“唾液酸(sialic acid, SA)”一词最早出现于1952年, 包括50余种神经氨酸衍生物[1]。在哺乳动物中, 最常见的SA衍生物为第5个碳原子(C5) 上连接1个乙酰基的N-乙酰神经氨酸(Neu5Ac)。通常, SA由不同的糖苷键(α-2, 3-、α-2, 6-、α-2, 8-或α-2, 9-) 连接到细胞表面糖脂或糖蛋白糖链的末端[2]。SA在细胞膜上的有利位置决定了其在机体感染、细胞膜结构稳定性调节、细胞间通讯、介导细胞与细胞外基质间相互作用等众多生理病理过程中扮演重要角色[3, 4]。在肿瘤部位, SA的异常高表达通过至少3种不同的机制实现, 包括SA合成原料可用性的提高、唾液酸转移酶(sialyltransferase) 和神经氨酸酶(neuraminidase) 表达水平的改变等[5, 6]。
大约在50年前, 研究者们就已认识到, 肿瘤细胞可以通过高表达SA修饰聚糖实现免疫逃逸。但当时的观点局限地认为, SA只是机械地阻碍了肿瘤细胞表面抗原与免疫受体间的相互作用, 使肿瘤细胞达到伪装自身、逃避免疫系统杀伤的目的[7]。随着糖生物学和肿瘤免疫学的不断发展, 最近的研究结果显示, 肿瘤环境的高度唾液酸化能够直接调节肿瘤相关免疫细胞的功能, 通过抵抗凋亡和增强侵袭等多种途径促进肿瘤细胞的生存与发展[8-10]。在这些过程中, 以SA为末端的聚糖结构承载着特定的生物学信息, 这些信息被称作“凝集素”的蛋白质家族解码, 包括SA结合的免疫球蛋白样凝集素(sialic acid-binding immunoglobulin-like lectins, Siglecs) 和C型凝集素受体(C-type lectin receptors, CLRs)[11, 12]。Siglecs主要在免疫细胞表面表达, 与SA配体结合后, 可以向免疫细胞传递正向或负向调节信号, 并积极参与先天性和适应性免疫反应[13]。肿瘤细胞表面Siglecs配体表达的增加可以通过调节多种肿瘤相关免疫细胞帮助肿瘤塑造炎症环境或实现免疫逃逸[14, 15]。CLRs中的选择素受体家族(selectins) 以SA依赖的方式结合包含SA-LewisX或SA-LewisA结构的糖缀合物, 已知这些糖缀合物在许多肿瘤类型中表达[16]。在正常生理条件下, 血小板、白细胞与内皮细胞上表达的selectins相互作用, 可以帮助免疫细胞外渗至炎症部位。在肿瘤存在情况下, selectins参与了肿瘤微环境中白细胞的募集和肿瘤细胞的转移[10, 17, 18] (图 1)。
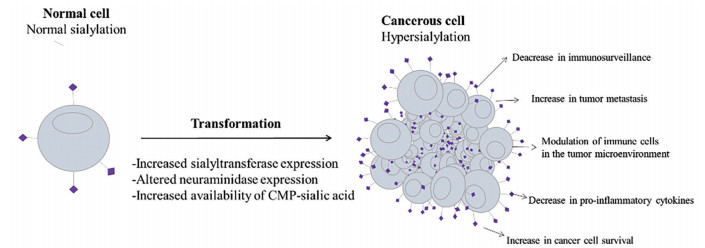
|
Figure 1 Hypersialylation in cancer: causes and effects. The purple bumps expressed on tumor cell surface are sialylated glycans |
近年来, 随着人们对SA过表达如何协助癌症发展, 特别是在唾液酸化配体如何通过与其受体相互作用, 调节宿主对肿瘤免疫反应方面的研究不断深入[2, 19], 凝集素家族的相关个体成员已成为设计新型抗肿瘤药物有希望的靶标。一个值得关注的研究领域是, 通过阻断SA与其受体间相互作用, 利用药物递送系统(drug delivery systems, DDS) 靶向SA结合受体, 实现相关疾病治疗目的[20-22]。本文概述了肿瘤环境的高度唾液酸化通过与Siglecs或selectins相互作用介导肿瘤发展、转移的最新机制, 并讨论了通过靶向这些SA结合受体治疗肿瘤的新策略, 为后续的研究提供依据。
1 SA结合受体参与肿瘤进展 1.1 Siglecs与肿瘤免疫逃逸Siglecs是一类经典的免疫球蛋白样(Ⅰ型) 凝集素, 分别由一个结合唾液酸化配体的免疫球蛋白结构域、一个跨膜结构域及不同长度的胞质内尾部组成[23]。Siglecs在免疫细胞表面广泛表达, 在维持免疫稳态和调节炎症反应过程中发挥重要作用[15, 24]。根据进化保守性和序列相似性, Siglecs可分为两类: 一类包括Siglec-1 (sialoadhesin, CD169)、Siglec-2 (CD22)、Siglec-4和Siglec-15, 在不同的哺乳动物间具有直系同源物, 并且这些保守的Siglecs之间具有较低的序列相似性; 另一类包括Siglec-3 (CD33) 及CD33相关Siglecs, 通过在Siglecs基因簇中的复制, 经历了快速的进化转变[25, 26]。CD22和大多数CD33相关Siglecs具有一个或多个胞质内免疫受体酪氨酸抑制基序(immunoreceptor tyrosine-based inhibitory motifs, ITIM), ITIM在被Src激酶激活后磷酸化, 募集SHP-1或SHP-2, 并向细胞内传递抑制信号。一些不具有ITIM基序的Siglecs在跨膜结构域中具有一个带正电的氨基酸, 能够与DAP12蛋白结合, 通过免疫受体酪氨酸激活基序(immunoreceptor tyrosine-based activation motifs, ITAM) 向细胞内传递活化信号[27]。这类激活性Siglecs往往与抑制性Siglecs成对进化, 例如在人类中经历了协同进化的Siglec-5/14和Siglec-11/16[14, 28]。尽管抑制性Siglecs的生理作用是通过识别“自我相关模式(self-associate pattern)”来保护机体免受过度反应的免疫系统侵袭[29], 但是某些病原体能够利用唾液酸化聚糖修饰自身, 通过与抑制性Siglecs结合逃避免疫监视, 从而导致相应配对受体的产生, 以及CD33相关Siglecs的快速进化。这一现象被称为“红皇后效应” (red queen effect), 用来描述有益于宿主免疫反应和被病原体利用的有害于宿主免疫反应之间对Siglecs的竞争[30]。病原体可通过与Siglecs的结合, 抑制免疫细胞激活并逃避免疫系统控制, 类似地, 肿瘤细胞也可通过高表达的唾液酸化配体与抑制性Siglecs结合实现免疫逃逸[31-34] (图 2)。例如, 表达在巨噬细胞表面的Siglec-1可以识别与内化肿瘤细胞来源的外泌体(exosomes), 通过阻止肿瘤相关抗原向树突状细胞及淋巴细胞的呈递, 抑制机体的抗肿瘤免疫反应[35]。研究发现, Siglec-2与B细胞的分化及淋巴瘤的发展相关[36]; Siglec-3在正常多能造血干细胞表面不表达, 而在髓系白血病细胞上表达, 可将Siglec-3作为人急性髓系白血病(acute myeloid leukemias, AMLs) 或慢性髓系白血病(chronic myeloid leukemias, CMLs) 的诊断和治疗靶标[37]。自然杀伤细胞(natural killer cell, NK) 参与机体对肿瘤细胞的鉴别和清除过程, 人NK细胞组成性表达抑制性受体Siglec-7, 一部分也表达抑制性受体Siglec-9, Siglec-7和Siglec-9在NK细胞的失活及肿瘤免疫逃逸中发挥重要作用[38, 39]。此外, 在使用Siglec-E (人Siglec-9同源) 缺陷小鼠模型的研究中发现, Siglec-E可以促进中性粒细胞依赖性肿瘤细胞的外渗和聚集, 最终加速肺转移的形成[40]。另一些体外数据表明, 配体与Siglec-E的结合可以抑制中性粒细胞产生活性氧(reactive oxygen species, ROS), 进而抑制中性粒细胞对肿瘤细胞的细胞毒性[41]。Beatson等[42]研究表明, 肿瘤细胞黏蛋白MUC1与肿瘤相关巨噬细胞(tumor-associated macrophages, TAMs) 表面Siglec-9的结合, 可增强TAMs向促瘤表型的极化。有研究结果显示, 尽管正常T细胞表面通常不表达Siglec-9, 但是在晚期实体瘤中浸润的T细胞表面发现了Siglec-9的表达, 并且往往与其他免疫检查点抑制受体(PD-1) 共表达, 协同发挥肿瘤免疫抑制作用[13]。在卵巢癌和三阴性乳腺癌中, 肿瘤细胞表面表达的免疫检查点CD24可以通过与TAMs表面表达的Siglec-10相互作用, 抑制巨噬细胞对肿瘤细胞的清除[43]。在另一项关于TAMs的研究中发现, Siglec-15可以选择性结合肿瘤相关的SA-Tn抗原(Neu5Acα2-6GalNAc), 诱导单核细胞或巨噬细胞产生免疫抑制性细胞因子TGF-β[44]。更有趣的是, Siglecs不止在肿瘤微环境中发挥作用, 甚至会参与宿主全身环境对肿瘤生长的贡献。Engblom等[45]研究表明, 肺腺癌会增加骨基质中Ocn+成骨细胞的产生, 远程提供可以浸润到肿瘤内且具有促瘤作用的Siglec-F (人Siglec-8同源) 高表达的中性粒细胞。综上所述, 虽然抑制性Siglecs是用来保护机体免受过度反应的免疫系统攻击, 但是肿瘤细胞可以利用不同种类的Siglecs, 来抑制机体的抗肿瘤免疫反应。同时在肿瘤微环境中的免疫细胞也会在Siglecs的帮助下, 促进肿瘤的进展与侵袭。

