2. 药物靶点研究与药效学评价湖北省重点实验室, 湖北 武汉 430030
2. Hubei Key Laboratory of Drug Targeting and Pharmacokinetic Evaluation, Wuhan 430030, China
二甲双胍源于Galega officinalis L., 名称为山羊豆, 该草药含有丰富的胍类化合物。1918年, 二甲双胍被证明可降低血糖[1], 目前被推荐为治疗2型糖尿病的一线药物[2]。其主要通过有机阳离子转运蛋白1 (organic cation transporter 1, OCT1) 转运到肝细胞并作用于线粒体[3, 4], 继而通过腺苷酸活化蛋白激酶[adenosine 5'-monophosphate (AMP)-activated protein kinase, AMPK] 依赖途径和非AMPK依赖途径共同抑制胞内糖异生以调节血糖[5] (图 1)。
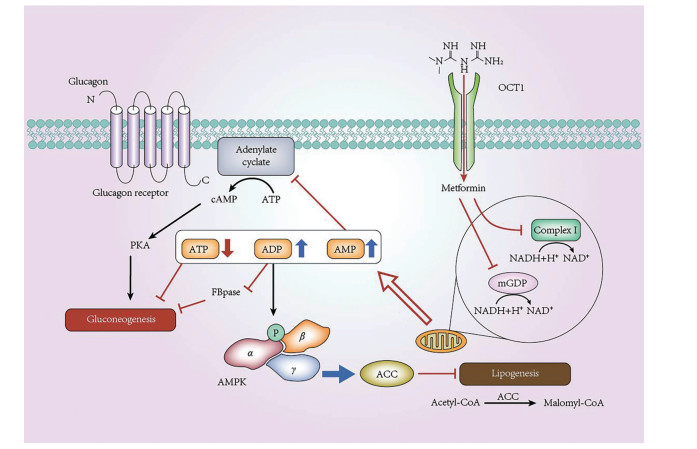
|
Figure 1 Molecular mechanism of metformin inhibition effects in hepatocyte gluconeogenesis. Metformin enters hepatocytes and then partially inhibits the intracellular mitochondrial respiratory chain complex Ⅰ, one of the most important proteins in the intracellular mitochondrial NADH respiratory chain, leading to a decrease in the transformation from ADP to ATP, which results low-generation of ATP and accumulation of ADP and AMP. ATP deficiency directly inhibits gluconeogenesis; high level of ADP reduces the activity of FBPase, the key enzyme in gluconeogenesis, to inhibit gluconeogenesis; elevated level of AMP inhibits adenylate cyclase and blocks the cAMP-PKA pathway of gluconeogenesis. At the same time, metformin decreases mGPD activity in the mitochondria, resulting in the alteration of redox states and reduction of NADH/H+ producing to inhibit gluconeogenesis. The change in AMP/ATP ratio mediated by metformin also activates AMPK and reduces ACC activity, which inhibits lipid synthesis and enhances cellular sensitivity to insulin. ATP: Adenosine triphosphate; ADP: Adenosine diphosphate; AMP: Adenosine monophosphate; NADH: Nicotinamide adenine dinucleotide; FBPase: Fructose-1, 6-bisphosphatase; mGPD: Mitochondrial glycerol phosphate dehydrogenase; ACC: Acetyl coenzyme A carboxylase; cAMP: Cyclic AMP; PKA: Protein kinase A; AMPK: Adenosine 5'-monophosphate-activated protein kinase; OCT1: Organic cation transporter 1 |
研究发现, 二甲双胍除降低血糖外, 还有其他的药理作用。如二甲双胍对多囊卵巢综合征、糖尿病肾病和妊娠期糖尿病、代谢综合征如非酒精性脂肪性肝病有一定疗效[6]。二甲双胍靶向衰老(targeting ageing with metformin, TAME) 的研究计划正在进行中, 旨在观察二甲双胍延缓衰老的作用[7]。近年来, 二甲双胍降低罹患癌症风险和改善癌症预后[3, 8], 对多种中枢神经系统疾病的改善作用[9]及对肠道菌群的调节作用受到越来越多的关注。因此, 本文围绕最新发现的二甲双胍药理作用及其机制进行综述。
1 二甲双胍对神经精神疾病的治疗作用 1.1 二甲双胍对焦虑障碍的治疗作用焦虑障碍是目前临床上最常见的心境障碍性疾病之一, 目前主要治疗药物为苯二氮䓬类、选择性5-羟色胺再摄取抑制药和选择性5-羟色胺/去甲肾上腺素再摄取抑制药等, 但存在药物依赖性、起效慢等不良反应[10]。
相关证据表明, 二甲双胍对原发性焦虑有较好的缓解效果。Fan等[11]在动物实验中发现大鼠腹腔注射二甲双胍(100 mg·kg-1) 30 min后即可产生快速的抗焦虑作用, 且与地西泮相比, 该作用不产生耐受性和成瘾性, 也不影响正常大鼠的血糖水平。其机制是二甲双胍通过激活海马脑区AMPK, 增加人类叉头框O蛋白3a (forkhead box class O 3a, FOXO3a) 表达, 后者与γ-氨基丁酸A型受体相关蛋白(gamma-aminobutyric acid receptor-associated protein, GABARAP) 启动子结合, 促进GABARAP的表达, 并增加GABARAP与γ-氨基丁酸A型受体(gamma-aminobutyric acid type A receptor, GABAA receptor) 的结合, 最终使GABAA受体在细胞膜表达增加, 从而增大微小抑制性突触后电流(miniature inhibitory postsynaptic currents, mIPSCs) 幅度, 提高抑制性突触传递效率来发挥抗焦虑作用。
