2. 西南民族大学化学与环境学院, 四川 成都 610041;
3. 中国科学院成都生物研究所, 四川 成都 610041
2. College of Chemistry and Environment, Southwest Minzu University, Chengdu 610041, China;
3. Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China
粉防己为防己科(Menispermaceae) 千金藤属植物粉防己(Stephania tetrandra S. Moore) 的干燥根, 主要分布于浙江、安徽、福建等地。味苦、性寒, 具有利水消肿、祛风止痛的功效, 主治水肿脚气、小便不利、湿疹疮毒、风湿痹通、高血压等症[1]。该植物含有大量的生物碱成分, 主要包括苄基异喹啉生物碱(monobenzyltetrahydroisoquinolines)、双苄基异喹啉生物碱(bisbenzyltetrahydroisoquinolines)、阿朴菲(aporphines) 和原小檗碱(protoberberines) 等结构类型[2]。丰富的次生代谢产物也造就了其生物活性的多样性, 如抗寄生虫[3]、抗菌[4]、抗真菌[5]、抗病毒[6]、抗炎[7]、抗肿瘤[8]、神经保护[9]、安眠[10]、抗高血压[11]等。
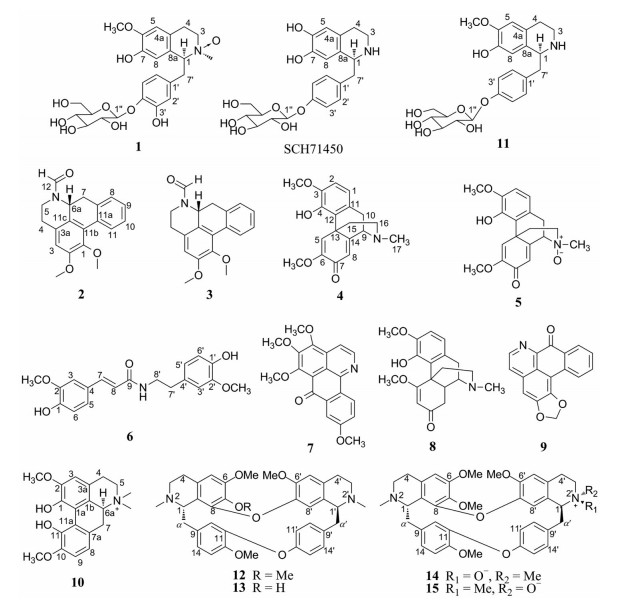
为了寻找人肺癌耐药细胞株H1299细胞毒活性先导化合物, 本研究对粉防己总生物碱部分进行了成分分离。经过反复柱色谱和高效液相色谱分离, 共分离得到15个生物碱类化合物, 分别鉴定为tetrandraside A (1)、(Z)-N-formyl-nornuciferin (2)、(E)-N-formyl-nornuciferin (3)、salutaridine (4)、salutaridine N-oxide (5)、(E)-3-(4-hydroxy-3-methoxyphenyl)-N-[2-(4-hydroxy-3-methoxyphenyl)ethyl]-2 propenamide (6)、dauriporphine (7)、sinomenine (8)、liriodenine (9)、α-magnoflorine (10)、(1S)-4'-β-glucosylcoclaurine (11)、tetrandrine (12)、fangchinoline (13)、tetrandrine 2'-β-oxide (14) 和tetrandrine 2'-α-oxide (15) (图 1)。其中, 化合物1为新化合物, 也是该属植物中分离出的极为少见的苷类化合物。细胞毒活性筛选结果显示, 这些化合物对人肺癌细胞耐药株H1299均有较好的效果, 其中新化合物的IC50为10.56 μmol·L-1, 化合物9活性最好, IC50为5.38 μmol·L-1 (阳性对照吉非替尼IC50为29.85 μmol·L-1)。
|
Figure 1 Structures of compounds 1-15 and SCH71450 |
化合物1 灰白色粉末, 在紫外254 nm下, 薄层色谱显示有暗斑, Wagner试剂呈阳性, HR-ESI-MS准分子离子峰m/z: 494.202 7 [M+H]+ (计算值494.202 6, C24H32O10N) 给出分子式为C24H31NO10。1H NMR (表 1, 400 MHz, CD3OD) 显示有1个1, 2, 4, 5-四取代苯环, 芳香氢质子为δH 6.76 (s, 1H, H-5) 和δH 6.30 (br s, 1H, H-8); 1个1, 3, 4-三取代苯环, 芳香氢质子为δH 6.76 (d, J = 1.8 Hz, 1H, H-2'), δH 7.16 (d, J = 8.4 Hz, 1H, H-5'), δH 6.40 (dd, J = 8.4, 1.8 Hz, 1H, H-6'); 1个葡萄糖端基质子为δH 4.78 (d, J = 7.4 Hz, 1H, H-1''); 4个葡萄糖次甲基质子δH 3.50, 3.42 (峰重叠, 4H, H-2'', 3", 4", 5"); 1个脂肪族次甲基δH 4.50 (dd, J = 3.0, 9.0 Hz, H-1); 1个葡萄糖亚甲基为δH 3.91 (dd, J = 1.0, 12.0 Hz, 1H, H-6"a), δH 3.73 (峰重叠, 1H, H-6"b); 两个脂肪族亚甲基分别为δH 3.50 (峰重叠, 2H, H-3), δH 3.26 (t, 7.8 Hz, 1H, H-4a), δH 2.99 (ddd, 17.3, 17.3, 5.8 Hz, 1H, H-4b); 1个甲氧基为δH 3.84 (s, 3H) 和1个NCH3为δH 3.24 (s, 3H)。结合13C NMR (表 1, 100 MHz, CD3OD) 和DEPT, 显示有7个芳香季碳为δC 147.6 (C-4')、147.2 (C-6)、144.6 (C-3')、144.5 (C-7)、132.8 (C-1')、125.4 (C-4a)、121.3 (C-8a), 5个芳香次甲基为δC 120.3 (C-6')、117.6 (C-5')、116.5 (C-2')、114.0 (C-8)、110.8 (C-5), 1个葡萄糖端基碳δC 102.9 (C-1"), 4个葡萄糖次甲基为δC 76.9 (C-3")、76.2 (C-5")、73.5 (C-2")、69.9 (C-4"), 1个脂肪族次甲基为δC 78.0 (C-1), 4个亚甲基分别为3个脂肪族亚甲基δC 61.6 (C-3)、37.6 (C-7')、24.9 (C-4) 和1个葡萄糖亚甲基δC 61.0 (C-6"), 两个甲基分别为δC 54.9 (6-OCH3)、53.2 (NCH3)。上述数据与文献化合物SCH71450[12, 13]结构(图 1) 相似, 不同的是SCH71450缺少1个甲氧基和1个NCH3的信号。仔细比较两个化合物的碳谱数据, 发现化合物1中C-1和C-3的化学位移分别向低场位移了20.1和20.7, 同时NCH3的化学位移也向低场位移, 提示化合物1为N氧化物, 另外高分辨质谱同样显示化合物1应该是一个氮氧化物。
| Table 1 1H and 13C NMR data of compound 1 (400 MHz for 1H NMR and 100 MHz for 13C NMR with methanol-d4 as solvent. J in Hz). *Signals overlapped |
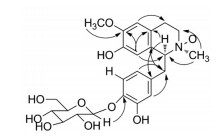