|
Figure 2 Structural features of functional human and murine sialic acid binding immunoglobulin-like lectins (Siglecs, Sig). ITIM: Immunoreceptor tyrosine-based inhibitory motifs; ITAM: Immunoreceptor tyrosine-based activation motifs |
Selectins是另一类重要的SA结合受体, 以SA依赖的方式优先识别含有SA-LewisX和SA-LewisxA结构的糖缀合物[46], 在内皮细胞(E-selectin)、白细胞(L-selectin) 和血小板(P-selectin) 上广泛表达[47]。在生理条件下, selectins介导炎症过程中白细胞滚动、黏附及外渗的早期步骤; 介导血小板参与的血栓形成; 还可以通过传递细胞内信号, 影响白细胞的活化[16, 48, 49]。在肿瘤微环境中, selectins参与了免疫细胞的募集与肿瘤相关炎症的发展过程, 特别是在帮助肿瘤细胞获得转移潜能方面有着重要贡献[21, 50]。通过高表达唾液酸化配体, 肿瘤细胞能够利用类似炎症反应中白细胞外渗的相关机制进行扩散和定植。在肿瘤转移过程中, selectins及其配体间相互作用的复杂程度远远超过了类似白细胞的外渗过程, 还包括肿瘤“栓子”的形成、转移生态位的形成及肿瘤细胞在生态位中的滞留等步骤[51-53]。
在转移性恶性肿瘤患者中, 肿瘤细胞可以利用多种机制诱导血小板的活化[54]。凭借P-selectin介导的相互作用, 功能亢进的血小板与肿瘤细胞形成“栓子”, 并通过多种方式促进转移, 包括阻止NK细胞等免疫细胞对肿瘤细胞的清除, 增强微血管对肿瘤细胞的阻滞, 通过分泌蛋白酶使肿瘤细胞获得侵袭性, 以及分泌血管内皮生长因子(vascular endothelial growth factor, VEGF) 等细胞因子促进血管生成[55, 56]。P-selectin介导的肿瘤细胞与血小板间的相互作用还可以导致内皮细胞局部炎症反应的激活。首先, 血小板通过释放细胞因子和趋化因子来支持炎症反应; 血小板介导的肿瘤“栓子”的形成可能造成微血管阻塞, 进而导致局部缺氧, 促进局部炎症反应; 其次, 聚集于肿瘤细胞上的血小板会诱导凝血级联反应, 导致凝血酶沉积水平增加, 并通过蛋白酶激活受体(protease activated receptors, PAR) 信号增强内皮细胞的局部炎症反应[57, 58]。最后, 局部炎性环境中细胞因子及促血管生成因子诱导了E-selectin在血管内皮细胞上的表达。E-selectin已被证实有助于肿瘤细胞的停滞、黏附及外渗过程[59-61]。局部炎性环境还能够诱导白细胞的募集和转移生态位的形成[62]。早在1889年, Paget提出了“种子和土壤”假说, 使人们意识到形成“适宜”的微环境或生态位对循环肿瘤细胞在远端器官的定植和转移的建立至关重要[63, 64]。鉴于生理条件下, selectins是介导白细胞外渗的主要介质, 可以想象, selectins积极参与了转移部位对白细胞的募集。相关研究指出, L-selectin介导的相互作用有助于将中性粒细胞和单核细胞募集至转移生态位[65, 66]。这些髓样细胞通过塑造局部免疫抑制环境(即“土壤”), 帮助肿瘤细胞(即“种子”) 存活并获得侵袭性等来支持转移的发展。例如, L-selectin介导的单核细胞的募集可以通过增加血管通透性来帮助肿瘤细胞跨内皮迁移[67] (图 3)。

|
Figure 3 Potential interactions that occur between selectins and cancer cells during hematogeneous metastasis |
最近越来越多的研究发现, 除了介导肿瘤转移过程中细胞间的相互作用外, selectins还可以通过激活信号传导级联反应来调节肿瘤微环境内的免疫状态。例如, 配体与白细胞表面L-selectin的结合可以诱导白细胞的活化, 并进一步调节白细胞与血小板、内皮细胞间的相互作用[68]。血小板上的P-selectin或内皮细胞上的E-selectin与白细胞表面selectins配体PSGL-1的结合同样可以触发细胞内信号的传导[69]。此外, 有研究指出, selectins可能会影响肿瘤免疫治疗的效果。例如, 血小板上P-selectin与调节性T细胞(regulatory T cells, Treg) 上PSGL-1的结合抑制了Treg向产生IL-17的细胞表型分化[70]; NK细胞表面L-selectin的表达与这些细胞在肿瘤环境内的持续存在有关。这些研究进一步证明, selectins可以作为肿瘤治疗中重要的潜在靶点[60]。
2 靶向SA结合受体的肿瘤治疗策略 2.1 Siglecs作为肿瘤治疗靶点鉴于Siglecs在免疫系统中的广泛表达及在各种免疫细胞亚群中的差异表达, 目前越来越多的研究将其视为肿瘤免疫治疗中有潜力的靶向目标[37, 38]。大部分Siglecs是内吞性受体, 这使得通过靶向Siglecs将细胞毒性药物或免疫调节剂递送至靶细胞成为可能[14, 71] (图 4)。
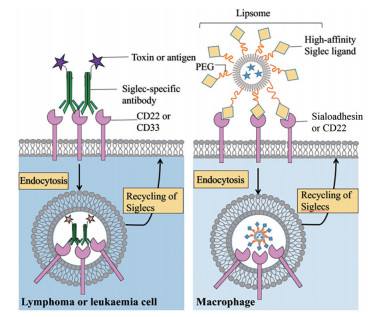
|
Figure 4 The endocytic property of the Siglecs expressed on lymphoma and leukaemia cells (CD22 and CD33) or macrophages (sialoadhesin) has been exploited to deliver cargo to the cell type of interest |
Siglec-1在单核细胞与巨噬细胞表面特异性表达, 并在与配体结合后迅速介导内吞。因此, 很多研究者通过靶向Siglec-1将肿瘤抗原从巨噬细胞呈递至T细胞[39, 72]。例如, 通过修饰Siglec-1配体(9-N-biphenylcarbonyl NeuAc) 递送抗原可以激活NKT细胞对肿瘤细胞的杀伤活性[73]。另一种策略是制备SA衍生物配体修饰的脂质体作为疫苗, 将抗原呈递给T细胞[74]。
靶向Siglecs的特异性抗体用于治疗白血病和淋巴瘤的相关临床研究已有20余年的历史, 这为将抗体作为靶向治疗剂提供了丰富经验[75]。特异性靶向Siglec-3的吉妥珠单抗(Mylotarg) 已于2002年在美国获批用于治疗急性髓系白血病(acute myeloid leukemias, AMLs), 是全球第一个上市的抗体偶联药物。出于对疗效和安全性的担忧, 辉瑞公司于2010年将其自主撤市。2017年, FDA再度批准Mylotarg上市, 用于治疗特定人群的CD33+ AMLs[76]。利用CD22特异性抗体治疗B细胞淋巴瘤和髓性白血病的抗体偶联药物包括偶联加利车霉素(galicamycin) 的Besponsa和偶联细菌蛋白毒素的Lumoxiti[77, 78], 其他治疗策略还包括使用裸抗体或放射性标记抗体, 分别利用抗体诱导的细胞毒作用或通过辐射杀伤白血病细胞[79, 80]。
