2. 中国科学院上海药物研究所, 药物制剂研究中心, 上海 201203
2. Center for Pharmaceutics Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China
炎症性肠病(inflammatory bowel disease, IBD) 是一种多因素诱导的慢性免疫介导性疾病, 包括溃疡性结肠炎(ulcerative colitis, UC) 和克罗恩病(Crohn's disease, CD)[1, 2], 其发病机制复杂不清, 研究多认为由遗传易感、免疫因素、肠道菌群、环境因素、焦虑及情绪障碍等多种因素导致该病发生[3, 4]。巨噬细胞在免疫系统激活和炎症发生过程中起到关键作用[5-7], 其有可塑性和功能多样性, 可以极化为M1或M2亚型[8, 9]。M1型巨噬细胞与促炎及抗菌能力有关, 而M2型巨噬细胞则与抗炎、伤口愈合及纤维化作用相关[10]。研究证实, 在活动期IBD患者和疾病动物肠黏膜中, 炎性M1型巨噬细胞的数量明显增加[11, 12], 因此巨噬细胞可被作为治疗IBD的靶细胞。
广藿香油(patchouli oil) 为广藿香Pogostemon cablin (Blanco) Benth.的干燥地上部分经水蒸气蒸馏提取的挥发油[13], 包含广藿香醇、广藿香酮、β-广藿香烯和α-愈创木烯等挥发性化学成分[14], 具有抗炎[15]、免疫调节[16]、抗菌[17]和保护肠屏障功能[18]等药理作用。课题组前期发现, 广藿香醇不仅能够降低UC大鼠血清中肿瘤坏死因子α (tumor necrosis factor α, TNF-α)、白介素6 (interleukin-6, IL-6) 和白介素1β (interleukin-1β, IL-1β) 等促炎因子水平, 抑制IκBα和p65的磷酸化水平, 通过阻断NF-κB (nuclear factor kappa-B) 信号通路抑制炎症反应[19], 并且还构建了炎性巨噬细胞靶向的乳铁蛋白修饰广藿香醇脂质体, 通过主动靶向递送药物提高了抗炎药效[20]。亦有研究表明广藿香油中其他成分同样具有抗炎作用, 如广藿香酮具有抑制T细胞增殖、减少致炎性Th细胞组织浸润、调节细胞因子分泌体现出抗炎作用[21-23], β-广藿香烯能够调节免疫功能、减缓葡聚糖硫酸钠(dextran sodium sulfate, DSS) 诱导的肠黏膜损伤[24]。虽然诸多研究发现广藿香油及其主要成分的抗炎作用, 但是基于油中多成分组合的作用机制仍很复杂, 抗炎作用的分子机制研究甚少, 因此, 本文拟通过构建药物分子-靶点-通路-疾病网络, 富集有关信号通路及生物进程, 并结合体外实验考察广藿香油及其主要成分的抗炎作用和肠上皮修复功能, 阐述广藿香油治疗IBD的潜在分子生物学机制, 为下一步新药研发提供基础研究。
材料与方法广藿香油活性成分及靶点 前期对广藿香油进行GC-MS (gas chromatography-mass spectrometer) 分析, 得到鉴定的14种有效成分(中国发明专利申请号: 202010317649.1)。在TCMSP (traditional Chinese medicine systems pharmacology) 数据库中检索活性成分靶点, 将靶点通过UniProt数据库中的UniProt KB功能搜索相应基因。经Swiss Target Prediction平台预测成分的潜在靶点。将两次获得的靶点合并去重, 则为化合物靶点基因。
炎症性肠病靶点及交集靶点 以“inflammatory bowel disease”为关键词, 物种设定为“Homo sapiens”, 利用DrugBank、GeneCards、OMIM、PharmGkb和TTD数据库获取IBD的相关靶点。交集靶点通过借助Venny 2.1.0在线绘制韦恩图获得。
