanoma[J]. ACS Nano, 2018, 12: 5241-5252. DOI:10.1021/acsnano.7b08355 |
| [46] |
Liu C, Zhang W, Li YK, et al. Microfluidic sonication to assemble exosome membrane-coated nanoparticles for immune evasion-mediated targeting[J]. Nano Lett, 2019, 19: 7836-7844. DOI:10.1021/acs.nanolett.9b02841 |
| [47] |
Rao L, Cai B, Bu LL, et al. Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy[J]. ACS Nano, 2017, 11: 3496-3505. DOI:10.1021/acsnano.7b00133 |
| [48] |
Li C, Yang XQ, An J, et al. Red blood cell membrane-enveloped O2 self-supplementing biomimetic nanoparticles for tumor imaging-guided enhanced sonodynamic therapy[J]. Theranostics, 2020, 10: 867-879. DOI:10.7150/thno.37930 |
| [49] |
Pei Q, Hu XL, Zheng XH, et al. Light-activatable red blood cell membrane-camouflaged dimeric prodrug nanoparticles for synergistic photodynamic/chemotherapy[J]. ACS Nano, 2018, 12: 1630-1641. DOI:10.1021/acsnano.7b08219 |
| [50] |
Xu HL, Shen BX, Lin MT, et al. Homing of ICG-loaded liposome inlaid with tumor cellular membrane to the homologous xenografts glioma eradicates the primary focus and prevents lung metastases through phototherapy[J]. Biomater Sci, 2018, 6: 2410-2425. DOI:10.1039/C8BM00604K |
| [51] |
Kang T, Zhu QQ, Wei D, et al. Nanoparticles coated with neutrophil membranes can effectively treat cancer metastasis[J]. ACS Nano, 2017, 11: 1397-1411. DOI:10.1021/acsnano.6b06477 |
| [52] |
Dehaini D, Wei XL, Fang RH, et al. Erythrocyte-platelet hybrid membrane coating for enhanced nanoparticle functionalization[J]. Adv Mater, 2017, 29: 1606209. DOI:10.1002/adma.201606209 |
| [53] |
Fang RH, Hu CMJ, Chen KN, et al. Lipid-insertion enables targeting functionalization of erythrocyte membrane-cloaked nanoparticles[J]. Nanoscale, 2013, 5: 8884-8888. DOI:10.1039/c3nr03064d |
| [54] |
Wu HH, Jiang XC, Li YS, et al. Engineering stem cell derived biomimetic vesicles for versatility and effective targeted delivery[J]. Adv Funct Mater, 2020, 30: 2006169. DOI:10.1002/adfm.202006169 |
| [55] |
Fan Z, Zhou H, Li PY, et al. Structural elucidation of cell membrane-derived nanoparticles using molecular probes[J]. J Mater Chem B, 2014, 2: 8231-8238. DOI:10.1039/C4TB00980K |
| [56] |
Ren XQ, Zheng R, Fang XL, et al. Red blood cell membrane camouflaged magnetic nanoclusters for imaging-guided photothermal therapy[J]. Biomaterials, 2016, 92: 13-24. DOI:10.1016/j.biomaterials.2016.03.026 |
| [57] |
Chen Z, Zhao PF, Luo ZY, et al. Cancer cell membrane-biomimetic nanoparticles for homologous-targeting dual-modal imaging and photothermal therapy[J]. ACS Nano, 2016, 10: 10049-10057. DOI:10.1021/acsnano.6b04695 |
| [58] |
Xue JW, Zhao ZK, Zhang L, et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence[J]. Nat Nanotechnol, 2017, 12: 692-700. DOI:10.1038/nnano.2017.54 |
| [59] |
Bu LL, Rao L, Yu GT, et al. Cancer stem cell‐platelet hybrid membrane‐coated magnetic nanoparticles for enhanced photothermal therapy of head and neck squamous cell carcinoma[J]. Adv Funct Mater, 2019, 29: 1807733. DOI:10.1002/adfm.201807733 |
| [60] |
Fller M, Huber SM, Lang F. Erythrocyte programmed cell death[J]. IUBMB Life, 2008, 60: 661-668. DOI:10.1002/iub.106 |
| [61] |
Godfrin Y, Horand F, Franco R, et al. International seminar on the red blood cells as vehicles for drugs[J]. Expert Opin Biol Ther, 2012, 12: 127-133. DOI:10.1517/14712598.2012.631909 |
| [62] |
Xu CH, Ye PJ, Zhou YC, et al. Cell membrane-camouflaged nanoparticles as drug carriers for cancer therapy[J]. Acta Biomater, 2020, 105: 1-14. DOI:10.1016/j.actbio.2020.01.036 |
| [63] |