鉴于Siglecs在肿瘤浸润T细胞、NK细胞、中性粒细胞、树突状细胞和巨噬细胞上广泛表达, 并在与肿瘤细胞表面过表达的SA聚糖结合后常介导免疫抑制作用, 越来越多的研究者开始关注开发Siglecs作为替代或补充的免疫检查点[38]。最初人们认为, 只有少量的人外周血幼稚T细胞亚群表达Siglec-7和-9, 而最近研究结果显示, 抑制性Siglecs受体在许多肿瘤浸润的CD4+和CD8+ T细胞表面上调, 并可以帮助肿瘤细胞抑制T细胞反应[38, 81]。Quentin等[82]发现, 用神经氨酸酶破坏Siglec-9配体可以增强肿瘤浸润的Siglec-9+ CD8+ T细胞对靶细胞的杀伤能力, 用抗Siglec-9抗体的Fab片段同样可以激活CD8+ T细胞的细胞毒性。但完整的抗Siglec-9抗体却抑制了这些细胞的细胞毒性, 这可能与其诱导了抑制信号有关。除T细胞外, NK细胞也参与了对肿瘤细胞的鉴别和杀伤过程。NK细胞表面表达多种抑制性受体, 其中包括Siglec-7和-9, 用抗Siglec-7/-9抗体的Fab片段与这些受体相互作用可以增强NK细胞对肿瘤细胞的细胞毒性。但正如在CD8+ T细胞中观察到的那样, 完整的抗Siglecs抗体抑制了细胞毒性, 表明这些抗体可能激活了Siglec-7/-9受体的抑制功能[83]。这些结果提示, 尽管大量数据支持Siglec-7和-9可以作为增强T细胞和NK细胞抗肿瘤反应的重要靶标, 但是能否像使用抗PD-1抗体那样使用抗Siglecs抗体调节T细胞和NK细胞的功能目前仍未明确, 这需要大量的研究才能更好地理解抑制性Siglecs受体信号调节的具体过程。
TAMs可以吞噬肿瘤细胞, 进而激活TAMs以及其他肿瘤浸润白细胞的免疫反应[84]。Barkal等[43]研究表明, 乳腺癌细胞上表达的CD24与TAMs上的Siglec-10相互作用后, 能够阻止TAMs对肿瘤细胞的吞噬, 从而介导肿瘤免疫逃逸。基于这一研究, 针对Siglec-10和CD24的双特异性抗体似乎是更好的肿瘤靶向治疗方案。Wang等[44]在TAMs上检测到了另一种Siglecs, Siglec-15, 其在与肿瘤细胞表面的SA-Tn抗原结合后, 能够诱导TAMs产生TGF-β并抑制T细胞的增殖与活化。已有研究指出, 无论是单独使用抗Siglec-15抗体还是联合抗PD-1抗体, 都可以显著抑制肿瘤的生长, 并改善现有的肿瘤免疫疗法。
与抗体方面的研究相比, 利用聚糖配体作为Siglecs抑制剂治疗肿瘤的相关报道较少, 文献中提到的有前途的策略包括对C4和C9位进行修饰的SA衍生物[55, 85]。Attrill等[86]报道了作为Siglec-7信号转导抑制剂的SA衍生物的设计。其中一种配体, oxamido-Neu5Ac [methyl α-9-(amino-oxalyl-amino)-9-deoxy-Neu5Ac], 在体外表现出比标准配体methyl-α-Neu5Ac对Siglec-7受体低1/2的IC50值。Kelm等[87]则合成了一种Siglec-2抑制剂, 这是一种C9位修饰的Neu5Ac, 即BPC-Neu5Ac [methyl-α-9-N-(biphenyl-4-carbonyl)-amino-9-deoxy-Neu5Ac], 其对人Siglec-2受体的相对抑制效力可达methyl-α-Neu5Ac的200倍以上。随后, Madge等[88]使用这种Siglec-2高亲和力配体修饰多柔比星脂质体, 靶向治疗B细胞淋巴瘤。有趣的是, 这种递送策略在毛细胞白血病、边缘区淋巴瘤和慢性淋巴细胞性白血病患者中均表现出了针对恶性B细胞的细胞毒作用。2013年, Kelm等[87]又进一步优化了针对Siglec-2的聚糖抑制剂, 通过同时修饰Neu5Ac的C4和C9位, 得到了对Siglec-2表现出更低IC50值的9-BPC-4-mNPC-Neu5 (methyl9-biphenylcarboxamido-4-m-nitrophenylcarboxamido-4, 9-dideoxy Neu5Ac), 其有望成为一种有前途的靶头或治疗剂。
2.2 靶向selectins抑制肿瘤转移越来越多的研究表明, selectins及其配体的相互作用可影响肿瘤发展的多个方面, 包括从肿瘤细胞的扩散到转移生态位的构建[54, 56]。现已开发了多种通过干预selectins及其配体的相互作用的抑制剂, 包括硫酸化多糖、小分子聚糖、拟糖物和针对selectins及其配体的抗体等, 用于肿瘤的治疗[21, 51, 89]。
肝素是一种天然存在于肥大细胞中的、由葡萄糖胺、L-艾杜糖醛苷、N-乙酰葡萄糖胺和D-葡萄糖醛酸交替组成的黏多糖硫酸脂[90]。临床上最早将肝素及小分子肝素用作抗凝剂, 随后的研究发现, 肝素及其衍生物可以通过选择性结合P-及L-selectin, 抑制肿瘤转移[57, 91]。肝素与selectins的结合需要独立于其抗凝活性的结构特性, 因此, 为了降低抗凝活性并增强对selectins的亲和力, 研究者们对肝素进行一系列化学修饰, 并在黑色素瘤、乳腺癌和结肠癌等肿瘤模型中证明了这些肝素衍生物对肿瘤转移的抑制作用[92, 93]。临床试验表明, 在肿瘤患者术后使用低分子肝素进行辅助治疗(NCT00475098、NCT00967148), 可改善患者的预后状况。当然, 这不仅归功于肝素及其衍生物抑制了selectins在转移过程中介导的相互作用, 还可能与其影响了肝素酶活性、生长因子信号传导及包括整联蛋白在内的其他细胞黏附分子有关[51, 94]。考虑到肝素及其衍生物诱导抗凝相关不良反应的风险, 以及selectins在维持机体稳态中的重要作用, 选择合理的治疗窗口至关重要。较为合理的用药方案是, 在肿瘤初步确诊后立即应用肝素及其衍生物抑制P-和L-selectin介导的肿瘤细胞血源性转移, 直到手术切除原发肿瘤一段时间后再停止用药[95-98]。其他一些带负电荷的多糖也可作为selectins抑制剂用于肿瘤治疗。例如已在小鼠结肠癌及黑色素瘤模型中得到验证的硫酸皮肤素和6-O-硫酸化壳聚糖, 可作为P-selectin的有效抑制剂[51]。因为天然葡聚糖肝素具有结构不均、纯度不高等缺点, Fritzsche等[99]合成了葡聚糖硫酸酯, 这种半合成硫酸化多糖对P-selectin的抑制效果更佳, 在临床上可取代肝素作为抗转移药物。褐藻多糖是一种由L-岩藻糖单元和硫酸化酯基组成的多糖, 其可以利用带负电荷的羟基和硫酸基来提高对P-selectin的靶向性, 与硫酸葡聚糖和肝素相比, 褐藻多糖靶向P-selectin的能力提高了2个数量级[100]。类似的其他糖类化合物或分子如硫酸化六糖和海鞘硫酸盐对P-selectin具有相似的作用。
对所有selectins具有靶向作用的四糖SA-LewisX是第1种被测试用作selectins抑制剂的聚糖结构, 但低亲和力、高生产成本和不良的药代动力学行为阻碍了SA-LewisX的进一步开发[101]。随后, 一些基于路易斯型糖结构的拟糖物被开发用来干扰selectins介导的病理过程[102]。在多发性骨髓瘤患者体内, P-selectin与PSGL-1的结合可以调节恶性细胞与微环境之间的相互作用, 并且介导了骨髓瘤细胞向骨髓小生境的归巢和对化疗药物的抗性。使用拟糖物GMI-1070或针对P-selectin/PSGL-1的抗体可以有效阻断P-selectin与PSGL-1间的相互作用, 进而抑制骨髓瘤细胞向骨髓部位的归巢并恢复其对硼替佐米的敏感性[103]。除了P-selectin外, E-selectin同样介导了多发性骨髓瘤细胞的侵袭和对硼替佐米的耐药, 拟糖物GMI-1271可以特异性抑制E-selectin, 并显著提高多发性骨髓瘤小鼠模型的存活率及对硼替佐米的敏感性。更令人惊喜的是, GMI-1271与标准化疗的联合应用能够降低小鼠模型AMLs负担, 也可有效抑制胰腺癌和乳腺癌在小鼠模型中的转移潜能, 并且GMI-1271能够抑制MCF-7乳腺癌干细胞(CD44+CD24-) 在骨髓中的扩散。在早期临床试验中, GMI-1271表现出良好的安全性, 并有助于减弱化疗带来的不良反应, 进而有助于改善患者的生存质量。基于这样令人振奋的研究结果, GlycoMimetics公司后续推进了GMI-1271与化疗药物联合治疗AMLs的Ⅱ期临床试验。2017年, 美国FDA授予GMI-1271突破性疗法认定, 用于治疗成年患者的复发性或难治性AMLs, 目前正处于Ⅲ期临床试验阶段[104, 105]。研究者们还开发了其他类似GMI-1271的拟糖物, 例如, 对E-selectin和CXCR4具有双重特异性的GMI-1359, 与标准化疗联合应用, 不但可以改善AMLs小鼠模型的存活率, 还可以抑制胰腺癌和前列腺癌在小鼠模型中的转移能力[106]。