网络构建与分析 为深入探讨广藿香油治疗IBD的作用机制, 使用Cytoscape3.7.2构建活性成分-交集靶点网络。通过Metascape在线平台[25] (https://metascape.org/) 输入交集靶点, 设置P < 0.01, 进行GO (gene ontology) 功能富集分析及KEGG (Kyoto encyclopedia of genes and genomes) 通路富集分析。为分析靶蛋白之间的联系, 利用String平台[26] (https://string-db.org/) 上传交集靶点研究蛋白质-蛋白质相互作用(protein-protein interaction, PPI), 将置信度 > 0.7的数据输入Cytoscape3.7.2可视化, 根据degree值调整颜色、大小筛选核心靶点, 构建PPI图。
成分-靶点分子对接 选取活性成分-交集靶点网络图中度值靠前的化合物与PPI网络中核心靶点使用AutoDock Vina 1.1.2软件分子对接。具体方法从PubChem数据库和PDB网站下载成分和靶点的结构, 利用ChemOffice、AutoDockTools 1.5.6和PyMol分别进行能量最小化、加氢、去除水分子和小分子配体, 文件均保存为pdbqt格式。随后用Vina进行分子对接和构象能量计算。通过PyMol、LigPlot+ v.2.2软件对以上对接结果中结合能最低的化合物与靶点, 进行可视化绘图。
巨噬细胞极化差异基因筛选 从NCBI的GEO (gene expression omnibus) 数据库中搜索巨噬细胞相关芯片数据, 获取Derlindati E等提交的GSE57614基因芯片数据集。此数据为GPL6480平台Agilent-014850 Whole Human Genome Microarray 4x44K G4112F (probe name version) 基因芯片, 选取其中经干扰素-γ和脂多糖(LPS) 诱导巨噬细胞极化至M1型9例样本, 白细胞介素-4诱导巨噬细胞极化至M2型9例样本。利用R x64 3.6.3软件, 引用limma包分析芯片数据的差异表达基因, 过滤条件为P < 0.05及差异倍数|log2FC| > 1, 绘制热图, 将M1、M2型巨噬细胞的差异基因导出到Excel表格。
差异表达基因KEGG富集分析 DAVID (the database for annotation, visualization and integrated discovery) 是一套全面的功能性注释工具。将筛选的差异基因导入DAVID在线数据库进行KEGG通路富集分析, 按照P值排序, 选取前20条信号通路可视化。
细胞与试药 NCM460人正常结肠上皮细胞(广州吉妮欧生物科技有限公司)。RAW264.7巨噬细胞(北纳生物细胞库)。DMEM培养基、胎牛血清(FBS) (Gibco公司); LPS (Sigma公司); 青霉素-链霉素(P/S)、0.25%胰蛋白酶(上海碧云天生物科技有限公司); RNA提取试剂、RNA逆转录试剂盒(上海翊圣生物科技有限公司); 广藿香挥发油(20180628, 江西恒诚天然香料油有限公司); 广藿香醇(MUST-17073111)、广藿香酮(MUST-19112812) (成都曼斯特生物科技有限公司)。
细胞培养及分组干预 NCM460人正常结肠上皮细胞作为细胞模型, 在DMEM培养基(含10% FBS、1% P/S), 37 ℃、5% CO2培养箱培养, 考察广藿香油、广藿香醇和广藿香酮在细胞上的作用。通过q-PCR检测药物对LPS诱导的NCM460人正常结肠上皮细胞释放的炎症因子及紧密连接蛋白的影响。将NCM460人正常结肠上皮细胞按每孔1×106个细胞种于12孔板中, 细胞贴壁后, 设置空白组、LPS (1 μg·mL-1) 刺激组以及广藿香油、广藿香醇、广藿香酮各给药组(4 μg·mL-1) 孵育24 h。Trizol法提取细胞总RNA, 使用逆转录试剂盒, 按照试剂盒说明书合成cDNA, 进行荧光实时定量PCR检测。借助PubMed Primer Blast设计引物, 引物序列详见表 1。
| Table 1 PCR primer sequence of each gene |
将对数生长期的RAW 264.7巨噬细胞消化, 细胞计数, 以每孔1×105细胞密度接种于6孔板中。设置空白组、LPS组、广藿香醇低剂量组(5 μmol·L-1)、广藿香醇中剂量组(10 μmol·L-1) 和广藿香醇高剂量组(20 μmol·L-1), 加入不同浓度的广藿香醇预处理2 h, 用LPS (100 ng·mL-1) 诱导24 h, 收集细胞进行后续检测实验。提取细胞总RNA, 逆转录合成cDNA, 根据试剂盒说明书进行q-PCR检测, 变性引物序列见表 2。