远程相关(HMBC, 图 2) 给出H-5与C-4相关, H-8与C-1、C-4a、C-6相关, H-1与C-4a、C-1'相关, H-7'与C-8a相关, H-2'、H-6'均与C-7'相关, 上述相关提示化合物基本骨架与SCH71450[1, 2]一致。此外, NCH3中的氢与C-3和C-1相关, 提示N上应有一个甲基, 甲氧基氢与C-6相关, 说明甲氧基连在C-6上, H-1"与C-4'相关, 说明葡萄糖连在C-4'上, 通过H-1"的偶合常数判断葡萄糖为β-葡萄糖。
|
Figure 2 Core HMBC correlations of compound 1 |
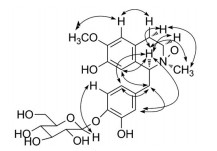
为了确定化合物1母核的相对构型, 考察了该化合物的NOESY相关谱(图 3)。在NOESY相关中, H-1与H-4α、NCH3相关, NCH3与H-1、H-4α相关, 说明H-1与H-4α、NCH3为相同朝向。H-1"与H-5'相关, 印证了葡萄糖连在C-4'上, 甲氧基与H-5相关, 说明甲氧基连在C-6上, H-5与H-4相关, H-1与H-2'、H-6'相关, H-8与H-1、H-8与H-7'相关, H-2'、H-6'与H-7'相关, 上述相关在此验证了化合物基本骨架的合理性。由于该化合物量的限制, 没有能够获得其水解产物, 从而无法确定糖的绝对构型。鉴于同属植物和该植物中都分离到了D-葡萄糖(11), 因此, 推测化合物1中的葡萄糖为D-构型, 命名为tetrandraside A。
|
Figure 3 Key NOESY correlations of compound 1 |
细胞毒活性测试结果(表 2) 显示, 这些化合物对人肺癌耐药细胞株H1299均有较好的效果, 其中新化合物1的IC50为10.56 μmol·L-1, 化合物9活性最好, IC50为5.38 μmol·L-1 (阳性对照吉非替尼IC50为29.85 μmol·L-1)。
| Table 2 Cytotoxicity test results of compounds against H1299 |
质谱(HR-ESI-MS) 使用Waters Vion IMS QTof; 核磁共振为Bruker, 400 MHz、600 MHz, 以methanol-d4或CDCl3为溶剂, TMS为内标; 红外光谱为傅里叶变换红外光谱仪FTS 3000; 紫外光谱为普析TU1810DPC; 旋光为SGW®-533; 制备色谱仪为江苏汉邦DAC80, 色谱柱为30 mm × 250 mm, 薄层色谱(TLC) 硅胶(GF254) 和柱色谱硅胶(200~300目, 青岛海洋化工有限公司) 用于柱色谱。反相色谱柱(Cosmosil 75, C18-OPN) 为Nacalai Tesque公司产品。葡聚糖凝胶Sephadex LH-20购自于当地生产商。TLC显色剂用Wagner试剂(碘1 g和碘化钾10 g溶于50 mL水中, 加热, 加冰醋酸2 mL用水稀释到100 mL)。实验室所用的有机溶剂以及其他耗材等从成都科龙化工试剂公司购买。
粉防己原料于2018年6月购于成都市荷花池中药材市场, 经王晓玲教授鉴定为Stephania tetrandra S. Moore, 样本存放于西南民族大学少数民族药物标本馆, 编号St-2019-1。
1 提取分离将10 kg粉防己粉碎(5~10目), 用3.0 L 70%乙醇水(盐酸调节pH = 3) 常温浸泡24 h。抽滤得总浸提液, 真空浓缩除去乙醇, 氨水调节pH = 9~10, 用氯仿萃取, 得氯仿萃取物90 g。水相部分上大孔树脂柱, 用30%和70%的乙醇水洗脱, 分别得到60 g的30%乙醇水洗脱物和30 g的70%乙醇水洗脱物。将氯仿萃取物部分上硅胶柱, 以氯仿-甲醇15∶1到5∶1梯度洗脱, 点板、合并相似组分, 共得到10个组分(STC-1到STC-10)。样品STC-1经过硅胶柱色谱(石油醚-乙酸乙酯= 4∶1) 得到2和3 (23.4 mg) 的异构体混合物、化合物5 (13.8 mg), 样品STC-2经过反复硅胶柱色谱得到化合物4 (14.1 mg) 和6 (18.3 mg), 样品STC-3经过反复硅胶柱色谱得到化合物7 (8.3 mg)、9 (7.3 mg), 样品STC-4经过硅胶柱色谱(石油醚-丙酮= 1∶1) 得到化合物12 (6.3 g)、13 (8.6 g), 样品STC-5经过高效液相色谱(HPLC) 梯度洗脱(65%~95%, 0~25 min), 流动相甲醇水, 流速35 mL·min-1, 柱子规格为C18, Φ 30 mm × 250 mm, 得化合物14 (tR = 10.7 min, 6.8 mg)、15 (tR = 12.2 min, 2.9 mg), 将70%乙醇水洗脱物上LH-20, 以纯甲醇洗脱, 薄层监测、合并相似组分, 共得到5个组分(STC-701到STC-705)。STC-702在HPLC上以甲醇(45)~水(55) (氨水0.1%) 等度洗脱(0~40 min), 流速35 mL·min-1, 柱子规格为C18, Φ 30 mm × 250 mm, 得到化合物8 (tR = 23.7 min, 5.6 mg), 将30%乙醇水洗脱物上反相柱, 分别以15%、75%甲醇水洗脱, 分别得20 g STC301和15 g STC302, 将样品STC-301上LH-20, 以甲醇洗脱, 得到STC-3011 (17.1 g)、STC-3012 (1.8 g), 样品3011上硅胶柱, 以氯仿甲醇3∶1等度洗脱, 得到10个组分(STC-3011-1到STC-3011-10)。样品STC-3011-6经HPLC梯度洗脱, 甲醇水35%到45% (0~50 min), 流速35 mL·min-1, 柱子规格为C18, Φ 30 mm × 250 mm, 得化合物11 (tR = 32.2 min, 200 mg), 样品STC-3011-10经HPLC梯度洗脱, 甲醇水5%到15% (0~50 min), 流速35 mL·min-1, 柱子规格为C18, Φ 30 mm × 250 mm, 得化合物1 (tR = 8 min, 5.2 mg)、10 (tR = 31 min, 11.3 g)。
2 结构鉴定化合物1 浅黄色粉末, [α]