通过靶向selectins将药物递送至转移部位是另一种有吸引力的肿瘤治疗策略。Shamay等[107]将E-selectin结合肽与多柔比星或促凋亡肽偶联, 该聚合物可以有效抑制黑色素瘤的肺转移, 并显著提高小鼠的存活率。同样, Shamay等[108]制备了一种使用褐藻多糖包被多柔比星的纳米载体, 该多糖与P-selectin的亲和力很强。与未修饰药物载体相比, 靶向P-selectin能够显著促进药物在肺转移部位的积累, 从而消灭癌细胞。Chu等[109]制备了一种透明质酸与褐藻多糖双靶向纳米粒, 以肿瘤血管上的P-selectin为靶点靶向递送药物, 抑制了前列腺癌的发展和转移。此外, 低分子量肝素包被的多柔比星脂质体可以通过阻断P-selectin介导的肿瘤细胞与血小板间的相互作用, 从而抑制黑色素瘤的肺转移[103]。在另一项研究中, Wayne等[110]制备了E-selectin包被的装载肿瘤坏死因子相关的凋亡诱导配体(tumor necrosis factor related apoptosis inducing ligand, TRAIL) 的脂质体, 利用脂质体表面E-selectin与血液循环免疫细胞表面的E-selectin配体间的相互作用, 使免疫细胞“装配”有TRAIL。结果表明, 这些“装配”TRAIL的白细胞能够有效清除循环中的结肠癌和前列腺癌细胞。
2.3 SA及衍生物修饰的纳米药物递送系统纳米药物递送系统(nanosized drug delivery systems, nano-DDS) 凭借其在时间和空间上控制药物递送以改进难治性疾病治疗方法的优势, 受到世界各国医药研究人员的广泛关注[111, 112]。在nano-DDS的设计中存在2个主要问题: 首先, 由于调理蛋白的结合, 纳米载体通常在血液中会被单核吞噬细胞系统(mononuclear phagocyte system, MPS) 快速清除, 而足够长的血液循环时间往往是这些载体成功递送药物的先决条件[113]。此外, 缺乏特异性靶向作用的纳米载体, 会减少药物在治疗部位的高浓度累积, 并导致不良反应的产生。自1906年, 德国著名的血液学家、免疫学说的奠基人Ehrlich提出“魔弹”靶向给药的概念以来, 研究者们不断努力通过改变纳米载体的表面性质, 或利用“配体”修饰载体, 实现“免疫伪装”或“主动靶向”的目的[114]。SA是哺乳动物细胞膜上含量最丰富的碳水化合物分子之一, 其在寡糖、糖蛋白和糖脂上的末端位置, 决定了SA及其衍生物在体内既能扮演“避免识别”的角色, 也能扮演“介导识别”的角色[115]。目前已有许多研究者将SA及其衍生物用于nano-DDS的构建, 并取得了相应的研究成果[116, 117]。
研究者们首先注意到的是SA及其衍生物的“免疫伪装”作用。研究指出, 人红细胞表面高度唾液酸化, 经唾液酸酶处理后, 红细胞的寿命会从原来的120天锐减到几个小时[118]。另外, 许多病原体利用SA“装扮”自身, 掩蔽其抗原表位, 从而成功逃脱宿主免疫系统的攻击[119]。神经节苷脂(ganglioside, Gg) 是一种含SA的酸性鞘糖脂, 其结构由亲水的寡糖链和亲脂的神经酰胺两部分组成[120]。其中, 单唾液酸四己糖神经节苷脂(monosialoteterahexosyl ganglioside, GM1) 是最早被用来构建“免疫伪装”载体的SA衍生物, 其用于长循环载体修饰的历史超过PEG修饰。1987年, Allen等[121]发现将GM1插入磷脂双分子层中可有效延长脂质体的血液循环时间, 认为GM1上的SA基团是产生长循环效应的关键, 从而提出“长循环脂质体(long circulating liposome)”的概念。聚唾液酸(polysialic acid, PSA) 是SA单体以α-2, 8-、α-2, 9-或α-2, 8-/α-2, 9-交替的酮苷键连接而成的无分支直链聚合物, 利用PSA构建“免疫伪装”载体比GM1得到了更广泛的应用。1993年的报道指出, PSA具有低免疫原性、可生物降解性等优势, 是PEG的最佳替代物[122]。至今, PSA已被广泛用于修饰脂质体和胶束等纳米载体, 或与小分子抗癌药物偶联以及修饰多肽类和蛋白类物质, 从而改善药物的动力学参数, 减少药物的降解或降低药物引起的免疫反应[123, 124]。更重要的是, 目前PSA修饰的多肽已经进入临床, 主要有PSA化胰岛素SuliXen®和PSA化促红细胞生长素ErepoXen®, 均由Xenetic Bioscience (原Lipoxen) 公司开发[125, 126]。
除“避免识别”外, SA在体内更多时候起到“介导识别”的作用。因此, 研究者们尝试将SA及其衍生物用于“主动靶向”治疗多种疾病[127, 128]。特别是利用SA及其衍生物作为肿瘤检测或治疗的靶向配体[129, 130]。SA修饰的纳米粒可以特异性靶向肿瘤血管内皮细胞上表达的E-selectin, 通过因肿瘤部位通透性异常增加的血管壁间隙, 进入肿瘤部位并被肿瘤细胞摄取[131]。此外, 由于selectins在肿瘤细胞表面过度表达, SA可以直接靶向肿瘤细胞, 增加药物的细胞毒性[132]。首先, 研究者们试图利用SA及其衍生物的修饰实现载体在肿瘤部位的特异性蓄积。现已有多项研究报道了利用SA-LewisX修饰装载不同药物(顺铂、甲氨蝶呤和消旋苯丙氨酸氮芥等) 的脂质体表面。与游离药物相比, 这些脂质体显著提高了小鼠模型内肿瘤部位的药物蓄积量, 并显著降低了药物的毒副作用[133, 134]。由于E-selectin在肿瘤细胞和炎性血管内皮细胞(inflammatory vascular endothelial cells, VECs) 上高表达, Xu等[135]制备了具有肿瘤细胞和VECs双重靶向性的SA-PEG-DOX胶束。SA-PEG-DOX通过SA与VECs上E-selectin的特异性结合, 实现药物在肿瘤部位的大量累积。在进入肿瘤部位后, 这些胶束会被E-selectin高表达的肿瘤细胞选择性地摄取。Zheng等[136]利用物理吸附的方式制备了SA修饰的硒纳米粒, 与无修饰的硒纳米粒相比, SA的修饰使纳米粒在HeLa细胞中的摄取量增加了338%, 并促进了细胞的凋亡。
在另一些研究中, SA及其衍生物被用来抑制肿瘤转移。Zeisig等[137]开展的体外研究结果显示, SA-LewisX修饰的脂质体可以通过抑制E-selectin来阻止肿瘤细胞的黏附。在一项类似的研究中, Saiki等[138]发现, 给小鼠静脉注射SA-LewisX修饰的脂质体能够抑制黑色素瘤细胞B16-BL6在肺部的外渗。除E-selectin外, 血小板上的P-selectin能够通过介导肿瘤“栓子”的形成, 促进肿瘤转移。Keil等[139]研究表明, SA-LewisX修饰的脂质体可以通过阻止血小板和肿瘤细胞的聚集, 抑制HT29肿瘤细胞在小鼠肺、肝和肠中的转移。
高度唾液酸化的肿瘤细胞往往会上调肿瘤微环境甚至循环系统中的免疫细胞表面SA结合受体Siglecs和selectins的表达, 并利用对这些促瘤表型免疫细胞的招募实现有助于肿瘤发展的局部免疫抑制环境和转移生态位的构建。因而, SA及其衍生物修饰的纳米载体, 可以通过免疫细胞介导的吞噬和递送作用, 实现肿瘤靶向治疗。She等[140]首先合成了一种唾液酸-十八胺衍生物(SA-ODA) 用于修饰装载匹杉琼的脂质体, 并借助MPS系统中的单核细胞作为“特洛伊木马”实现肿瘤靶向递药, 最终实现了“肿瘤脱落”的治疗效果。随后, Zhou等[141]注意到SA-ODA在小鼠体内具有一定毒性, 因而制备了一种生物相容性更好的唾液酸-胆固醇衍生物(SA-CH), 用于修饰装载表柔比星的脂质体, 同样在小鼠S180肿瘤模型中表现出了良好的抑瘤效果。Qiu等[142]则制备了SA衍生物修饰的依鲁替尼纳米复合物, 以期通过Siglecs-1受体靶向TAMs治疗肿瘤。有趣的是, 体外实验表明, 依鲁替尼对S180肿瘤细胞和RAW264.7巨噬细胞模型几乎没有细胞毒性, 该纳米复合物在小鼠体内表现出的良好抑瘤作用是通过抑制TAMs中布鲁顿酪氨酸激酶(Bruton's tyrosine kinase, BTK) 的磷酸化、缓解TAMs诱导的免疫抑制作用实现的。这一结果提示, 肿瘤治疗不应仅仅局限于肿瘤细胞本身, 对肿瘤赖以生存的微环境的破坏, 切断肿瘤细胞营养的供给, 可能会获得更加令人惊喜的治疗效果。除巨噬细胞外, 中性粒细胞在肿瘤炎性环境中会高表达SA结合受体L-selectin, 并且这些活化的中性粒细胞具有自主迁移到肿瘤炎性环境中的能力。因而, Li等[143]设计了合成一种新的SA衍生物(SA-ODC), 将SA的C9位与十八酰氯偶联, 最大程度地暴露在与selectins受体结合中起关键作用的C1位羧基。用该衍生物修饰的多柔比星脂质体, 可以在静脉注射后, 被血液循环中高表达L- selectin的活化中性粒细胞快速摄取, 并利用这些中性粒细胞向肿瘤炎性部位的趋化能力将药物递送至肿瘤部位, 实现优异的肿瘤靶向性及抑瘤效果(图 5)。