| Table 2 PCR primer sequences |
统计学分析 使用GraphPad Prism 8软件分析数据。通过t检验及one-way ANOVA进行数据统计学差异分析。数据表示为
前期对广藿香油的化学成分的分析结果见表 3。从TCMSP数据库和Swiss Target Prediction平台共获得236个成分靶点, 去重得到112个靶点。
| Table 3 Information on the main chemical constituents of patchouli oil |
通过DrugBank和GeneCards、OMIM、PharmGkb、TTD数据库检索IBD靶点, 取其并集(图 1A) 并删除重复靶点, 获得IBD有关的疾病靶点5 800个, 将广藿香油的112个靶点和IBD的5 800个靶点取交集, 共获97个共同靶点(图 1B)。

|
Figure 1 Disease target union map (A), the intersection target of patchouli oil and inflammatory bowel disease (IBD) Venn diagram (B) |
将广藿香油活性成分与交集靶点导入到Cytoscape3.7.2软件中进行网络图的绘制和分析, 共有111个节点和207个边(图 2)。节点代表活性成分和靶点, 边代表活性成分与靶点之间的相互作用关系。经Network Analyzer工具计算节点的degree值, 值越大则在网络中越重要。其中排名前5的化合物分别是韦得醇(widdrol)、广藿香酮(pogostone)、α-布藜烯(α-bulnesene)、广藿香醇(patchouli alcohol)、2-甲基-4-(2, 2, 6-三甲基-1-环己烯基)-2-丁烯醛(boronal)。在靶点基因中, 位居前5的靶标是PPARA (peroxisome proliferator-activated receptor alpha)、CNR2 (cannabinoid receptor 2)、GABRA1 (gamma-aminobutyric acid receptor subunit alpha-1)、SLC6A2 (sodium-dependent noradrenaline transporter) 和NR1I3 (nuclear receptor subfamily 1 group I member 3), 其中PPARA、CNR2、GABRA1为degree值≥ 9的关键靶点, 提示这3个靶标对IBD的治疗具有关键作用。
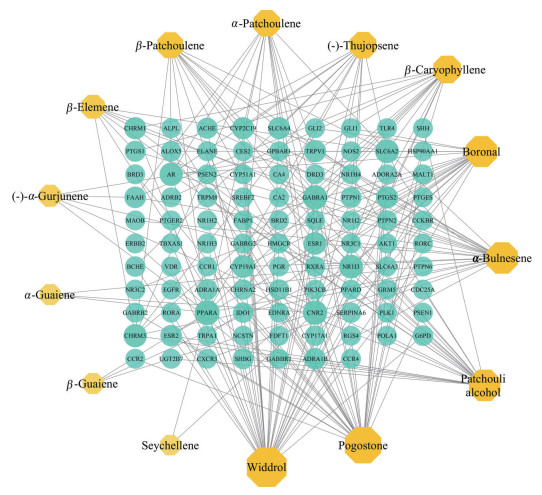
|
Figure 2 Patchouli oil active ingredient-intersection target network diagram |
在String数据库中上传97个交集靶点, 将置信度 > 0.7的数据结果导入到Cytoscape3.7.2软件中进行PPI的网络图的可视化(图 3)。该网络图共有86个节点和201条边, 依据degree值设定节点大小和颜色的深浅, 节点越大表示节点蛋白越重要, 颜色也越深。节点度值前5的是AKT1 (RAC-alpha serine/threonine-protein kinase)、RXRA (retinoic acid receptors alpha)、EGFR (epidermal growth factor receptor)、PTGS2 (prostaglandin-endoperoxide synthase 2) 和HSP90AA1 (heat shock protein 90 alpha family class A member 1), 对应degree值分别是15、14、14、14和12, 这些靶点预测可作为广藿香油治疗IBD的核心靶点。

|
Figure 3 Protein-protein interaction (PPI) network diagram |