Xia Q, Zhang YT, Li Z, et al. Red blood cell membrane-camouflaged nanoparticles: a novel drug delivery system for antitumor application[J]. Acta Pharm Sin B, 2019, 9: 675-689. DOI:10.1016/j.apsb.2019.01.011 |
| [64] |
Aryal S, Hu CMJ, Fang RH, et al. Erythrocyte membrane-cloaked polymeric nanoparticles for controlled drug loading and release[J]. Nanomedicine, 2013, 8: 1271-1280. DOI:10.2217/nnm.12.153 |
| [65] |
Piao JG, Wang L, Gao F, et al. Erythrocyte membrane is an alternative coating to polyethylene glycol for prolonging the circulation lifetime of gold nanocages for photothermal therapy[J]. ACS Nano, 2014, 8: 10414-10425. DOI:10.1021/nn503779d |
| [66] |
Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect[J]. Adv Drug Deliv Rev, 2011, 63: 136-151. DOI:10.1016/j.addr.2010.04.009 |
| [67] |
Li SY, Cheng H, Xie BR, et al. Cancer cell membrane camouflaged cascade bioreactor for cancer targeted starvation and photodynamic therapy[J]. ACS Nano, 2017, 11: 7006-7018. DOI:10.1021/acsnano.7b02533 |
| [68] |
Lang TQ, Yin Q, Li YP. Progress of cell‐derived biomimetic drug delivery systems for cancer therapy[J]. Adv Ther, 2018, 1: 1800053. DOI:10.1002/adtp.201800053 |
| [69] |
Zhang N, Li MH, Sun XT, et al. NIR-responsive cancer cytomembrane-cloaked carrier-free nanosystems for highly efficient and self-targeted tumor drug delivery[J]. Biomaterials, 2018, 159: 25-36. DOI:10.1016/j.biomaterials.2018.01.007 |
| [70] |
Sun HP, Su JH, Meng QS, et al. Cancer cell membrane‐coated gold nanocages with hyperthermia‐triggered drug release and homotypic target inhibit growth and metastasis of breast cancer[J]. Adv Funct Mater, 2017, 27: 1604300. DOI:10.1002/adfm.201604300 |
| [71] |
Jia Y, Wang XB, Hu DH, et al. Phototheranostics: active targeting of orthotopic glioma using biomimetic proteolipid nanoparticles[J]. ACS Nano, 2018, 13: 386-398. |
| [72] |
Zhang KM, Ye JJ, Li CX, et al. Cytomembrane-mediated transport of metal ions with biological specificity[J]. Adv Sci, 2019, 6: 1900835. DOI:10.1002/advs.201900835 |
| [73] |
Chen FM, Liu XH. Advancing biomaterials of human origin for tissue engineering[J]. Prog Polym Sci, 2016, 53: 86-168. |
| [74] |
Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells[J]. Science, 1994, 284: 143-147. |
| [75] |
Zhang TY, Li FY, Xu QH, et al. Ferrimagnetic nanochains‐based mesenchymal stem cell engineering for highly efficient post‐stroke recovery[J]. Adv Funct Mater, 2019, 29: 1900603. DOI:10.1002/adfm.201900603 |
| [76] |
Zhang TY, Xu QH, Huang T, et al. New insights into biocompatible Iron oxide nanoparticles: a potential booster of gene delivery to stem cells[J]. Small, 2020, 16: 2001588. DOI:10.1002/smll.202001588 |
| [77] |
Kim SM, Jeong CH, Woo JS, et al. In vivo near-infrared imaging for the tracking of systemically delivered mesenchymal stem cells: tropism for brain tumors and biodistribution[J]. Int J Nanomedicine, 2016, 11: 13-23. |
| [78] |
Nowakowski A, Drela K, Rozycka J, et al. Engineered mesenchymal stem cells as an anti-cancer trojan horse[J]. Stem Cells Dev, 2016, 25: 1513-1531. DOI:10.1089/scd.2016.0120 |
| [79] |
Lee MW, Ryu S, Kim DS, et al. Mesenchymal stem cells in suppression or progression of hematologic malignancy: current status and challenges[J]. Leukemia, 2019, 33: 597-611. DOI:10.1038/s41375-018-0373-9 |
| [80] |
Sage EK, Thakrar RM, Janes SM. Genetically modified mesenchymal stromal cells in cancer therapy[J]. Cytotherapy, 2016, 18: 1435-1445. DOI:10.1016/j.jcyt.2016.09.003 |
| [81] |
Wang M, Xin YF, Cao H, et al. Recent advances in mesenchymal stem cell membrane-coated nanoparticles for enhanced drug delivery[J]. Biomater Sci, 2021, 9: 1088-1103. DOI:10.1039/D0BM01164A |