化合物2 白色晶体(甲醇), 紫外254 nm有暗斑, Wagner试剂呈阳性, 高分辨质谱HR-ESI-MS准分子离子峰m/z: 332.125 4 [M+Na]+ (calculated 332.126 3, C19H19O3NNa) 给出分子式为C19H19NO3。1H NMR (400 MHz, CDCl3) δH 8.39 (br d, J = 8.0 Hz, 1H, H-11), 8.26 (s, 1H, H-12), 7.23~7.38 (峰重叠, 3H, H-8, 9, 10), 6.66 (s, 1H, H-3), 4.93 (dd, J = 14.0, 4.0 Hz, 1H, H-6a), 3.90 (s, 3H, 2-OCH3), 3.83 (dd, J = 12.9, 3.8 Hz, 1H, H-5a), 3.67 (s, 3H, 1-OCH3), 3.42 (ddd, J = 12.9, 12.9, 2.3 Hz, 1H, H-5b), 3.10~3.19 (峰重叠, H-7a), 2.94 (ddd, J = 15.0, 12.5, 4.4 Hz, 1H, H-4a), 2.73-2.84 (峰重叠, H-7b, H-4b)。13C NMR (100 MHz, CDCl3) δC 162.1 (C-12), 152.3 (C-2), 145.9 (C-1), 136.1 (C-7a), 131.4 (C-11a), 128.6 (C-8), 128.6 (C-11c), 128.4 (C-11), 127.9 (C-11b), 127.8 (C-9), 127.1 (C-10), 125.2 (C-3a), 111.4 (C-3), 60.0 (1-OCH3), 55.9 (2-OCH3), 49.4 (C-6a), 42.0 (C-5), 34.1 (C-7), 31.0 (C-4)。核磁数据与文献[14]化合物(Z)-N-formyl-nornuciferin一致, 化合物2被鉴定为(Z)-N-formyl-nornuciferin。
化合物3 白色粉末, 紫外254 nm有暗斑, Wagner试剂呈阳性, 高分辨质谱HR-ESI-MS准分子离子峰m/z: 332.125 4 [M+Na]+ (calculated 332.126 3, C19H19NO3Na) 给出分子式为C19H19NO3。1H NMR (400 MHz, CDCl3) δH 8.44 (br d, J = 8.0 Hz, 1H, H-11), 7.23~7.38 (峰重叠, 3H, H-8, 9, 10), 6.69 (s, 1H, H-3), 4.50 (dd, J = 14.0, 4.0 Hz, 1H, H-6a), 4.43 (ddd, J = 12.8, 4.0, 3.5 Hz, H-5a), 3.90 (s, 3H, 2-OCH3), 3.67 (s, 3H, 1-OCH3), 3.10~3.21 (峰重叠, H-5b), 3.10~3.19 (峰重叠, H-7a), 2.73~2.84 (峰重叠, H-4a, 4b, 7b)。13C NMR (100 MHz, CDCl3) δC 161.9 (C-12), 152.6 (C-2), 145.7 (C-1), 135.4 (C-7a), 131.6 (C-11a), 129.5 (C-11c), 128.7 (C-11), 128.1 (C-8), 127.9 (C-9), 127.5 (C-10), 127.4 (C-11b), 124.7 (C-3a), 111.7 (C-3), 60.1 (1-OCH3), 55.9 (2-OCH3), 53.4 (C-6a), 37.9 (C-7), 36.1 (C-5), 29.6 (C-4)。核磁数据与文献[1]化合物(E)-N-formyl-nornuciferin一致, 化合物3被鉴定为(E)-N-formyl-nornuciferin。化合物2和3为一组顺反异构体(Z/E = 2∶1), 单晶析出E-构型(Mo Kα, λ = 0.710 73, P212121, a = 6.287 7 (4), b = 15.884 6 (11), c = 16.921 6 (9), α = γ = β = 90°, V = 1 690.10 (17), deposition number 2074120, 晶体结构的详细数据可以通过网址www.ccdc.cam.ac.uk/data_request/cif免费获取)。
化合物4 白色晶体(甲醇), 紫外254 nm有暗斑, Wagner试剂呈阳性, 高分辨质谱HR-ESI-MS准分子离子峰m/z: 328.154 6 [M+H]+ (calculated 328.154 9, C19H22NO4+) 给出分子式为C19H21NO4。1H NMR (400 MHz, CDCl3) δH 7.59 (s, 1H, H-5), 6.75 (d, J = 8.3 Hz, 1H, H-2), 6.66 (d, J = 8.3 Hz, 1H, H-1), 6.33 (s, 1H, H-8), 3.87 (s, 3H, 3-OCH3), 3.74 (s, 3H, 6-OCH3), 3.70 (d, J = 5.5 Hz, 1H, H-9), 3.33 (d, J = 17.7 Hz, 1H, Hax-10), 3.00 (dd, J = 5.5, 17.7 Hz, 1H, Heq-10), 2.62 (dddd, J = 1.7, 2.8, 4.3, 12.5 Hz, 1H, Hax-16), 2.51 (dd, J = 2.8, 12.5 Hz, 1H, Heq-16), 2.44 (s, 3H, H-17), 2.37 (dt, J = 1.7, 12.5 Hz, 1H, Hax-15), 1.76 (td, J = 4.8, 12.5 Hz, 1H, Heq-15)。13C NMR (100 MHz, CDCl3) δC 181.4 (C-7), 161.5 (C-14), 150.7 (C-6), 154.3 (C-3), 143.2 (C-4), 129.2 (C-11), 123.6 (C-12), 122.0 (C-8), 120.6 (C-5), 118.6 (C-1), 109.4 (C-2), 60.7 (C-9), 56.1 (3-OCH3), 54.6 (6-OCH3), 46.7 (C-16), 43.5 (C-13), 41.3 (C-17), 37.2 (C-15), 32.5 (C-10)。核磁数据与文献[15, 16]化合物salutaridine一致, 同时, 其结构也通过单晶衍射得到了进一步确认(Mo Kα, λ = 0.710 73, P212121, a = 10.810 7 (5), b = 11.461 6 (6), c = 13.031 1 (7), α = γ = β = 90°, V = 1 614.66 (14), deposition number 2074119, 晶体结构的详细数据可以通过网址www.ccdc.cam.ac.uk/data_request/cif免费获取)。因此, 化合物4被鉴定为salutaridine。