此外, 二甲双胍对继发性焦虑也有较好的治疗效果。有研究指出脑缺血后焦虑症及抑郁症发病率会增加[12]。Vicentini等[13]报道了全脑缺血/再灌注损伤通过增加大脑氧化应激而促进焦虑样行为和抑郁样行为。给予二甲双胍(200 mg·kg-1) 14天可改善脑缺血大鼠的认知功能、焦虑和抑郁样行为[14]。同时, Ge等[15]发现从缺血/再灌注15 min开始给予二甲双胍(200 mg·kg-1·day-1) 可减少缺血大鼠海马脑区神经元死亡, 这与二甲双胍促进RAC-α丝氨酸/苏氨酸蛋白激酶(RAC-alpha serine/threonine-protein kinase, AKT1) 和磷脂酰肌醇3-激酶(phosphatidylinositol 3-kinase, PI3K) 蛋白表达, 从而抑制c-Jun氨基端激酶3 (c-Jun N-terminal kinase 3, JNK3) 磷酸化, 阻止胱天蛋白酶-3 (cysteine-requiring aspartate protease 3, caspase-3) 激活, 减少缺血诱导的细胞凋亡, 发挥神经保护作用有关[16]。
二甲双胍对其他疾病伴发的焦虑症也有治疗作用。如对于伴有焦虑和抑郁的糖尿病患者, 给予二甲双胍在降血糖的同时, 还可有效改善患者焦虑及抑郁表现, 增强其认知功能[17-21]。对于甲基苯丙胺成瘾患者伴发的焦虑和抑郁情况, 给予二甲双胍有缓解作用[17, 22]; 二甲双胍亦可改善患有多囊卵巢综合征的青春期或成年女性患者的焦虑等情绪障碍[23], 但其具体机制仍需进一步研究。
1.2 二甲双胍对阿尔茨海默病的治疗作用及争议流行病学资料显示, 2000~2010年台湾地区2型糖尿病(type 2 diabetes mellitus, T2DM) 或肥胖等代谢性疾病的患者中, 阿尔茨海默病(Alzheimer's disease, AD) 的发病率(11.00%~13.75%) 明显高于正常人(4.17%~6.09%)[24, 25]。Moreno-Gonzalez等[26]提出胰岛中的胰岛淀粉样多肽和大脑中的β淀粉样蛋白(β-amyloid protein, Aβ) 的错误折叠相互作用, 加速AD和T2DM的发生发展。此外, 胰岛素信号异常、线粒体功能障碍、能量稳态异常[27]等也可能导致T2DM和AD共患病。
Tu和Wahlqvist等[24, 25]在队列研究中均发现, T2DM患者联合使用磺酰脲类药物与二甲双胍可显著降低AD发生风险率约60%; 在动物实验[28, 29]中发现低剂量二甲双胍(20 mg·kg-1·day-1) 可降低淀粉样前体蛋白(amyloid precursor protein, APP) 和tau蛋白含量, 改善AD模型小鼠的记忆力, 为二甲双胍用于AD治疗提供实验依据。
但有资料显示, 二甲双胍可能会增加AD的发病风险。Picone等[30]报道使用50 mmol·L-1二甲双胍处理LAN5神经母细胞瘤细胞24 h后, 显著增加胞内APP和早老素含量, 使APP切割增加, 进而促进Aβ在细胞内聚集, 形成寡聚物或不溶性斑块或神经纤维缠结[31]。此外, 二甲双胍可激活核因子κB (nuclear factor kappa-B, NF-κB), 促进APP和早老素基因表达[30], APP会进入线粒体并阻塞线粒体外膜转位酶40 (translocases of the outer mitochondrial membrane 40, TOM40) 孔, 破坏线粒体氧化还原平衡, 引起线粒体功能障碍[32]。动物实验中, Picone等[30]连续使用高剂量二甲双胍(300 mg·kg-1·day-1) 7天可显著增加小鼠神经元内APP和早老素信使RNA (messenger RNA, mRNA) 的表达, 进一步表明高剂量二甲双胍可能会增加AD的发病风险。
因此, 目前对于二甲双胍在AD中的作用研究仍存在一定争议, 具体机制尚不明确, 其确切疗效仍有待进一步验证。
2 二甲双胍对心血管疾病的作用糖尿病是心血管系统疾病的危险因素之一, 二甲双胍在降低血糖的同时可降低心血管疾病的发生率。Hong等[33]报道相较于格列吡嗪, 连续服用二甲双胍3年能显著减少心血管事件发生率。最近研究发现[34-36], 二甲双胍有独立于血糖调节的改善心脏能量效率的作用。其在增加胰岛素抵抗的心力衰竭患者的心肌效率(每搏功/心肌耗氧量) 的同时, 可直接提高对胰岛素敏感性正常的心力衰竭患者的射血分数[35]。二甲双胍在维持慢性心衰患者每搏功的基础上降低心肌耗氧量, 从而提高心衰患者的心肌效率[34]。以上均提示二甲双胍对心脏能量效率的改善作用不依赖于血糖和代谢调节。同时, Cittadini等[35]指出二甲双胍不改变心肌能量底物的循环水平或全身呼吸交换率, 即二甲双胍对底物氧化改变并不是其提高心肌效率的原因, 同时其对左室收缩功能的具体影响也有待进一步研究。因此, 二甲双胍改善心脏能量效率的机制有待进一步研究。
二甲双胍对心衰的治疗效果存在明显的个体差异, 表现出一定的年龄和性别依赖性[34], 这可能是由于不同年龄和性别的个体激素分泌水平的差异影响二甲双胍的药物代谢动力学, 提示应用二甲双胍时需全面考虑疾病、衰老和损伤状态下的激素水平调节对治疗效果的影响。
值得一提的是, 个体遗传药物代谢动力学的差异也不可忽略。编码二甲双胍膜转运蛋白基因, 即OCT基因和MATE基因的遗传变异, 可能影响二甲双胍的药代动力学, 即随着MATE基因变异数的增加, 二甲双胍药物代谢减慢[34]。OCT基因和MATE基因等二甲双胍代谢相关基因的突变可能会抑制其对心输出量的改善作用, 减弱二甲双胍对心衰的治疗效果, 表明进一步探索药物遗传学对二甲双胍诱导的心脏效应的影响具有重要的临床意义。
3 二甲双胍对肿瘤的治疗作用回顾性研究显示, 二甲双胍可抑制肿瘤的发生和发展, 降低T2DM患者罹患肿瘤风险, 改善肿瘤患者的预后并延长生存期[37]。相较于常规抗肿瘤治疗, 联合使用二甲双胍治疗体重指数较大的非小细胞肺癌患者, 其生存率更高[38]。Wang等[39]指出使用二甲双胍的人群患食管鳞状细胞癌(esophageal squamous cell carcinoma, ESCC) 的风险显著降低, 尤其是在二甲双胍的新近使用者中, ESCC风险降低得更为明显, 提示短期使用二甲双胍的抗肿瘤效果更为明显。二甲双胍亦可和其他药物或疗法合用, 发挥协同抗肿瘤疗效。如二甲双胍与多柔比星联合治疗乳腺癌具有较好的增效减毒作用[40]; 当二甲双胍联合二氯乙酸靶向和放射治疗可降低线粒体DNA含量, 对儿童胶质瘤有良好的疗效[41]; 若二甲双胍联合奥沙利铂和光动力疗法可减轻胰腺癌中肿瘤相关成纤维细胞诱导的对吉西他滨靶向治疗耐药作用[42]; 二甲双胍和新型光敏剂IR780在采用PEG-PCL脂质体包装后进入胃癌组织内, 联合应用808 nm激光照射肿瘤病灶, 产生活性氧, 并释放二甲双胍和IR780, 抑制癌细胞线粒体的电子传递链, 使氧化磷酸化被抑制起到协同抗肿瘤效果[43]; 同时, 当二甲双胍和间歇性进食诱导的低血糖联合使用时可显著抑制肿瘤生长, 这提示二甲双胍在低糖环境中可具有抗肿瘤作用[44]。