|
Figure 5 The application of SA-modified liposomes transported in blood for the targeting of activated peripheral blood neutrophils to improve the tumor therapeutic efficacy. (Adapted from Ref. 143 with permission. Copyright © 2020 The Royal Society of Chemistry's) |
为了适应低氧的肿瘤微环境, 肿瘤细胞的代谢方式由氧化反应转变为糖酵解, 这便是众所周知的“Warburg效应”。在这种情况下, 肿瘤细胞中糖转运蛋白和糖酵解酶的相关基因表达增加, 这可能与肿瘤细胞表面高表达SA-LewisX或SA-LewisA有关[144]。相关研究报道, 在不同肿瘤患者(卵巢癌、乳腺癌、结肠直肠癌、神经胶质瘤、神经母细胞瘤和肺癌等) 的肿瘤细胞表面均发现了SA修饰聚糖的显著高表达[145-147]。高度唾液酸化的肿瘤细胞可以通过与外周Siglecs和selectins的结合, 促进免疫抑制环境的塑造和转移进程[148, 149]。然而, 肿瘤细胞还会“驯化”外周的免疫细胞等进一步高表达SA结合受体帮助自身发展。例如, 肿瘤细胞释放的炎性因子TNF-α、IL-1β等可以诱导E-selectin的表达, IL-3、IL-4等可以诱导P-selectin的表达[18, 150]。此外, 在肿瘤炎性环境中, 免疫细胞表面的L-selectin会发生构象改变, 提高对配体的亲和力[151]。Siglec-1也被证明在TAMs表面高表达, 并有助于TAMs进一步发挥促炎功能[152]。Siglec-9在正常T细胞表面的表达水平非常低, 而在非小细胞肺癌、结直肠癌和卵巢癌患者的肿瘤浸润T细胞上显著高表达[13]。在正常生理条件下, Siglec-15只在某些髓细胞上表达, 但其却在人类肿瘤细胞和肿瘤浸润髓细胞上广泛上调[44]。在卵巢癌和三阴性乳腺癌中, 肿瘤细胞表面高表达CD24, 肿瘤相关巨噬细胞表面高表达其受体Siglec-10[43]。SA结合受体甚至参与了宿主全身环境对肿瘤生长的贡献, 肺腺癌会增加骨基质中Ocn+成骨细胞的产生, 远程提供浸润到肿瘤中能够促瘤的Siglec-F (人Siglec-8同源) 高表达中性粒细胞[45]。因而, 研究者们试图通过靶向这些SA结合受体治疗肿瘤, 目前这类策略中应用较为广泛的包括阻断Siglecs的特异性抗体和靶向selectins的聚糖或拟糖物[22, 49]。
特别是近年来免疫检查点阻断治疗的成功, 使研究者们开始关注用特异性抗体阻断Siglecs受体治疗肿瘤。但值得注意的是, 相同的Siglecs往往会在不同的肿瘤相关免疫细胞上表达, 不同的Siglecs也可能在同一种细胞上表达。并且抗Siglecs抗体往往自身具有诱导免疫抑制反应的潜力, 限制了抗Siglecs抗体在肿瘤治疗中的应用[34]。靶向selectins的聚糖及拟糖物由于对糖苷酶水解的敏感性, 在血液循环中的快速清除和体内生物利用度低等缺点, 难以应用于临床[21]。相比于以上两类治疗方式, nano-DDS具有以下优势: ①可以通过粒径的控制及配体的修饰同时实现被动靶向和主动靶向; ②能够改善药物的溶解行为; ③改善药物的稳定性和生物相容性等; ④实现药物的可控释放; ⑤改善细胞对药物的内化及药物在细胞内的传递等[153-155]。特别是小分子碳水化合物配体修饰的靶向药物递送系统(targeted drug delivery system, TDDS), 与体积较大的复杂配体相比, 具有设计与合成更灵活、生产成本低、易于修饰和表征、无免疫原性、体内外稳定性好及肿瘤穿透能力强等优势, 更利于临床转化[156, 157]。
综上所述, SA及其衍生物修饰的TDDS可以通过多种机制发挥抗肿瘤作用: ①直接杀伤肿瘤细胞和肿瘤微环境局部高表达Siglecs或selectins的促瘤基质细胞; ②通过竞争性抑制肿瘤细胞与血小板、白细胞、内皮细胞通过selectins介导的结合, 抑制“转移簇”的形成和肿瘤细胞的外渗; ③可以利用SA修饰的纳米载体装载免疫激活剂来逆转高表达Siglecs免疫细胞的免疫抑制作用; ④利用血液循环中高表达SA结合受体, 具有吞噬能力并即将被招募到肿瘤微环境中的免疫细胞(单核细胞、中性粒细胞等) 作为“特洛伊木马”, 将药物递送至肿瘤部位; ⑤特异性杀伤机体内高表达Siglecs或selectins, 具有促瘤表型的免疫细胞, 利用机体免疫细胞可以自我补充的特点, 间接提高抑瘤表型免疫细胞的比例, 改善宿主全身免疫环境等。
随着肿瘤免疫学与纳米技术的不断发展, 研究者们更加深入地了解肿瘤发展过程中SA结合受体的表达水平与信号转导过程, 并在制剂设计的整个过程中, 充分将免疫学与药剂学有机地结合在一起, 从受体选择、配体设计到载体制备始终将免疫学放在首位。相信在不久的将来, 靶向SA结合受体的肿瘤治疗策略, 特别是SA及其衍生物修饰的DDS, 以及这些DDS与其他治疗方式的联合应用将给更多肿瘤患者带来希望。
作者贡献: 李聪负责文献调研和文章撰写; 范垂众和闫鑫杨负责文章思路梳理; 高新和赖晓雪负责参考文献格式整理; 刘欣荣、宋艳志和邓意辉负责文章指导和修改。
利益冲突: 所有作者均声明不存在利益冲突。
| [1] |
Blix G, Svennerholm L, Werner IM, et al. The isolation of chondrosamine from gangliosides and from submaxillary mucin[J]. Acta Chem Scand, 1952, 6: 358-362. DOI:10.3891/acta.chem.scand.06-0358 |
| [2] |
Schauer R, Kamerling JP. Exploration of the sialic acid world[J]. Adv Carbohyd Chem Biochem, 2018, 75: 1-213. |
| [3] |
Zhou X, Yang G, Guan F. Biological functions and analytical strategies of sialic acids in tumor[J]. Cell, 2020, 9: 273. DOI:10.3390/cells9020273 |
| [4] |
Van DE, Wall S, Santegoets KCM, et al. Sialoglycans and siglecs can shape the tumor immune microenvironment[J]. Trends Immunol, 2020, 41: 274-285. DOI:10.1016/j.it.2020.02.001 |
| [5] |
Szabo R, Skropeta D. Advancement of sialyltransferase inhibitors: therapeutic challenges and opportunities[J]. Med Res Rev, 2017, 37: 219-270. DOI:10.1002/med.21407 |
| [6] |
Quentin H, Cedric S, Stephan VG. A cartography of Siglecs and sialyltransferases in gynecologic malignancies: is there a road towards a sweet future[J]. Front Oncol, 2018, 8: 68. DOI:10.3389/fonc.2018.00068 |
| [7] |
Gabius HJ. Tumor lectinology: at the intersection of carbohydrate chemistry, biochemistry, cell biology, and oncology[J]. Angew Chem Int Edit, 1988, 27: 1267-1276. DOI:10.1002/anie.198812671 |
| [8] |
Pearce OMT, Heinz L. Sialic acids in cancer biology and immunity[J]. Glycobiology, 2016, 26: 111-128. DOI:10.1093/glycob/cwv097 |