化合物5 白色粉末, 紫外254 nm有暗斑, Wagner试剂呈阳性, 高分辨质谱HR-ESI-MS准分子离子峰m/z: 344.149 9 [M+H]+ (calculated 344.149 8, C19H22NO5+) 给出分子式为C19H21NO5。1H NMR (400 MHz, CDCl3) δH 7.80 (s, 1H, H-5), 6.91 (dd, J = 8.3, 1.0 Hz, 1H, H-2), 6.69 (br d, J = 8.3 Hz, 1H, H-1), 6.46 (s, 1H, H-8), 4.30 (d, J = 4.6 Hz, 1H, H-9), 3.87 (s, 3H, 3-OCH3), 3.76 (s, 3H, 6-OCH3), 3.32 (s, 3H, H-17)。13C NMR (100 MHz, CDCl3) δC 180.8 (C-7), 157.0 (C-14), 150.6 (C-6), 147.2 (C-3), 144.3 (C-4), 126.1 (C-8), 124.7 (C-11), 123.3 (C-12), 120.6 (C-5), 118.6 (C-1), 110.8 (C-2), 76.3 (C-9), 60.6 (C-16), 56.1 (3-OCH3), 55.3 (C-17), 54.0 (6-OCH3), 42.2 (C-13), 35.0 (C-15), 32.9 (C-10)。1H NMR数据与文献[17]化合物salutaridine N-oxide一致, 13C NMR数据参考化合物4, 因此, 化合物5被鉴定为salutaridine N-oxide。
化合物6 白色粉末, 紫外254 nm有暗斑, Wagner试剂呈阳性, 高分辨质谱HR-ESI-MS准分子离子峰m/z: 344.149 3 [M+H]+ (calculated 344.149 8, C19H22NO5+) 给出分子式为C19H21NO5。1H NMR (400 MHz, CDCl3) δH 7.52 (d, J = 15.6 Hz, 1H, H-7), 6.99 (dd, J = 8.1, 2.0 Hz, 1H, H-5), 6.93 (d, J = 2.0 Hz, 1H, H-3), 6.87 (d, J = 8.1 Hz, 1H, H-6), 6.84 (d, J = 7.9 Hz, 1H, H-6'), 6.70 (d, J = 1.6 Hz, 1H, H-3'), 6.68 (dd, J = 7.9, 1.6 Hz, 1H, H-3'), 6.19 (d, J = 15.6 Hz, 1H, H-8), 3.85 (s, 3H, 2-OCH3), 3.83 (s, 3H, 2'-OCH3), 3.60 (q, J = 6.9 Hz, 2H, H-8'), 2.79 (t, J = 6.9 Hz, 2H, H-7')。13C NMR (100 MHz, CDCl3) δC 166.5 (C-9), 147.5 (C-1), 146.8 (C-2), 146.7 (C-2'), 144.2 (C-1'), 141.1 (C-7), 130.6 (C-4'), 127.1 (C-4), 122.0 (C-5), 121.3 (C-5'), 117.9 (C-8), 114.8 (C-6), 114.4 (C-6'), 111.3 (C-3'), 109.7 (C-3), 55.85 (2-OCH3), 55.83 (2'-OCH3), 40.9 (C-8'), 35.2 (C-7')。1H、13C NMR数据与文献[18]化合物(E)-3-(4-hydroxy-3-methoxyphenyl)-N-[2-(4-hydroxy-3-methoxyphenyl)ethyl]-2 propenamide一致, 化合物6被鉴定为(E)-3-(4-hydroxy-3-methoxyphenyl)-N-[2-(4-hydroxy-3-methoxyphenyl)ethyl]-2 propenamide。
化合物7 白色粉末, 紫外254 nm有暗斑, Wagner试剂呈阳性, 高分辨质谱HR-ESI-MS准分子离子峰m/z: 352.118 6 [M+H]+ (calculated 352.118 5, C20H18NO5+) 给出分子式为C20H17NO5。1H NMR (400 MHz, CDCl3) δH 8.80 (d, J = 8.7 Hz, 1H, H-11), 8.68 (d, J = 5.4 Hz, 1H, H-2), 7.94 (d, J = 5.4 Hz, 1H, H-3), 7.87 (d, J = 1.6 Hz, 1H, H-8), 7.32 (dd, J = 8.7, 1.6 Hz, 1H, H-10), 4.27 (s, 3H, 4-OCH3), 4.17 (s, 3H, 6-OCH3), 4.05 (s, 3H, 5-OCH3), 3.98 (s, 3H, 9-OCH3)。13C NMR (100 MHz, CDCl3) δC 181.5 (C-7), 161.4 (C-9), 160.9 (C-6), 153.1 (C-4), 147.4 (C-11b), 146.3 (C-5), 143.1 (C-2), 135.0 (C-7a), 129.5 (C-11a), 128.5 (C-3a), 126.9 (C-11), 121.6 (C-10), 120.0 (C-3b), 116.5 (C-6a), 114.5 (C-3), 108.8 (C-8), 61.8 (4-OCH3), 61.8 (5-OCH3), 61.8 (6-OCH3), 55.7 (9-OCH3)。1H、13C NMR数据与文献[19]化合物dauriporphine一致, 化合物7被鉴定为dauriporphine。
化合物8 白色粉末, 紫外254 nm有暗斑, Wagner试剂呈阳性, 高分辨质谱HR-ESI-MS准分子离子峰m/z: 330.170 2 [M+H]+ (calculated 330.170 5, C19H24NO4+) 给出分子式为C19H23NO4。1H NMR (400 MHz, CD3OD) δH 6.73 (d, J = 8.3 Hz, 1H, H-2), 6.55 (d, J = 8.3 Hz, 1H, H-1), 5.77 (d, J = 1.8 Hz, 1H, H-6), 3.78 (s, 3H, 3-OCH3), 3.47 (s, 3H, 7-OCH3), 2.43 (s, 3H, H-17)。13C NMR (100 MHz, DMSO-d6) δC 193.3 (C-6), 151.8 (C-7), 145.8 (C-3), 145.4 (C-4), 131.0 (C-11), 123.7 (C-12), 118.2 (C-1), 116.9 (C-8), 110.1 (C-2), 56.1 (C-9), 56.0 (3-OCH3), 54.7 (7-OCH3), 49.0 (C-5), 47.1 (C-16), 45.5 (C-14), 42.9 (C-13), 35.7 (C-15), 24.3 (C-10)。13C NMR (100 MHz, CD3OD) δC 195.1 (C-6), 152.0 (C-7), 145.8 (C-3), 145.2 (C-4), 129.8 (C-11), 122.2 (C-12), 117.9 (C-1), 116.0 (C-8), 109.5 (C-2), 56.5 (C-9), 55.1 (3-OCH3), 53.9 (7-OCH3), 49.0 (C-5, 与溶剂重叠), 46.8 (C-16), 45.1 (C-14), 40.2 (C-13), 34.9 (C-15), 23.9 (C-10)。核磁数据与文献[20]化合物sinomenine一致, 化合物8被鉴定为sinomenine。