二甲双胍可能是通过抑制炎症反应、调节机体免疫系统以及直接抑制肿瘤细胞生长来发挥抗肿瘤作用。Morales等[45]发现二甲双胍激活AMPK抑制巨噬细胞和脂肪细胞等多种细胞中的促炎细胞因子, 如肿瘤坏死因子α (tumor necrosis factor α, TNF-α)、白介素6 (interleukin-6, IL-6)、白介素8 (interleukin-8, IL-8) 和血管内皮生长因子等, 该抑制作用与NF-κB、信号转导及转录激活蛋白3 (signal transducer and activator transcription 3, STAT3) 和缺氧诱导因子1β (hypoxia inducible factor-1β, HIF-1β) 的失活有关[46]。此外, 二甲双胍增加CD8+肿瘤浸润淋巴细胞的数量[47], 促进CD8+记忆T细胞的生成[48], 并降低调节性T细胞的免疫抑制作用[49], 进而抑制肿瘤进展和转移[50]。二甲双胍还可通过AMPK途径阻断肿瘤前表型巨噬细胞M2的极化, 抑制肿瘤进展[51]。
大多数肿瘤细胞通过糖酵解产生ATP[52], 而AMPK的沉默可促使肿瘤细胞在有氧条件下更倾向于进行有氧糖酵解(Warburg效应)[53]。二甲双胍通过激活AMPK, 对抗Warburg效应而抑制肿瘤细胞进行氧化磷酸化, 因此可作为代谢性肿瘤抑制药[54-56]。此外, 二甲双胍通过激活AMPK, 抑制脂肪合成酶, 如脂肪酸合酶[57]或乙酰基辅酶A羧化酶[58, 59]来抑制肿瘤细胞的脂质生物合成途径, 从而抑制乳腺癌和结肠癌的发生发展。此外, 二甲双胍激活AMPK也可使哺乳动物雷帕霉素靶蛋白敏感复合体1 (mammalian target of rapamycin complex 1, mTORC1) 受到抑制[60-63], 从而减少缺氧诱导因子1 (hypoxia inducible factor-1, HIF-1) 和血管内皮生长因子的合成, 使肿瘤细胞从代谢活跃转为能量储存, 起到抑制细胞生长作用[64]。
4 二甲双胍对肠道菌群的影响肠道菌群在心血管疾病、代谢性疾病、消化系统疾病以及肿瘤等疾病的发生发展中发挥关键作用。二甲双胍对上述疾病的治疗作用可能与其对肠道菌群的调节有关。临床试验发现[65, 66], 糖尿病患者口服二甲双胍后其粪便样本中大肠杆菌含量显著增加, 对胃肠功能有一定的改善作用, 包括改善粪便形态、促进肠胃蠕动、抑制病原菌在肠道内繁殖等方面。Wu等[65]观察到二甲双胍治疗2型糖尿病时, 用药者胃肠道内双歧杆菌的数量较安慰剂组有所增加。在荟萃分析研究[67]中发现肠道内乳酸杆菌属、双歧杆菌属数量的增加可改善2型糖尿病患者的高血压并降低血清胆固醇水平。此外, 乳酸杆菌属、双歧杆菌属可促进人巨噬细胞、树突状细胞成熟, 并从多途径调节各种细胞因子的产生以发挥免疫调节作用, 这可能与二甲双胍减少癌前病变发生有关[68, 69]。双歧杆菌和乳酸菌等肠道细菌则能抑制病原菌的生长, 合成人体需要的维生素, 促进人体对矿物质的吸收, 产生醋酸、丙酸、丁酸和乳酸等有机酸刺激肠道蠕动, 促进排便, 防止便秘以及抑制肠道蛋白质的腐败作用、激活免疫系统从而提高抗病能力等方面有着重要作用。
免疫球蛋白A (immunoglobulin A, IgA) 是调节肠道内稳态、缓解炎症发生的关键分子。Luck等[70]在对肥胖小鼠的治疗中发现, 二甲双胍可提高小鼠体内IgA合成分泌水平, 分泌型IgA能抑制细菌对肠道黏膜的黏附作用、中和毒素、灭活酶和病毒, 同时对革兰阴性杆菌具有特殊的亲和力, 对部分抗原物质具有封闭作用, 并能诱导嗜酸性、嗜碱性粒细胞脱颗粒作用和抗体依赖细胞介导的细胞毒作用。这提示二甲双胍可能是通过增加分泌型IgA水平, 进而提高肠道通透性, 改善肠道黏膜屏障功能以及调节免疫系统功能。
5 小结与展望二甲双胍作为治疗2型糖尿病的一线药物, 越来越多的研究发现其降血糖以外的临床应用, 包括二甲双胍对原发性、继发性以及其他疾病伴发的焦虑均有良好的治疗效果; 可有效延缓心衰患者进程, 降低T2DM患者罹患肿瘤风险, 改善肿瘤患者的预后并延长生存期; 调节人体肠道菌群而对多种疾病起到改善作用。但在研究过程中也发现一系列矛盾的结果, 如小剂量和大剂量二甲双胍分别对AD呈现出截然相反的作用效果。因此, 未来仍需深入揭示二甲双胍对AD的作用机制, 通过设计个性化用药方案及合理的临床试验以明确远期预后。
此外, 二甲双胍作用位点涉及多种疾病的交叉通路, 这可能使其在研究多种疾病并发机制及临床治疗中更具研究价值。必须指出的是, 在研究二甲双胍对其他疾病的治疗效果中, 2型糖尿病与这些疾病的关联性是不可忽视的, 这提示利用二甲双胍的多效性可为个体化治疗多种合并症提供新思路。二甲双胍的多效性可能与其可部分激活免疫系统以诱导相关免疫调节有关[71], 但其对免疫调节的具体机制仍未明确, 能否应用于肿瘤的联合免疫治疗仍需进一步的临床前瞻性研究。但毋庸置疑的是, 在后续研究支持下, 二甲双胍在其他多系统疾病治疗方面定有更广阔的临床前景。
作者贡献: 肖文铉负责文章构思及布局、文献检索、文章撰写、制图和文章修改; 罗雅丹负责文章撰写和文章修改; 陈建国和王芳进行文章的构思、布局和文章修改。
利益冲突: 所有作者均声明无利益冲突。
| [1] |
Bailey CJ, Day C. Avandamet: combined metformin-rosiglitazone treatment for insulin resistance in type 2 diabetes[J]. Int J Clin Pract, 2004, 58: 867-876. DOI:10.1111/j.1742-1241.2004.00318.x |
| [2] |
American Diabetes A. Standards of medical care in diabetes--2014[J]. Diabetes Care, 2014, 37 Suppl 1: S14-S80. |
| [3] |