| [9] |
Boligan KF, Mesa C, Fernandez LE, et al. Cancer intelligence acquired (CIA): tumor glycosylation and sialylation codes dismantling antitumor defense[J]. Cell Mol Life Sci, 2015, 72: 1231-1248. DOI:10.1007/s00018-014-1799-5 |
| [10] |
Emily R, Matthew M. Hypersialylation in cancer: modulation of inflammation and therapeutic opportunities[J]. Cancers, 2018, 10: 207. DOI:10.3390/cancers10060207 |
| [11] |
Brown GD, Willment J. C-Type lectins in immunity and homeostasis[J]. Nat Rev Immunol, 2018, 18: 374-389. DOI:10.1038/s41577-018-0004-8 |
| [12] |
Ding D, Yao Y, Zhang S, et al. C-Type lectins facilitate tumor metastasis[J]. Oncol Lett, 2017, 13: 13-21. DOI:10.3892/ol.2016.5431 |
| [13] |
Stanczak MA, Siddiqui SS, Trefny MP, et al. Self-associated molecular patterns mediate cancer immune evasion by engaging Siglecs on T cells[J]. J Clin Invest, 2018, 128: 4912-4923. DOI:10.1172/JCI120612 |
| [14] |
Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease[J]. Nat Rev Immunol, 2014, 14: 653-666. DOI:10.1038/nri3737 |
| [15] |
Daly J, Carlsten M, O'Dwyer M. Sugar free: novel immunotherapeutic approaches targeting siglecs and sialic acids to enhance natural killer cell cytotoxicity against cancer[J]. Front Immunol, 2019, 10: 1047. DOI:10.3389/fimmu.2019.01047 |
| [16] |
Barthel SR, Gavino JD, Descheny L, et al. Targeting selectins and selectin ligands in inflammation and cancer[J]. Expert Opin Ther Targets, 2007, 11: 1473-1491. DOI:10.1517/14728222.11.11.1473 |
| [17] |
Häuselmann I, Roblek M, Protsyuk D, et al. Monocyte induction of E-selectin–mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade[J]. Cancer Res, 2016, 76: 5302-5312. DOI:10.1158/0008-5472.CAN-16-0784 |
| [18] |
Li SS, Carman KMI, Matthew YHT, et al. Sialyl Lewisx-P-selectin cascade mediates tumor–mesothelial adhesion in ascitic fluid shear flow[J]. Nat Commun, 2019, 10: 2406. DOI:10.1038/s41467-019-10334-6 |
| [19] |
Shalapour S, Karin M. Immunity, inflammation, andcancer: an eternal fight between good and evil[J]. J Clin Invest, 2015, 125: 3347-3355. DOI:10.1172/JCI80007 |
| [20] |
Ding J, Zhao D, Hu Y, et al. Terminating the renewal of tumor-associated macrophages: a sialic acid-based targeted delivery strategy for cancer immunotherapy[J]. Int J Pharm, 2019, 571: 118706. DOI:10.1016/j.ijpharm.2019.118706 |
| [21] |
Natoni A, Macauley MS, O'dwyer ME. Targeting selectins and their ligands in cancer[J]. Front Oncol, 2016, 6: 93. |
| [22] |
Adams OJ, Stanczak MA, Von Gunten S, et al. Targeting sialic acid–Siglec interactions to reverse immune suppression in cancer[J]. Glycobiology, 2018, 28: 640-647. |
| [23] |
Powell DL, Varki A. Ⅰ-type lectins[J]. J Biol Chem, 1995, 270: 14243-14246. DOI:10.1074/jbc.270.24.14243 |
| [24] |
Bornhöfft KF, Goldammer T, Rebl A, et al. Siglecs: a journey through the evolution of sialic acid-binding immunoglobulin-type lectins[J]. Dev Comp Immunol, 2018, 86: 219-231. DOI:10.1016/j.dci.2018.05.008 |
| [25] |
Angata T. Possible influences of endogenous and exogenous ligands on the evolution of human Siglecs[J]. Front Immunol, 2018, 9: 2885. DOI:10.3389/fimmu.2018.02885 |
| [26] |
Walter RB, Raden BW, Zeng R, et al. ITIM-dependent endocytosis of CD33-related Siglecs: role of intracellular domain, tyrosine phosphorylation, and the tyrosine phosphatases, Shp1 and Shp2[J]. J Leukocyte Biol, 2008, 83: 200-211. DOI:10.1189/jlb.0607388 |
| [27] |
Crocker PR, Redelinghuys P. Siglecs as positive and negative regulators of the immune system[J]. Biochem Soc Trans, 2008, 36: 1467-1471. DOI:10.1042/BST0361467 |
| [28] |