化合物9 白色粉末, 紫外254 nm有暗斑, Wagner试剂呈阳性, 高分辨质谱HR-ESI-MS准分子离子峰m/z: 276.066 0 [M+H]+ (calculated 276.066 1, C17H11NO3+) 给出分子式为C17H10NO3。1H NMR (400 MHz, CDCl3) δH 8.90 (d, J = 5.3 Hz, 1H, H-8), 8.65 (br d, J = 8.0 Hz, 1H, H-6), 8.60 (dd, J = 8.0, 1.0 Hz, 1H, H-4), 7.74~7.79 (峰重叠, 2H, H-9, 11), 7.60 (t, J = 7.7 Hz, 1H, H-10), 7.20 (br s, 1H, 3), 6.39 (s, 2H, OCH2O)。核磁数据与文献[21]化合物liriodenine一致, 化合物9被鉴定为liriodenine。
化合物10 白色粉末, 紫外254 nm有暗斑, Wagner试剂呈阳性, 高分辨质谱HR-ESI-MS准分子离子峰m/z: 342.170 5 [M]+ (calculated 342.170 5, C20H24NO4) 给出准分子式为C20H24NO4。1H NMR (400 MHz, CD3OD) δH 6.67 (d, J = 8.0 Hz, 1H, H-9), 6.47 (d, J = 10.0 Hz, 1H, H-8), 6.46 (s, 1H, H-3), 3.84 (s, 3H, 10-OCH3), 3.77 (s, 3H, 2-OCH3), 3.25 (s, 3H, α-CH3), 2.80 (s, 3H, β-CH3)。13C NMR (100 MHz, DMSO-d6) δC 152.9 (C-2), 152.5 (C-10), 151.7 (C-1), 150.8 (C-11), 125.6 (C-7a), 123.6 (C-11a), 123.1 (C-1a), 120.5 (C-1b), 113.0 (C-8), 112.2 (C-3a), 110.2 (C-9), 109.1 (C-3), 69.6 (C-6a), 60.9 (C-5), 56.1 (2-OCH3), 55.6 (10-OCH3), 53.1 (α-CH3), 42.9 (β-CH3), 30.9 (C-7), 23.7 (C-4)。核磁数据与文献[22, 23]化合物α-magnoflorine一致, 化合物10被鉴定为α-magnoflorine。
化合物11 白色粉末, 紫外254 nm有暗斑, Wagner试剂呈阳性, 高分辨质谱HR-ESI-MS准分子离子峰m/z: 448.196 7 [M+H]+ (calculated 448.197 1, C23H30NO8+) 给出分子式为C23H29NO8。1H NMR (400 MHz, CD3OD) δH 7.18 (d, J = 8.4 Hz, 2H, H-2', 6'), 7.06 (d, J = 8.4 Hz, 2H, H-3', 5'), 6.67 (s, 1H, H-8), 6.64 (s, 1H, H-5), 4.89 (与溶剂峰重叠, H-1''), 4.08 (dd, J = 4.2, 9.2 Hz, 1H, H-1), 3.91 (dd, J = 1.6, 12.0 Hz, 1H, H-6''), 3.84 (s, 3H, 4-OCH3), 3.69 (dd, J = 4.2, 12.0 Hz, 1H, H-6''), 3.39~3.46 (m, 4H, H-2'', 3'', 4'', 5''), 3.14~3.18 (m, 2H, H-3, 7'), 2.81~2.87 (m, 2H, H-3, 7'), 2.71~2.74 (m, 2H, H-4)。13C NMR (100 MHz, CD3OD) δC 157.0 (C-4'), 146.9 (C-6), 144.7 (C-7), 132.6 (C-1'), 130.4 (C-2', 6'), 130.0 (C-8a), 125.9 (C-4a), 117.0 (C-2', 6'), 113.1 (C-8), 111.9 (C-5), 101.4 (C-1''), 77.1 (C-3''), 77.0 (C-5''), 73.9 (C-2''), 70.4 (C-4''), 61.5 (C-6''), 56.9 (C-1), 55.3 (6-OCH3), 41.2 (C-7'), 40.5 (C-3), 28.5 (C-4)。核磁数据与文献[24, 25]化合物(1S)-4'-β-glucosylcoclaurine一致, 化合物11被鉴定为(1S)-4'-β-glucosylcoclaurine。
化合物12 灰白色粉末, 紫外254 nm有暗斑, Wagner试剂呈阳性。ESI-MS: m/z 645 [M+Na]+。1H NMR (400 MHz, CDCl3) δH 7.34 (1H, dd, J = 2.4, 8.0 Hz, H-10'), 7.14 (1H, dd, J = 2.4, 8.0 Hz, H-11'), 6.86 (2H, m, H-13, 14), 6.80 (1H, dd, J = 2.4, 8.0 Hz, H-13'), 6.54 (1H, br s, H-10), 6.50 (1H, s, H-5'), 6.30 (1H, dd, J = 2.4, 8.0 Hz, H-14'), 6.30 (1H, s, H-5), 5.99 (1H, s, H-8'), 3.93 (3H, s, 12-OCH3), 3.73 (3H, s, 6-OCH3), 3.37 (3H, s, 6'-OCH3), 3.19 (3H, s, 7-OCH3), 2.62 (3H, s, 2'-NCH3), 2.33 (3H, s, 2-NCH3)。其谱学数据与文献[26]报道的粉防己碱的谱学数据对照基本一致, 故鉴定化合物12为粉防己碱(tetrandrine)。
化合物13 米白色固体, 紫外254 nm有暗斑, Wagner试剂呈阳性。ESI-MS: m/z 631 [M+Na]+。1H NMR (400 MHz, CDCl3) δH 7.33 (1H, dd, J = 2.4, 8.0 Hz, H-14'), 7.12 (1H, dd, J = 2.4, 8.0 Hz, H-13'), 6.85 (2H, m, H-13, 14), 6.80 (1H, dd, J = 2.4, 8.0 Hz, H-11'), 6.57 (1H, br s, H-10), 6.51 (1H, s, H-5'), 6.31 (1H, dd, J = 2.4, 8.0 Hz, H-10'), 6.28 (1H, s, H-5), 6.05 (1H, s, H-8'), 3.92 (3H, s, 12-OCH3), 3.75 (3H, s, 6-OCH3), 3.34 (3H, s, 6'-OCH3), 2.62 (3H, s, 2'-NCH3), 2.32 (3H, s, 2-NCH3)。其谱学数据与文献[27]报道的防己诺林碱的谱学数据对照基本一致, 故鉴定化合物13为防己诺林碱(fangchinoline)。