Viollet B, Guigas B, Sanz Garcia N, et al. Cellular and molecular mechanisms of metformin: an overview[J]. Clin Sci (Lond), 2012, 122: 253-270. DOI:10.1042/CS20110386 |
| [4] |
Vancura A, Bu P, Bhagwat M, et al. Metformin as an anticancer agent[J]. Trends Pharmacol Sci, 2018, 39: 867-878. DOI:10.1016/j.tips.2018.07.006 |
| [5] |
Foretz M, Guigas B, Bertrand L, et al. Metformin: from mechanisms of action to therapies[J]. Cell Metab, 2014, 20: 953-966. DOI:10.1016/j.cmet.2014.09.018 |
| [6] |
Vassilatou E. Nonalcoholic fatty liver disease and polycystic ovary syndrome[J]. World J Gastroenterol, 2014, 20: 8351-8363. DOI:10.3748/wjg.v20.i26.8351 |
| [7] |
Barzilai N, Crandall JP, Kritchevsky SB, et al. Metformin as a tool to target aging[J]. Cell Metab, 2016, 23: 1060-1065. DOI:10.1016/j.cmet.2016.05.011 |
| [8] |
Pollak MN. Investigating metformin for cancer prevention and treatment: the end of the beginning[J]. Cancer Discov, 2012, 2: 778-790. DOI:10.1158/2159-8290.CD-12-0263 |
| [9] |
Ashabi G, Khodagholi F, Khalaj L, et al. Activation of AMP-activated protein kinase by metformin protects against global cerebral ischemia in male rats: interference of AMPK/PGC-1α pathway[J]. Metab Brain Dis, 2014, 29: 47-58. DOI:10.1007/s11011-013-9475-2 |
| [10] |
Bandelow B, Michaelis S, Wedekind D. Treatment of anxiety disorders[J]. Dialogues Clin Neurosci, 2017, 19: 93-107. DOI:10.31887/DCNS.2017.19.2/bbandelow |
| [11] |
Fan J, Li D, Chen HS, et al. Metformin produces anxiolytic-like effects in rats by facilitating GABAA receptor trafficking to membrane[J]. Br J Pharmacol, 2019, 176: 297-316. DOI:10.1111/bph.14519 |
| [12] |
Sarkaki A, Farbood Y, Badavi M, et al. Metformin improves anxiety-like behaviors through AMPK-dependent regulation of autophagy following transient forebrain ischemia[J]. Metab Brain Dis, 2015, 30: 1139-1150. DOI:10.1007/s11011-015-9677-x |
| [13] |
Vicentini JE, Weiler M, Almeida SRM, et al. Depression and anxiety symptoms are associated to disruption of default mode network in subacute ischemic stroke[J]. Brain Imaging Behav, 2017, 11: 1571-1580. DOI:10.1007/s11682-016-9605-7 |
| [14] |
Fatemi I, Saeed-Askari P, Hakimizadeh E, et al. Long-term metformin therapy improves neurobehavioral functions and antioxidative activity after cerebral ischemia/reperfusion injury in rats[J]. Brain Res Bull, 2020, 163: 65-71. DOI:10.1016/j.brainresbull.2020.07.015 |
| [15] |
Ge XH, Zhu GJ, Geng DQ, et al. Metformin protects the brain against ischemia/reperfusion injury through PI3K/Akt1/JNK3 signaling pathways in rats[J]. Physiol Behav, 2017, 170: 115-123. DOI:10.1016/j.physbeh.2016.12.021 |
| [16] |
Qi D, Liu H, Niu J, et al. Heat shock protein 72 inhibits c-Jun N-terminal kinase 3 signaling pathway via Akt1 during cerebral ischemia[J]. J Neurol Sci, 2012, 317: 123-129. DOI:10.1016/j.jns.2012.02.011 |
| [17] |
Jiang T, Yu JT, Zhu XC, et al. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy[J]. Br J Pharmacol, 2014, 171: 3146-3157. DOI:10.1111/bph.12655 |