本研究对97个交集靶点利用Metascape在线数据库进行分析, 旨在揭示广藿香油治疗IBD的可能作用机制。按P < 0.01富集到18条通路(图 4)。结果表明, 广藿香油治疗IBD主要与雌激素信号通路(estrogen signaling pathway)、黏附连接(adherens junction)、cAMP信号通路(cAMP signaling pathway)、TRP通道的炎性介质调节(inflammatory mediator regulation of TRP channels) 等有关。一个靶点对应多条通路, 一条通路上又存在多个靶点, 因此分析表明广藿香油治疗IBD可能是通过多靶点及多通路调节。另外, 发现广藿香油活性成分也参与了Th17细胞分化、趋化因子、神经活性配体-受体相互作用等多条通路, 提示广藿香油还可治疗其他种类疾病。
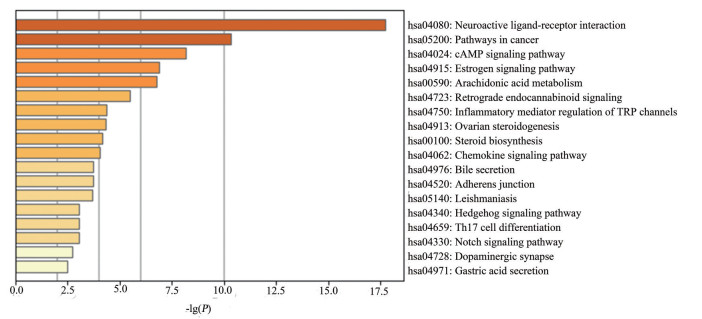
|
Figure 4 Enrichment analysis diagram of Kyoto encyclopedia of genes and genomes (KEGG) pathway in the treatment of IBD by patchouli oil |
使用Metascape在线数据库对广藿香油治疗IBD的97个交集靶点进行GO注释分析, GO富集分析由生物过程(biological process, BP)、细胞组成(cellular component, CC)、分子功能(molecular function, MF) 三部分组成。生物过程主要集中于细胞对有机环状化合物的反应(cellular response to organic cyclic compound)、类固醇代谢过程(steroid metabolic process)、化学突触传递(chemical synaptic transmission) 等方面(图 5); 细胞组成主要包括突触膜(synaptic membrane)、受体复合体(receptor complex)、RNA聚合酶II转录因子复合物(RNA polymerase II transcription factor complex) 等部位(图 6); 分子功能主要涉及类固醇激素受体活性(steroid hormone receptor activity)、激素结合(hormone binding)、神经递质受体活性(neurotransmitter receptor activity) 等方面(图 7)。

|
Figure 5 Bioprocess diagram of patchouli oil in the treatment of IBD |

|
Figure 6 Cell composition diagram of patchouli oil in the treatment of IBD |
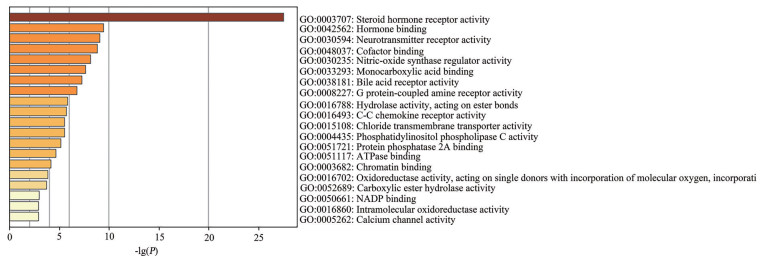
|
Figure 7 Molecular function diagram of patchouli oil in the treatment of IBD |
将广藿香油中活性成分与PPI网络中核心靶点进行Vina分子对接, 结果见表 4。阳性对照药为5-氨基水杨酸, 该药作用于炎症黏膜[27], 对引起炎症的前列腺素合成和炎症介质白三烯形成有抑制作用。广藿香醇、广藿香酮、α-布藜烯、韦得醇等4个活性成分与PTGS2、AKT1、RXRA等5个degree值最高的关键性靶点对接, 结果发现有4组对接(广藿香醇与AKT1、广藿香酮与PTGS2、韦得醇与AKT1、韦得醇与PTGS2) 的结合能小于-7.0 kcal·mol-1, 且结合程度均优于阳性对照药5-氨基水杨酸, 其中广藿香醇与AKT1受体(PDB ID: 6HHF) 结合最稳定。使用PyMol、LigPlot+ v.2.2绘制广藿香醇与AKT1对接模式图(图 8)。由图可知, AKT1与广藿香醇在氨基酸残基Tyr272处形成氢键, 与Val217、Val270、Trp80、Asp292、Asp274、Gln79、Thr82、Asn54形成疏水作用。