化合物14 白色固体, 紫外254 nm有暗斑, Wagner试剂呈阳性。ESI-MS: m/z 639 [M+H]+。1H NMR (600 MHz, CDCl3) δH 7.37 (1H, dd, J = 2.0, 8.2 Hz, H-14'), 7.20 (1H, dd, J = 2.5, 8.2 Hz, H-13'), 6.90 (dd, J = 2.0, 8.2 Hz, 1H, H-14), 6.86 (d, J = 8.2 Hz, 1H, H-13), 6.83 (dd, J = 2.5, 8.3 Hz, 1H, H-11'), 6.57 (s, 1H, H-5'), 6.53 (d, J = 2.0 Hz, 1H, H-10), 6.30 (s, 1H, H-5), 6.24 (dd, J = 2.0, 8.3, Hz, 1H, H-10'), 6.04 (s, 1H, H-8'), 4.57 (dd, J = 5.0, 11.0 Hz, 1H, H-1'), 3.96 (m, 1H, H-3'), 3.93 (3H, s, 12-OCH3), 3.73 (3H, s, 6-OCH3), 3.73 (m, 1H, H-1), 3.57 (s, 1H, 2'-NCH3), 3.57 (m, 2H, H-3', α'), 3.53 (m, 1H, H-4'), 3.50 (m, 1H, H-3), 3.35 (s, 3H, 6'-OCH3), 3.24 (s, 3H, 7-OCH3), 2.91 (m, 1H, H-3), 2.90 (m, 2H, H-4, 4'), 2.70 (m, 1H, H-α), 2.69 (m, 1H, H-α'), 2.50 (d, J = 14.0 Hz, 1H, H-α), 2.40 (dd, J = 6.0, 16.0 Hz, 1H, H-4), 2.33 (3H, s, 2-NCH3)。13C NMR (150 MHz, CDCl3) δC 154.9 (C-12'), 151.6 (C-6), 149.6 (C-6'), 149.1 (C-11), 148.5 (C-12), 147.1 (C-8), 144.6 (C-7'), 138.2 (C-7), 135.1 (C-9), 132.6 (C-10'), 130.9 (C-4'a), 130.4 (C-14'), 128.0 (C-4a), 124.3 (C-8'a), 123.4 (C-9'), 123.0 (C-14, 8a), 122.5 (C-11', 13'), 120.9 (C-8'), 116.4 (C-10), 112.2 (C-5'), 111.7 (C-13), 106.2 (C-5), 76.9 (C-1'), 61.6 (C-1), 60.4 (7-OCH3), 57.6 (2'-NCH3), 57.3 (C-3'), 56.2 (12-OCH3), 55.8 (6-OCH3), 55.7 (6'-OCH3), 44.1 (C-3), 42.3 (2-NCH3), 42.0 (C-α), 40.4 (C-α'), 24.4 (C-4'), 22.0 (C-4)。上述1H、13C NMR经过HSQC、HMBC、DEPT进行了归属, 解析出结构与文献[28]报道的tetrandrine 2'-β-oxide一致, 故鉴定化合物14为tetrandrine 2'-β-oxide。
化合物15 白色固体, 紫外254 nm有暗斑, Wagner试剂呈阳性。ESI-MS: m/z 639 [M+H]+。1H NMR (600 MHz, CDCl3) δH 7.42 (1H, dd, J = 2.0, 8.2 Hz, H-14'), 7.19 (1H, dd, J = 2.5, 8.2 Hz, H-13'), 6.93 (br d, J = 8.2 Hz, 1H, H-14), 6.86 (d, J = 8.2 Hz, 1H, H-13), 6.83 (dd, J = 2.5, 8.3 Hz, 1H, H-11'), 6.56 (s, 1H, H-5'), 6.52 (d, J = 1.7 Hz, 1H, H-10), 6.29 (s, 1H, H-5), 6.24 (dd, J = 2.0, 8.3, Hz, 1H, H-10'), 6.06 (s, 1H, H-8'), 4.75 (dd, J = 6.0, 12.0 Hz, 1H, H-1'), 4.00 (dd, J = 6.0, 10.0 Hz, 1H, H-3'), 3.92 (3H, s, 12-OCH3), 3.83 (t, J = 10.0 Hz, 1H, H-3'), 3.73 (m, 1H, H-1), 3.72 (s, 3H, 6-OCH3), 3.71 (s, 1H, 2'-NCH3), 3.60 (dd, J = 6.0, 12.0 Hz, 1H, H-α'), 3.52 (m, 1H, H-3), 3.43 (m, 1H, H-4'), 3.35 (s, 3H, 6'-OCH3), 3.25 (s, 3H, 7-OCH3), 2.98 (m, 2H, H-4, 4'), 2.90 (m, 1H, H-4), 2.75 (dd, J = 3.2, 9.9 Hz, 1H, H-α), 2.72 (dd, J = 6.0, 12.0 Hz, 1H, H-α'), 2.50 (d, J = 14.0 Hz, 1H, H-α), 2.44 (dd, J = 3.6, 14.2 Hz, 1H, H-4), 2.35 (3H, s, 2-NCH3)。上述1H NMR数据与文献[29]报道的tetrandrine 2'-α-oxide的一致, 故鉴定化合物15为tetrandrine 2'-α-oxide。
3 活性测试采用MTT法测试了部分化合物对人肺癌耐药细胞株H1299的细胞毒活性。细胞株用含10%胎牛血清的DMEM培养基(100 u·mL-1青霉素、100 μg·mL-1链霉素) 在37 ℃、5% CO2培养箱中培养。取对数期的细胞株, 按每孔大约3 000个细胞的量接种于96孔板, 置于37 ℃恒温培养箱中孵育24 h。24 h后, 将不同浓度梯度的样品分别加入到培养孔中, 并设置空白对照, 置于细胞培养箱中继续培养72 h。再向培养基中加入20 μL MTT培养液(5 mg·mL-1) 染色4 h。移去MTT溶液, 每孔加入150 μL DMSO, 震荡10 min后, 用酶标仪检测在570 nm波长下各孔的吸光值, 并计算抑制率及IC50。