| [18] |
Guo M, Mi J, Jiang QM, et al. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus[J]. Clin Exp Pharmacol Physiol, 2014, 41: 650-656. |
| [19] |
Pintana H, Apaijai N, Pratchayasakul W, et al. Effects of metformin on learning and memory behaviors and brain mitochondrial functions in high fat diet induced insulin resistant rats[J]. Life Sci, 2012, 91: 409-414. DOI:10.1016/j.lfs.2012.08.017 |
| [20] |
Zhao RR, Xu XC, Xu F, et al. Metformin protects against seizures, learning and memory impairments and oxidative damage induced by pentylenetetrazole-induced kindling in mice[J]. Biochem Biophys Res Commun, 2014, 448: 414-417. DOI:10.1016/j.bbrc.2014.04.130 |
| [21] |
Kessing LV, Rytgaard HC, Ekstrom CT, et al. Antidiabetes agents and incident depression: a nationwide population-based study[J]. Diabetes Care, 2020, 43: 3050-3060. DOI:10.2337/dc20-1561 |
| [22] |
El-Mir MY, Detaille D, R-Villanueva G, et al. Neuroprotective role of antidiabetic drug metformin against apoptotic cell death in primary cortical neurons[J]. J Mol Neurosci, 2008, 34: 77-87. DOI:10.1007/s12031-007-9002-1 |
| [23] |
Erensoy H, Niafar M, Ghafarzadeh S, et al. A pilot trial of metformin for insulin resistance and mood disturbances in adolescent and adult women with polycystic ovary syndrome[J]. Gynecol Endocrinol, 2019, 35: 72-75. DOI:10.1080/09513590.2018.1498476 |
| [24] |
Tu HP, Lin CH, Hsieh HM, et al. Prevalence of anxiety disorder in patients with type 2 diabetes: a nationwide population-based study in Taiwan 2000-2010[J]. Psychiatr Q, 2017, 88: 75-91. DOI:10.1007/s11126-016-9436-0 |
| [25] |
Wahlqvist ML, Lee MS, Chuang SY, et al. Increased risk of affective disorders in type 2 diabetes is minimized by sulfonylurea and metformin combination: a population-based cohort study[J]. BMC Med, 2012, 10: 150. DOI:10.1186/1741-7015-10-150 |
| [26] |
Moreno-Gonzalez I, Edwards Iii G, Salvadores N, et al. Molecular interaction between type 2 diabetes and Alzheimer's disease through cross-seeding of protein misfolding[J]. Mol Psychiatry, 2017, 22: 1327-1334. DOI:10.1038/mp.2016.230 |
| [27] |
Sims-Robinson C, Kim B, Rosko A, et al. How does diabetes accelerate Alzheimer disease pathology?[J]. Nat Rev Neurol, 2010, 6: 551-559. DOI:10.1038/nrneurol.2010.130 |
| [28] |
Farr SA, Roesler E, Niehoff ML, et al. Metformin improves learning and memory in the SAMP8 mouse model of Alzheimer's disease[J]. J Alzheimers Dis, 2019, 68: 1699-1710. DOI:10.3233/JAD-181240 |
| [29] |
Pera M, Larrea D, Guardia-Laguarta C, et al. Increased localization of APP-C99 in mitochondria-associated ER membranes causes mitochondrial dysfunction in Alzheimer disease[J]. EMBO J, 2017, 36: 3356-3371. DOI:10.15252/embj.201796797 |
| [30] |