Ali SR, Fong JJ, Carlin AF, et al. Siglec-5 and Siglec-14 are polymorphic paired receptors that modulate neutrophil and amnion signaling responses to group B streptococcus[J]. J Exp Med, 2014, 211: 1231-1242. DOI:10.1084/jem.20131853 |
| [29] |
Crunkhorn S. Inflammation: Siglec-targeting nanoparticle treats sepsis[J]. Nat Rev Drug Discov, 2015, 14: 750. |
| [30] |
Angata T, Margulies EH, Green ED, et al. Large-scale sequencing of the CD33-related Siglec gene cluster in five mammalian species reveals rapid evolution by multiple mechanisms[J]. Proc Natl Acad Sci U S A, 2004, 101: 13251-13256. DOI:10.1073/pnas.0404833101 |
| [31] |
Fraschilla I, Pillai S. Viewing Siglecs through the lens of tumor immunology[J]. Immunol Rev, 2017, 276: 178-191. DOI:10.1111/imr.12526 |
| [32] |
Angata T. Associations of genetic polymorphisms of Siglecs with human diseases[J]. Glycobiology, 2014, 24: 785-793. DOI:10.1093/glycob/cwu043 |
| [33] |
Fehervari Z. Targeting cancer by siglecs[J]. Nat Immunol, 2018, 19: 1148. |
| [34] |
Duan S, Paulson JC. Siglecs as immune cell checkpoints in disease[J]. Annu Rev Immunol, 2020, 38: 365-395. DOI:10.1146/annurev-immunol-102419-035900 |
| [35] |
Morishita M, Takahashi Y, Nishikawa M, et al. Pharmacokinetics of exosomes—an important factor for elucidating the biological roles of exosomes and for the development of exosome-based therapeutics[J]. J Pharm Sci, 2017, 106: 2265-2269. DOI:10.1016/j.xphs.2017.02.030 |
| [36] |
Chen WC, Sigal DS, Saven A, et al. Targeting B lymphoma with nanoparticles bearing glycan ligands of CD22[J]. Leuk Lymphoma, 2012, 53: 208-210. DOI:10.3109/10428194.2011.604755 |
| [37] |
Herrmann H, Cerny-Reiterer S, Gleixner KV, et al. CD34+/CD38- stem cells in chronic myeloid leukemia express Siglec-3 (CD33) and are responsive to the CD33-targeting drug gemtuzumab/ozogamicin[J]. Haematologica, 2011, 97: 219-226. |
| [38] |
Ikehara Y, Ikehara SK, Paulson JC. Negative regulation of T cell receptor signaling by Siglec-7 (p70/AIRM) and Siglec-9[J]. J Biol Chem, 2004, 279: 43117-43125. DOI:10.1074/jbc.M403538200 |
| [39] |
Nicoll G, Ni J, Liu D, et al. Identification and characterization of a novel siglec, siglec-7, expressed by human natural killer cells and monocytes[J]. J Biol Chem, 1999, 274: 34089-34095. DOI:10.1074/jbc.274.48.34089 |
| [40] |
Siddiqui S, Schwarz F, Springer S, et al. Studies on the detection, expression, glycosylation, dimerization, and ligand binding properties of mouse Siglec-E[J]. J Biol Chem, 2017, 292: 1029-1037. DOI:10.1074/jbc.M116.738351 |
| [41] |
Mcmillan SJ, Sharma RS, Mckenzie EJ, et al. Siglec-E is a negative regulator of acute pulmonary neutrophil inflammation and suppresses CD11b beta2-integrin-dependent signaling[J]. Blood, 2013, 121: 2084-2094. DOI:10.1182/blood-2012-08-449983 |
| [42] |
Beatson R, Tajadura-Ortega V, Achkova D, et al. The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9[J]. Nat Immunol, 2016, 17: 1273-1281. DOI:10.1038/ni.3552 |
| [43] |
Barkal AA, Brewer RE, Markovic M, et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy[J]. Nature, 2019, 572: 392-396. DOI:10.1038/s41586-019-1456-0 |
| [44] |
Wang J, Sun J, Liu LN, et al. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy[J]. Nat Med, 2019, 25: 656-666. DOI:10.1038/s41591-019-0374-x |
| [45] |
Engblom C, Pfirschke C, Zilionis R, et al. Osteoblasts remotely supply lung tumors with cancer-promoting Siglec Fhigh neutrophils[J]. Science, 2017, 358: 5081. DOI:10.1126/science.aal5081 |
| [46] |
Tedder TF, Steeber DA, Chen A, et al. The selectins: vascular adhesion molecules[J]. FASEB J, 1995, 9: 866-873. DOI:10.1096/fasebj.9.10.7542213 |
| [47] |
Ley K. The role of selectins in inflammation and disease[J]. Trends Mol Med, 2003, 9: 263-268. DOI:10.1016/S1471-4914(03)00071-6 |