| Table 4 Molecular docking results of active components in patchouli oil |
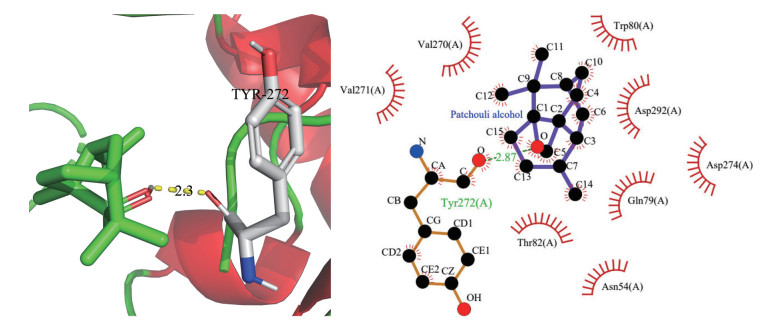
|
Figure 8 2D and 3D models of the interaction between fulmar alcohol and AKT1 protein |
GSE57614共获得2 523个差异基因。其中, 1 227个基因在M1型巨噬细胞中表达上调, 在M2型巨噬细胞中表达下调; 1 296个基因在M1型巨噬细胞中表达下调, 在M2型巨噬细胞中表达上调。图 9的热图显示样本中基因在M1、M2型巨噬细胞中表达量有显著差异。
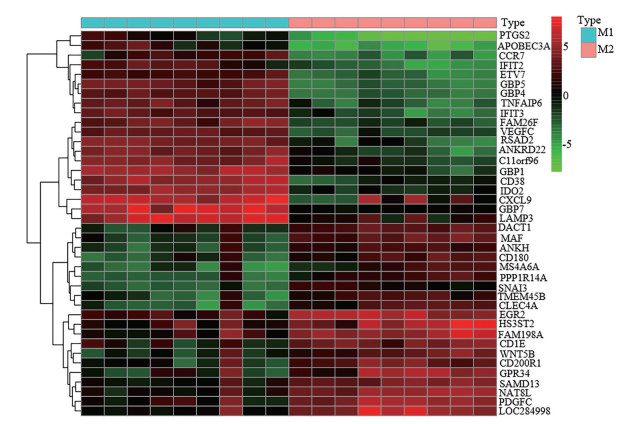
|
Figure 9 The heat map of the differential gene between M1 and M2 macrophages |
差异表达基因富集得到77条信号通路, 炎症反应相关通路主要涉及TNF (tumor necrosis factor)、NF-κB、Jak-STAT (Just another kinase-signal transducer and activator of transcription) 及Toll样受体(Toll-like receptors) 等信号通路, 显著富集通路结果见图 10。
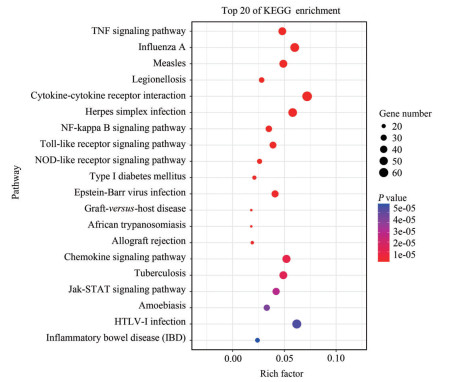
|
Figure 10 The top 20 signal pathways with the most significant differential gene enrichment |
广藿香油、广藿香醇和广藿香酮(4 μg·mL-1) 等剂量实验结果显示, LPS组中促炎细胞因子(TNF-α、IL-1β、IL-6和IL-23) 的mRNA水平均显著高于正常组。采用广藿香油、广藿香醇和广藿香酮处理后均能显著降低细胞因子mRNA水平, 但各给药组无显著性差异, 如图 11所示。此外, LPS组紧密连接蛋白相关mRNA水平均下降, 各给药组均能提高紧密连接蛋白mRNA水平, 但给药组间基本无统计学差异, 其中广藿香酮对紧密连接蛋白的促进作用相对较强, 见图 12。
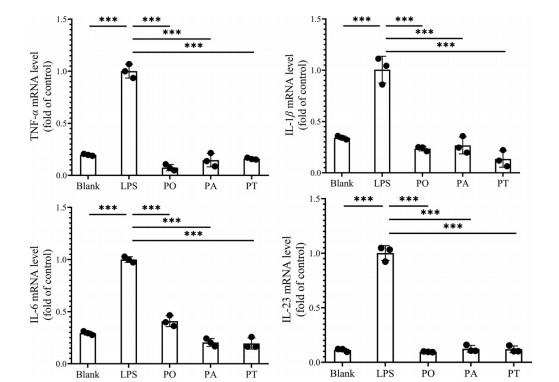
|
Figure 11 The mRNA levels of proinflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-23) by q-PCR. n = 3, |
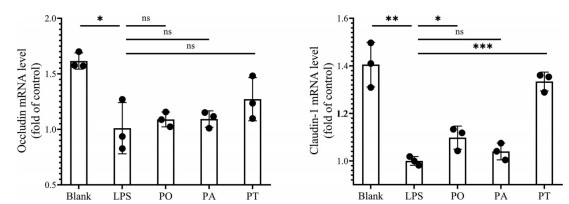
|
Figure 12 The mRNA levels of tight junction protein by q-PCR. n = 3, |
q-PCR结果显示, 经LPS处理后的RAW264.7巨噬细胞TNF-α、IL-6、IL-1β mRNA显著上调(P < 0.001), 见图 13。经广藿香醇处理后, 巨噬细胞炎症因子水平与LPS组相比显著下降; 广藿香醇高剂量组抑制炎症因子能力最强。广藿香醇可以剂量依赖性地抑制炎症因子的表达。
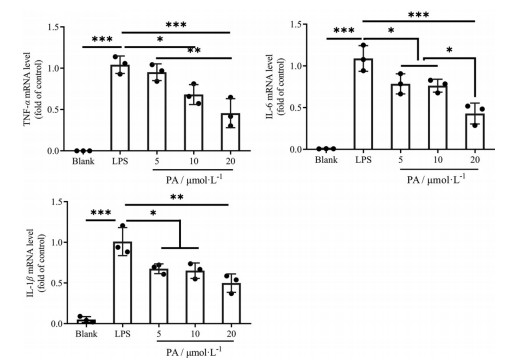
|
Figure 13 The mRNA levels of TNF-α, IL-6, IL-1β by q-PCR. n = 3, |