Picone P, Nuzzo D, Caruana L, et al. Metformin increases APP expression and processing via oxidative stress, mitochondrial dysfunction and NF-κB activation: use of insulin to attenuate metformin's effect[J]. Biochim Biophys Acta, 2015, 1853: 1046-1059. DOI:10.1016/j.bbamcr.2015.01.017 |
| [31] |
Selkoe DJ. The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer's disease[J]. Trends Cell Biol, 1998, 8: 447-453. DOI:10.1016/S0962-8924(98)01363-4 |
| [32] |
Hayden KM, McEvoy JM, Linnertz C, et al. A homopolymer polymorphism in the TOMM40 gene contributes to cognitive performance in aging[J]. Alzheimers Dement, 2012, 8: 381-388. DOI:10.1016/j.jalz.2011.10.005 |
| [33] |
Hong J, Zhang Y, Lai S, et al. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease[J]. Diabetes Care, 2013, 36: 1304-1311. DOI:10.2337/dc12-0719 |
| [34] |
Larsen AH, Jessen N, Norrelund H, et al. A randomised, double-blind, placebo-controlled trial of metformin on myocardial efficiency in insulin-resistant chronic heart failure patients without diabetes[J]. Eur J Heart Fail, 2020, 22: 1628-1637. DOI:10.1002/ejhf.1656 |
| [35] |
Cittadini A, Napoli R, Monti MG, et al. Metformin prevents the development of chronic heart failure in the SHHF rat model[J]. Diabetes, 2012, 61: 944-953. DOI:10.2337/db11-1132 |
| [36] |
Ruddy RM, Adams KV, Morshead CM. Age- and sex-dependent effects of metformin on neural precursor cells and cognitive recovery in a model of neonatal stroke[J]. Sci Adv, 2019, 5: eaax1912. DOI:10.1126/sciadv.aax1912 |
| [37] |
Quinn BJ, Kitagawa H, Memmott RM, et al. Repositioning metformin for cancer prevention and treatment[J]. Trends Endocrinol Metab, 2013, 24: 469-480. DOI:10.1016/j.tem.2013.05.004 |
| [38] |
Yendamuri S, Barbi J, Pabla S, et al. Body mass index influences the salutary effects of metformin on survival after lobectomy for stage I NSCLC[J]. J Thorac Oncol, 2019, 14: 2181-2187. DOI:10.1016/j.jtho.2019.07.020 |
| [39] |
Wang QL, Santoni G, Ness-Jensen E, et al. Association between metformin use and risk of esophageal squamous cell carcinoma in a population-based cohort study[J]. Am J Gastroenterol, 2020, 115: 73-78. DOI:10.14309/ajg.0000000000000478 |
| [40] |
Zheng S, Yu JD, Chen LY, et al. Preparation of lipid membrane-wrapped nanoparticle loaded with metformin polymer and doxorubicin and evaluation of their therapeutic effect on breast cancer[J]. Acta Pharm Sin (药学学报), 2019, 54: 2316-2325. |
| [41] |
Shen H, Yu M, Tsoli M, et al. Targeting reduced mitochondrial DNA quantity as a therapeutic approach in pediatric high-grade gliomas[J]. Neuro Oncol, 2020, 22: 139-151. DOI:10.1093/neuonc/noz140 |
| [42] |
Han H, Hou Y, Chen X, et al. Metformin-induced stromal depletion to enhance the penetration of gemcitabine-loaded magnetic nanoparticles for pancreatic cancer targeted therapy[J]. J Am Chem Soc, 2020, 142: 4944-4954. DOI:10.1021/jacs.0c00650 |
| [43] |