| [82] |
Gao CY, Lin ZH, Jurado‐Sánchez B, et al. Stem cell membrane‐coated nanogels for highly efficient in vivo tumor targeted drug delivery[J]. Small, 2016, 12: 4056-4062. DOI:10.1002/smll.201600624 |
| [83] |
Gao CY, Lin ZH, Wu ZG, et al. Stem-cell-membrane camouflaging on near-infrared photoactivated upconversion nanoarchitectures for in vivo remote-controlled photodynamic therapy[J]. ACS Appl Mater Inter, 2016, 8: 34252-34260. DOI:10.1021/acsami.6b12865 |
| [84] |
Gao CY, Wu ZG, Lin ZH, et al. Polymeric capsule-cushioned leukocyte cell membrane vesicles as a biomimetic delivery platform[J]. Nanoscale, 2016, 8: 3548-3554. DOI:10.1039/C5NR08407E |
| [85] |
Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation[J]. Nature, 2008, 454: 436-444. DOI:10.1038/nature07205 |
| [86] |
Wang QL, Ren Y, Mu JY, et al. Grapefruit-derived nanovectors use an activated leukocyte trafficking pathway to deliver therapeutic agents to inflammatory tumor sites[J]. Cancer Res, 2015, 75: 2520-2529. DOI:10.1158/0008-5472.CAN-14-3095 |
| [87] |
Oroojalian F, Beygi M, Baradaran B, et al. Immune cell membrane‐coated biomimetic nanoparticles for targeted cancer therapy[J]. Small, 2021, 17: 2006484. DOI:10.1002/smll.202006484 |
| [88] |
Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis[J]. Cell, 2010, 141: 39-51. DOI:10.1016/j.cell.2010.03.014 |
| [89] |
Xuan MJ, Shao JX, Dai LL, et al. Macrophage cell membrane camouflaged mesoporous silica nanocapsules for in vivo cancer therapy[J]. Adv Healthc Mater, 2015, 4: 1578. DOI:10.1002/adhm.201570064 |
| [90] |
Zhang LR, Li RT, Chen H, et al. Human cytotoxic T-lymphocyte membrane-camouflaged nanoparticles combined with low-dose irradiation: a new approach to enhance drug targeting in gastric cancer[J]. Int J Nanomedicine, 2017, 12: 2129-2142. DOI:10.2147/IJN.S126016 |
| [91] |
Deng GJ, Sun ZH, Li SP, et al. Cell-membranes immunotherapy based on natural killer cell-membranes-coated nanoparticles for effective inhibition of primary and abscopal tumor growth[J]. ACS Nano, 2018, 12: 12096-12108. DOI:10.1021/acsnano.8b05292 |
| [92] |
Jiang Q, Liu Y, Guo RR, et al. Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors[J]. Biomaterials, 2019, 192: 292-308. DOI:10.1016/j.biomaterials.2018.11.021 |
| [93] |
Rao L, Meng QF, Huang QQ, et al. Platelet-leukocyte hybrid membrane‐coated immunomagnetic beads for highly efficient and highly specific isolation of circulating tumor cells[J]. Adv Funct Mater, 2018, 28: 1803531. DOI:10.1002/adfm.201803531 |
| [94] |
Wang YC, Luan, ZY, Zhao CY, et al. Target delivery selective CSF-1R inhibitor to tumor-associated macrophages via erythrocyte-cancer cell hybrid membrane camouflaged pH-responsive copolymer micelle for cancer immunotherapy[J]. Eur J Pharm Sci, 2020, 142: 105136. DOI:10.1016/j.ejps.2019.105136 |
| [95] |
Gao WW, Zhang LF. Coating nanoparticles with cell membranes for targeted drug delivery[J]. J Drug Target, 2015, 23: 619-626. DOI:10.3109/1061186X.2015.1052074 |
| [96] |
Fang RH, Jiang Y, Fang JC, et al. Cell membrane-derived nanomaterials for biomedical applications[J]. Biomaterials, 2017, 128: 69-83. |
| [97] |
Zai WJ, Kang L, Dong TJ, et al. E. coli membrane vesicles as a catalase carrier for long-term tumor hypoxia relief to enhance radiotherapy[J]. ACS Nano, 2021, 15: 15381-15394. |
| [98] |
Pei WY, Li XQ, Bi RL, et al. Exosome membrane-modified M2 macrophages targeted nanomedicine: treatment for allergic asthma[J]. J Control Release, 2021, 338: 253-267. |
 2022, Vol. 57
2022, Vol. 57