PPI网络显示AKT1、RXRA、EGFR、PTGS2和HSP90AA1等度值较大, 可能是广藿香油治疗IBD的关键性靶标。郭爱丽[28]研究发现, 在UC和LPS刺激的巨噬细胞中, 类视黄醇X受体α (RXRA) 在基因及蛋白水平表达下降, 提示可能与UC发病有关。核受体RXRA的下调可抑制NF-κB的活性, 介导衰老巨噬细胞中环氧合酶2 (cyclooxygenase-2, COX-2) 的表达和PGE2产生[29]。RXRA与肠道免疫和炎症反应密切相关, 维生素A缺乏大鼠肠道黏膜炎症因子表达水平升高, RXRA mRNA表达水平降低[30]。前列腺素内过氧化物合酶2 (PTGS2), 也称COX-2, 是前列腺素生物合成的关键酶, 几乎不在正常肠黏膜组织表达, 而在IBD中表达明显增强[31], 这种表达在损伤愈合过程中可促进上皮细胞的修复和肠上皮细胞增生。此外, 前列腺素也参与炎症过程, 增强5-脂氧合酶的活性和促进炎性白三烯的释放[32]。据报道, PTGS2抑制剂塞来昔布和罗非昔布对UC患者和动物模型均有效[33]。因此, RXRA和PTGS2可能是广藿香油治疗IBD的重要靶标之一。
KEGG通路富集分析表明, 广藿香油治疗IBD与神经活性配体-受体相互作用、Notch信号通路、TRP通道的炎性介质调节及Th17细胞分化等通路有关。在IBD患者的炎症发病机制中, 肠-脑轴是中枢神经系统和胃肠道之间的重要生理联系[34]。IBD患者存在交感迷走失衡、功能性肠神经缺损和下丘脑-垂体-肾上腺轴活性降低等临床症状, 迷走神经可在系统和肠道局部层面发挥多效的抗炎作用[35]。Chen等[36]研究表明TRP通道与IBD的内脏高敏感性(visceral hypersensitivity, VHS) 和免疫发病机制相关, TRP通道的激活可导致VHS或肠道炎症的改善。Th17细胞是一类能分泌IL-17的CD4+细胞, 参与上皮细胞和中性粒细胞介导的对细胞外微生物的免疫应答以及自身免疫性疾病的发病机制。裴中美等[37]发现UC和CD患者的肠黏膜组织IL-17表达为阳性, 且血浆中IL-17表达水平显著高于对照组, 提示其可能参与IBD的发病过程。Notch信号通路是巨噬细胞重要的分化调节器, 活化Notch信号促使巨噬细胞向M1型极化, 抑制此通路调节巨噬细胞向M2型分化[38]。巨噬细胞极化相关差异基因的KEGG通路富集中, NF-κB信号通路富集了33个基因。课题组前期研究发现, 广藿香醇脂质体能抑制MAPK (mitogen-activated protein kinase) 以及NF-κB信号通路, 降低IL-6和IL-12等促炎细胞因子的释放, 同时该载药系统使M1型巨噬细胞重极化, 转化为M2型巨噬细胞, 缓解结肠炎症[39]。因此, 广藿香油可能是通过激活或抑制以上通路而治疗IBD。从分子对接结合能可知, 与抗炎阳性药5-氨基水杨酸相比, 广藿香醇、广藿香酮、韦得醇、α-布藜烯与5个关键靶点蛋白的结合程度均较为稳定, 推测以上4个成分有抗炎活性[21, 40], 但韦得醇、α-布藜烯仍未见抗炎作用的文献报道。q-PCR实验结果显示, 在广藿香油、广藿香醇和广藿香酮干预LPS诱导的NCM460人正常结肠上皮细胞炎症模型中, 相对于LPS组, 各药物组中TNF-α、IL-1β、IL-6和IL-23的mRNA水平降低, 证实药物对IBD的潜在治疗作用。
本文基于先前研究发现, 靶向巨噬细胞的广藿香醇脂质体对结肠炎有好的治疗效果, 在此借助多种生物信息学手段, 将广藿香油的活性成分进行靶点预测、通路分析, 联合IBD中免疫细胞—巨噬细胞极化差异表达基因, 进一步预测了中药成分群治疗复杂疾病的分子机制, 为广藿香创新药物的成分与机制关联性研究提供了前期基础。
作者贡献: 王冰和张彤负责本文设计思路及讨论; 何毅豪和汪颖舒具体参与成分靶点、疾病靶点、通路分析、分子对接以及巨噬细胞极化差异基因分析工作; 杜瑶瑶进行体外实验并处理数据; 何毅豪负责文章撰写及修改。
利益冲突: 所有作者均声明本文不存在利益冲突。
| [1] |
Yao J, Gao RY, Luo MH, et al. Close homolog of L1-deficient ameliorates inflammatory bowel disease by regulating the balance of Th17/Treg[J]. Gene, 2020, 757: 144931. DOI:10.1016/j.gene.2020.144931 |
| [2] |
Xia B, Deng CS, Wu KC, et al. Inflammatory Bowel Disease (炎症性肠病学)[M]. Beijing: People's Medical Publishing House, 2014: 1-700.