Yang Z, Wang J, Liu S, et al. Defeating relapsed and refractory malignancies through a nano-enabled mitochondria-mediated respiratory inhibition and damage pathway[J]. Biomaterials, 2020, 229: 119580. DOI:10.1016/j.biomaterials.2019.119580 |
| [44] |
Elgendy M, Ciro M, Hosseini A, et al. Combination of hypoglycemia and metformin impairs tumor metabolic plasticity and growth by modulating the PP2A-GSK3beta-MCL-1 axis[J]. Cancer Cell, 2019, 35: 798-815. DOI:10.1016/j.ccell.2019.03.007 |
| [45] |
Morales DR, Morris AD. Metformin in cancer treatment and prevention[J]. Annu Rev Med, 2015, 66: 17-29. DOI:10.1146/annurev-med-062613-093128 |
| [46] |
Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3[J]. Nat Rev Cancer, 2009, 9: 798-809. DOI:10.1038/nrc2734 |
| [47] |
Eikawa S, Nishida M, Mizukami S, et al. Immune-mediated antitumor effect by type 2 diabetes drug, metformin[J]. Proc Natl Acad Sci U S A, 2015, 112: 1809-1814. DOI:10.1073/pnas.1417636112 |
| [48] |
Pearce EL, Walsh MC, Cejas PJ, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism[J]. Nature, 2009, 460: 103-107. DOI:10.1038/nature08097 |
| [49] |
Memmott RM, Mercado JR, Maier CR, et al. Metformin prevents tobacco carcinogen--induced lung tumorigenesis[J]. Cancer Prev Res, 2010, 3: 1066-1076. DOI:10.1158/1940-6207.CAPR-10-0055 |
| [50] |
Pereira FV, Melo ACL, Low JS, et al. Metformin exerts antitumor activity via induction of multiple death pathways in tumor cells and activation of a protective immune response[J]. Oncotarget, 2018, 9: 25808-25825. DOI:10.18632/oncotarget.25380 |
| [51] |
Ding L, Liang G, Yao Z, et al. Metformin prevents cancer metastasis by inhibiting M2-like polarization of tumor associated macrophages[J]. Oncotarget, 2015, 6: 36441-36455. DOI:10.18632/oncotarget.5541 |
| [52] |
Menendez JA, Joven J, Cufi S, et al. The Warburg effect version 2.0: metabolic reprogramming of cancer stem cells[J]. Cell Cycle, 2013, 12: 1166-1179. DOI:10.4161/cc.24479 |
| [53] |
Faubert B, Boily G, Izreig S, et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo[J]. Cell Metab, 2013, 17: 113-124. DOI:10.1016/j.cmet.2012.12.001 |
| [54] |
Hardie DG, Alessi DR. LKB1 and AMPK and the cancer-metabolism link - ten years after[J]. BMC Biol, 2013, 11: 36. DOI:10.1186/1741-7007-11-36 |
| [55] |
Menendez JA, Vellon L, Oliveras-Ferraros C, et al. mTOR-regulated senescence and autophagy during reprogramming of somatic cells to pluripotency: a roadmap from energy metabolism to stem cell renewal and aging[J]. Cell Cycle, 2011, 10: 3658-3677. DOI:10.4161/cc.10.21.18128 |
| [56] |
Salani B, Del Rio A, Marini C, et al. Metformin, cancer and glucose metabolism[J]. Endocr Relat Cancer, 2014, 21: R461-R471. DOI:10.1530/ERC-14-0284 |
| [57] |
Lettieri Barbato D, Vegliante R, Desideri E, et al. Managing lipid metabolism in proliferating cells: new perspective for metformin usage in cancer therapy[J]. Biochim Biophys Acta, 2014, 1845: 317-324. |