作者贡献: 何达海进行了实验研究方案的设计、研究过程实施及论文的起草; 刘军、王晓玲为本文工作提供了技术及材料支持; 方东梅负责了化合物的核磁共振测试工作; 李丽梅为研究选题的提出、研究方案的设计及论文的修改提供了支持。
利益冲突: 本研究不存在任何利益冲突。
| [1] |
Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China: Vol I (中华人民共和国药典: 第一部) [S]. Beijing: Chemical Industry Press, 2005: 19.
|
| [2] |
Jiang Y, Liu M, Liu H, et al. A critical review: traditional uses, phytochemistry, pharmacology and toxicology of Stephania tetrandra S. Moore (Fen Fang Ji)[J]. Phytochem Rev, 2020, 19: 1-41. DOI:10.1007/s11101-019-09655-7 |
| [3] |
García Díaz J, Tuenter E, Arranz JE, et al. Antimicrobial activity of leaf extracts and isolated constituents of Croton linearis[J]. J Ethnopharmacol, 2019, 236: 250-257. DOI:10.1016/j.jep.2019.01.049 |
| [4] |
Zhang Z, Yan J, Xu KJ, et al. Tetrandrine reverses drug resistance in isoniazid and ethambutol dual drug-resistant Mycobacterium tuberculosis clinical isolates[J]. BMC Infect Dis, 2015, 15: 153. DOI:10.1186/s12879-015-0905-0 |
| [5] |
Zhao YJ, Liu WD, Shen YN, et al. The efflux pump inhibitor tetrandrine exhibits synergism with fluconazole or voriconazole against Candida parapsilosis[J]. Mol Biol Rep, 2019, 46: 5867-5874. DOI:10.1007/s11033-019-05020-1 |
| [6] |
Schafer A, Cheng H, Lee C, et al. Development of potential small molecule therapeutics for treatment of Ebola virus disease[J]. Curr Med Chem, 2018, 25: 5177-5190. |
| [7] |
Guo C, Wang M, Li J, et al. Effect of inflammatory cytokines in the LPS induced RAW264.7 cells by the decoction and its split components from Stephania tetrandra S. Moore[J]. Acta Chin Med Pharmacol, 2015, 43: 33-36. |
| [8] |
Payon V, Kongsaden C, Ketchart W, et al. Mechanism of cepharanthine cytotoxicity in human ovarian cancer cells[J]. Plant Med, 2019, 85: 41-47. DOI:10.1055/a-0706-7503 |
| [9] |
Lv YL, Wu ZZ, Chen LX, et al. Neuroprotective effects of tetrandrine against vascular dementia[J]. Neural Regen Res, 2016, 11: 454-459. DOI:10.4103/1673-5374.179058 |
| [10] |
Huang YL, Cui SY, Cui XY, et al. Tetrandrine, an alkaloid from S. tetrandra exhibits anti-hypertensive and sleep enhancing effects in SHR via different mechanisms[J]. Phytomedicine, 2016, 23: 1821-1829. DOI:10.1016/j.phymed.2016.10.021 |
| [11] |
Wang X, Yang Y, Yang D, et al. Tetrandrine prevents monocrotaline-induced pulmonary arterial hypertension in rats through regulation of the protein expression of inducible nitric oxide synthase and cyclic guanosine monophosphate-dependent protein kinase type 1[J]. J Vasc Surg, 2016, 64: 1468-1477. DOI:10.1016/j.jvs.2015.09.016 |
| [12] |
Hegde VR, Dai P, Ladislaw C, et al. D-4 dopamine receptor-selective compounds from the Chinese plant Phoebe chekiangensis[J]. Bioorg Med Chem Lett, 1997, 7: 1207-1212. DOI:10.1016/S0960-894X(97)00194-7 |
| [13] |
Wu HP, Lu TN, Hsu NY, et al. Absolute stereochemical assignment of SCH 71450, a selective dopamine D4 receptor antagonist, through enantioselective epimer synthesis[J]. Eur J Org Chem, 2013, 2013: 2898-2905. DOI:10.1002/ejoc.201300072 |