|
| [3] |
Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease[J]. Nature, 2007, 448: 427-434. DOI:10.1038/nature06005 |
| [4] |
Graff LA, Walker JR, Bernstein CN. Depression and anxiety in inflammatory bowel disease: a review of comorbidity and management[J]. Inflamm Bowel Dis, 2009, 15: 1105-1118. DOI:10.1002/ibd.20873 |
| [5] |
Grainger JR, Konkel J, Shaw TN, et al. Macrophages in gastrointestinal homeostasis and inflammation[J]. Pflugers Arch, 2017, 469: 527-539. DOI:10.1007/s00424-017-1958-2 |
| [6] |
Gren ST, Grip O. Role of monocytes and intestinal macrophages in Crohn's disease and ulcerative colitis[J]. Inflamm Bowel Dis, 2016, 22: 1992-1998. DOI:10.1097/MIB.0000000000000824 |
| [7] |
Lissner D, Schumann M, Batra A, et al. Monocyte and M1 macrophage-induced barrier defect contributes to chronic intestinal inflammation in IBD[J]. Inflamm Bowel Dis, 2015, 21: 1297-1305. |
| [8] |
Zhang J, Zhao Y, Hou T, et al. Macrophage-based nanotherapeutic strategies in ulcerative colitis[J]. J Control Release, 2020, 320: 363-380. DOI:10.1016/j.jconrel.2020.01.047 |
| [9] |
Geissmann F, Manz MG, Jung S, et al. Development of monocytes, macrophages, and dendritic cells[J]. Science, 2010, 327: 656-661. DOI:10.1126/science.1178331 |
| [10] |
Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis[J]. Semin Liver Dis, 2010, 30: 245-257. DOI:10.1055/s-0030-1255354 |
| [11] |
Grimm MC, Pullman WE, Bennett GM, et al. Direct evidence of monocyte recruitment to inflammatory bowel disease mucosa[J]. J Gastroenterol Hepatol, 1995, 10: 387-395. DOI:10.1111/j.1440-1746.1995.tb01589.x |
| [12] |
Platt AM, Bain CC, Bordon Y, et al. An independent subset of TLR expressing CCR2-dependent macrophages promotes colonic inflammation[J]. J Immunol, 2010, 184: 6843-6854. DOI:10.4049/jimmunol.0903987 |
| [13] |
Pharmacopoeia Commission of the People's Republic of China. Pharmacopoeia of the People's Republic of China (Part 1) [中华人民共和国药典(一部)] [S]. Beijing: China Medical Science and Technology Press, 2020, 415.
|
| [14] |
Verma RS, Padalia RC, Chauhan A, et al. Chemical composition of leaves, inflorescence, whole aerial-parts and root essential oils of patchouli {Pogostemon cablin (Blanco) Benth}[J]. J Essent Oil Res, 2019, 31: 319-325. DOI:10.1080/10412905.2019.1566100 |
| [15] |
Xian YF, Suo Juan, Huang XD, et al. A Pharmacological study on anti-inflammatory effects of refined Huodan Recipe[J]. Chin J Exp Tradit Med Form (中国实验方剂学杂志), 2007, 13: 54-56. |
| [16] |
Qi SS, Hu LP, Chen WN, et al. Immunological regulation effects of essential oil in leaves of Cablin Patchouli Herb on mice[J]. Chin Arch Tradit Chin Med (中华中医药学刊), 2009, 27: 774-776. |
| [17] |
Yang X, Zhang X, Yang SP, et al. Evaluation of the antibacterial activity of patchouli oil[J]. Iran J Pharm Res, 2013, 12: 307-316. |
| [18] |
Xie XC, Tang F. Protective effect of volatile oil from patchouli on intestinal barrier function[J]. China Tradit Herb Drugs (中草药), 2009, 40: 942-944. |
| [19] |
Wu Z, Zeng H, Zhang L, et al. Patchouli alcohol: a natural sesquiterpene against both inflammation and intestinal barrier damage of ulcerative colitis[J]. Inflammation, 2020, 43: 1423-1435. DOI:10.1007/s10753-020-01219-8 |
| [20] |
Zhao Y, Yang Y, Zhang J, et al. Lactoferrin-mediated macrophage targeting delivery and patchouli alcohol-based therapeutic strategy for inflammatory bowel diseases[J]. Acta Pharm Sin B, 2020, 10: 1966-1976. DOI:10.1016/j.apsb.2020.07.019 |
| [21] |
Su JY. Pogostone Ameliorates TNBS-induced Inflammatory Bowel Disease: An Immunomodulation Study on T Cell (从T细胞角度探讨广藿香酮对炎症性肠病的免疫调节作用) [D]. Guangzhou: Guangzhou University of Chinese Medicine, 2015.