| [58] |
Brown KA, Samarajeewa NU, Simpson ER. Endocrine-related cancers and the role of AMPK[J]. Mol Cell Endocrinol, 2013, 366: 170-179. DOI:10.1016/j.mce.2012.06.016 |
| [59] |
Loubiere C, Goiran T, Laurent K, et al. Metformin-induced energy deficiency leads to the inhibition of lipogenesis in prostate cancer cells[J]. Oncotarget, 2015, 6: 15652-15661. DOI:10.18632/oncotarget.3404 |
| [60] |
Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival[J]. Cell, 2003, 115: 577-590. DOI:10.1016/S0092-8674(03)00929-2 |
| [61] |
Alexander A, Walker CL. The role of LKB1 and AMPK in cellular responses to stress and damage[J]. FEBS Lett, 2011, 585: 952-957. DOI:10.1016/j.febslet.2011.03.010 |
| [62] |
Sinnett-Smith J, Kisfalvi K, Kui R, et al. Metformin inhibition of mTORC1 activation, DNA synthesis and proliferation in pancreatic cancer cells: dependence on glucose concentration and role of AMPK[J]. Biochem Biophys Res Commun, 2013, 430: 352-357. DOI:10.1016/j.bbrc.2012.11.010 |
| [63] |
Gwinn DM, Shackelford DB, Egan DF, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint[J]. Mol Cell, 2008, 30: 214-226. DOI:10.1016/j.molcel.2008.03.003 |
| [64] |
Choi YK, Park KG. Metabolic roles of AMPK and metformin in cancer cells[J]. Mol Cells, 2013, 36: 279-287. DOI:10.1007/s10059-013-0169-8 |
| [65] |
Wu H, Esteve E, Tremaroli V, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug[J]. Nat Med, 2017, 23: 850-858. DOI:10.1038/nm.4345 |
| [66] |
Forslund K, Hildebrand F, Nielsen T, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota[J]. Nature, 2015, 528: 262-266. DOI:10.1038/nature15766 |
| [67] |
He J, Zhang F, Han Y. Effect of probiotics on lipid profiles and blood pressure in patients with type 2 diabetes: a meta-analysis of RCTs[J]. Medicine, 2017, 96: e9166. DOI:10.1097/MD.0000000000009166 |
| [68] |
Vulevic J, Drakoularakou A, Yaqoob P, et al. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers[J]. Am J Clin Nutr, 2008, 88: 1438-1446. |
| [69] |
Depeint F, Tzortzis G, Vulevic J, et al. Prebiotic evaluation of a novel galactooligosaccharide mixture produced by the enzymatic activity of Bifidobacterium bifidum NCIMB 41171, in healthy humans: a randomized, double-blind, crossover, placebo-controlled intervention study[J]. Am J Clin Nutr, 2008, 87: 785-791. DOI:10.1093/ajcn/87.3.785 |
| [70] |
Luck H, Khan S, Kim JH, et al. Gut-associated IgA+ immune cells regulate obesity-related insulin resistance[J]. Nat Commun, 2019, 10: 3650. DOI:10.1038/s41467-019-11370-y |
| [71] |
Wang S, Lin Y, Xiong X, et al. Low-dose metformin reprograms the tumor immune microenvironment in human esophageal cancer: results of a phase Ⅱ clinical trial[J]. Clin Cancer Res, 2020, 26: 4921-4932. DOI:10.1158/1078-0432.CCR-20-0113 |
 2021, Vol. 56
2021, Vol. 56