| [14] |
Pachaly P, Adnan AZ, Will G. NMR-assignments of N-acylaporphine alkaloids from Tinospora crispa[J]. Planta Med, 1992, 58: 184-187. DOI:10.1055/s-2006-961425 |
| [15] |
Szantay C, Barczai-Beke M, Pechy P, et al. Studies aimed at the synthesis of morphine. 3. Synthesis of (±)-salutaridine via phenolic oxidative coupling of (±)-reticuline[J]. J Org Chem, 1982, 47: 594-596. DOI:10.1021/jo00342a051 |
| [16] |
Yu R, Ye Q, Chen B, et al. Chemical study on the Mitrephora Maingayi[J]. Nat Prod Res Dev (天然产物研究与开发), 2003, 1: 212-215. |
| [17] |
Sartyar G, Gulgeze HB, Gözler B. Salutaridine N-oxide from the capsules of Papaver bracteatum[J]. Planta Med, 1992, 58: 368-369. DOI:10.1055/s-2006-961487 |
| [18] |
Suzuki T, Holden I, Casida JE. Diphenyl ether herbicides remarkably elevate the content in Spinacia oleracea of (E)-3-(4-hydroxy-3-methoxyphenyl)-N-[2-(4-hydroxy-3-methoxyphenyl)ethyl]-2 propenamide[J]. J Agric Food Chem, 1981, 29: 992-995. DOI:10.1021/jf00107a027 |
| [19] |
Sugimoto Y, Babiker HAA, Inanaga S, et al. Oxoisoaporphines from Menispermum dauricum[J]. Phytochemistry, 1999, 52: 1431-1435. DOI:10.1016/S0031-9422(99)00330-1 |
| [20] |
Gong YH, Ding LS. 13C NMR Analysis of Natural Products (天然产物核磁共振碳谱分析) [M]. Kunming: Yunnan Science and Technology Press, 2006: 733.
|
| [21] |
Zheng B, Qu HY, Meng TZ, et al. Novel total syntheses of oxoaporphine alkaloids enabled by mild Cu-catalyzed tandem oxidation/aromatization of 1-Bn-DHIQs[J]. RSC Adv, 2018, 8: 28997-29007. DOI:10.1039/C8RA05338C |
| [22] |
Holzbach JC, Lopes LMX. Aristolactams and alkamides of Aristolochia gigantea[J]. Molecules, 2010, 15: 9462-9472. DOI:10.3390/molecules15129462 |
| [23] |
Chen JH, Du ZZ, Shen YM, et al. Aporphine alkaloids from Clematis parviloba and their antifungal activity[J]. Arch Pharm Res, 2009, 32: 3-5. DOI:10.1007/s12272-009-1111-7 |
| [24] |
Maier UH, Rodl W, Deusneumann B, et al. Biosynthesis of erythrina alkaloids in Erythrina crista-galli[J]. Phytochemistry, 1999, 52: 373-382. DOI:10.1016/S0031-9422(99)00230-7 |
| [25] |
Liu S, Dou XT, Liu Y, et al. Chemical constituents from Stephania cepharantha[J]. Chin Tradit Pat Med (中成药), 2020, 42: 1498-1503. DOI:10.3969/j.issn.1001-1528.2020.06.020 |
| [26] |
Pan JX, Zhuo JC, Han GQ, et al. Solution conformation of tetrandrine[J]. Chin J Magn Reson (波谱学杂志), 1989, 6: 163-168. |
| [27] |
Yang JS, Zhang Q, Zhou Y, et al. 2D NMR spectra of bisbenzylisoquinoline alkaloid of fangchinoline[J]. West China J Pharm Sci (华西药学杂志), 2001, 16: 161-164. |
| [28] |
Dahmen K, Pachaly P, Zymalkowski F. Alkaloids from the Thai drug "Krung Kha Mao" (Cyclea Barbata, Menispermaceae), V: isolation and structural elucidation of further bisbenzytiquinoline alkaloids[J]. Arch Pharm (Weinheim), 1977, 310: 95-102. DOI:10.1002/ardp.19773100204 |
| [29] |
Lin MB, Zhang W, Zhao XW, et al. Chemical studies on tetradrandrine-oxides[J]. Acta Chim Sin (化学学报), 1984, 42: 199-204. DOI:10.3321/j.issn:0251-0790.1984.02.011 |
 2021, Vol. 56
2021, Vol. 56