|
| [22] |
Su J, Li C, Yu X, et al. Protective effect of pogostone on 2, 4, 6-trinitrobenzenesulfonic acid-induced experimental colitis via inhibition of T helper cell[J]. Front Pharmacol, 2017, 8: 829. DOI:10.3389/fphar.2017.00829 |
| [23] |
Li YC, Xian YF, Su ZR, et al. Pogostone suppresses proinflammatory mediator production and protects against endotoxic shock in mice[J]. J Ethnopharmacol, 2014, 157: 212-221. DOI:10.1016/j.jep.2014.09.023 |
| [24] |
Liu YH. β-Patchoulene, A Transformation Product of Patchouli Alcohol in Gastric Juice, Protect against Gastric Ulcer and Ulcerative Colitis (广藿香醇胃液代谢物β-广藿香烯抗胃溃疡及溃疡性结肠炎的研究) [D]. Guangzhou: Guangzhou University of Chinese Medicine, 2018.
|
| [25] |
Zhou Y, Zhou B, Pache L, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets[J]. Nat Commun, 2019, 10: 1523. DOI:10.1038/s41467-019-09234-6 |
| [26] |
Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets[J]. Nucleic Acids Res, 2019, 47: D607-D613. DOI:10.1093/nar/gky1131 |
| [27] |
Bo YZ, Luo J, Miao YL. Research progress and chemoprophylaxis of 5-aminosalicylic acid in inflammatory bowel disease[J]. J Intern Med (内科理论与实践), 2017, 12: 205-208. |
| [28] |
Guo AL. Retinoic Acid Receptor Alpha and Retinoid X Receptor Alpha Status in Human Ulcerative and Murine Colitis (维甲酸受体α和类视黄醇X受体α在溃疡性结肠炎患者和小鼠结肠炎模型中的表达及其在炎症中的作用) [D]. Wuhan: Huazhong University of Science and Technology, 2016.
|
| [29] |
Chen H, Ma F, Hu X, et al. Elevated COX2 expression and PGE2 production by downregulation of RXRα in senescent macrophages[J]. Biochem Biophys Res Commun, 2013, 440: 157-162. DOI:10.1016/j.bbrc.2013.09.047 |
| [30] |
Dong P, Tao Y, Yang Y, et al. Expression of retinoic acid receptors in intestinal mucosa and the effect of vitamin A on mucosal immunity[J]. Nutrition, 2010, 26: 740-745. DOI:10.1016/j.nut.2009.08.011 |
| [31] |
Xu CM, Dong WG, Yu BP, et al. Expression of HIF-1α and COX-2 protein in inflammatory bowel disease[J]. J Clin Med (临床内科杂志), 2005, 22: 82-84. |
| [32] |
Ferrer MD, Busquets-Cortés C, Capó X, et al. Cyclooxygenase-2 inhibitors as a therapeutic target in inflammatory diseases[J]. Curr Med Chem, 2019, 26: 3225-3241. DOI:10.2174/0929867325666180514112124 |
| [33] |
Mahadevan U, Loftus EV, Tremaine WJ, et al. Safety of selective cyclooxygenase-2 inhibitors in inflammatory bowel disease[J]. Am J Gastroenterol, 2002, 97: 910-914. DOI:10.1111/j.1572-0241.2002.05608.x |
| [34] |
Gracie DJ, Hamlin PJ, Ford AC. The influence of the brain-gut axis in inflammatory bowel disease and possible implications for treatment[J]. Lancet Gastroenterol Hepatol, 2019, 4: 632-642. DOI:10.1016/S2468-1253(19)30089-5 |
| [35] |
Tamara M, Rebecca B, Qasim A, et al. The role of the autonomic nervous system in the pathogenesis and therapy of IBD[J]. Aliment Pharmacol Ther, 2019, 50: 720-737. DOI:10.1111/apt.15433 |
| [36] |
Chen YD, Mu JX, Zhu M, et al. Transient receptor potential channels and inflammatory bowel disease[J]. Front Immunol, 2020, 11: 180. DOI:10.3389/fimmu.2020.00180 |
| [37] |
Pei ZM, Zhao ZY, Xiao JB, et al. Expression and significance of interleukin-17 in plasma and intestinal mucosa of patients with active inflammatory bowel disease[J]. Gastroenterology (胃肠病学), 2016, 21: 549-553. |
| [38] |
Li HR, Sun Y, Chang CC, et al. Notch signaling pathway with the polarization of macrophages[J]. J Med Postgra (医学研究生学报), 2015, 28: 1316-1321. |
| [39] |
Zhao YG. Study on the Lactoferrin-modified Patchouli Alcohol Liposomes Forcolitis Treatment (乳铁蛋白修饰广藿香醇脂质体治疗结肠炎的研究) [D]. Nanchang: Nanchang University, 2020.
|
| [40] |
He JJ. Experimental Study on Anti-inflammatory, Anti-allergic and Immunomodulatory Activities of Patchouli Oil and Pogostone (广藿香油和广藿香酮的抗炎抗过敏和免疫调节作用研究) [D]. Guangzhou: Guangzhou University of Chinese Medicine, 2013.
|
 2021, Vol. 56
2021, Vol. 56