| [48] |
Patel KD, Cuvelier SL, Wiehler S. Selectins: critical mediators of leukocyte recruitment[J]. Semin Immunol, 2002, 14: 73-81. DOI:10.1006/smim.2001.0344 |
| [49] |
Ehrhardt C, Kneuer C, Bakowsky U. Selectins-an emerging target for drug delivery[J]. Adv Drug Deliv Rev, 2004, 56: 527-549. DOI:10.1016/j.addr.2003.10.029 |
| [50] |
Lubor B. Selectins in cancer immunity[J]. Glycobiology, 2018, 28: 648-655. DOI:10.1093/glycob/cwx105 |
| [51] |
Cagnoni AJ, Pérez SJM, Rabinovich GA, et al. Turning-off signaling by Siglecs, selectins and galectins: chemical inhibition of glycan-dependent interactions in cancer[J]. Front Oncol, 2016, 6: 109. |
| [52] |
Korniluk A, Kamińska J, Kiszo P, et al. Lectin adhesion proteins (P-, L- and E-selectins) as biomarkers in colorectal cancer[J]. Biomarkers, 2017, 22: 629-634. |
| [53] |
Läubli H, Borsig L. Selectins promote tumor metastasis[J]. Semin Cancer Biol, 2010, 20: 169-177. DOI:10.1016/j.semcancer.2010.04.005 |
| [54] |
Lai XX, Wang S, Yan XY, et al. Strategies and reflections on platelet-based targeted therapy for tumor[J]. Acta Pharm Sin (药学学报), 2021, 56: 1025-1034. |
| [55] |
Mezouar S, Frère C, Darbousset R, et al. Role of platelets in cancer and cancer-associated thrombosis: experimental and clinical evidences[J]. Thromb Res, 2016, 139: 65-76. DOI:10.1016/j.thromres.2016.01.006 |
| [56] |
Becker KA, Beckmann N, Adams C, et al. Melanoma cell metastasis via P-selectin-mediated activation of acid sphingomyelinase in platelets[J]. Clin Exp Metastas, 2017, 34: 25-35. DOI:10.1007/s10585-016-9826-6 |
| [57] |
Borsig L, Wong R, Feramisco J, et al. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis[J]. Proc Natl Acad Sci U S A, 2001, 98: 3352-3357. DOI:10.1073/pnas.061615598 |
| [58] |
Ferber S, Tiram G, Sousa-Herves A, et al. Co-targeting the tumor endothelium and P-selectin-expressing glioblastoma cells leads to a remarkable therapeutic outcome[J]. eLife, 2017, 6: 1-34. |
| [59] |
Carrascal MA, Silva M, Ramalho JS, et al. Inhibition of fucosylation in human invasive ductal carcinoma reduces E-selectin ligand expression, cell proliferation and ERK1/2 and p38 MAPK activation[J]. Mol Oncol, 2018, 12: 579-593. DOI:10.1002/1878-0261.12163 |
| [60] |
Esposito M, Mondal N, Greco TM, et al. Bone vascular niche E-selectin induces mesenchymal–epithelial transition and Wnt activation in cancer cells to promote bone metastasis[J]. Nat Cell Biol, 2019, 21: 627-639. DOI:10.1038/s41556-019-0309-2 |
| [61] |
Mohammadalipour A, Showalter C, Muturi HT, et al. Cell membrane cholesterol modulates lung cancer cell adhesion and rolling on E-selectin[J]. Cancer Res, 2018, 78: 98. |
| [62] |
Liubomirski Y, Lerrer S, Meshel T, et al. Tumor-stroma-inflammation networks promote pro-metastatic chemokines and aggressiveness characteristics in triple-negative breast cancer[J]. Front Immunol, 2019, 10: 757. DOI:10.3389/fimmu.2019.00757 |
| [63] |
Paget S. The distribution of secondary growths in cancer of the breast[J]. Cancer Metast Rev, 1989, 8: 98-101. |
| [64] |
Han T, Chen Y, Zhai D. Research progress of natural polysaccharides and their nano-sized drug delivery systems in regulating tumor microenvironment[J]. Acta Pharm Sin (药学学报), 2021. DOI:10.16438/j.0513-4870.2019-0396 |
| [65] |
Cellars NJ, Lanier AL, Burdick MM. Breast and colon cancer cells express L-selectin ligands that interact with L-selectin on white blood cells under flow conditions[J]. Cancer Res, 2019, 79: 63. |
| [66] |
Ivetic A, Green HLH, Hart SJ. L-selectin: a major regulator of leukocyte adhesion, migration and signaling[J]. Front Immunol, 2019, 10: 1068. DOI:10.3389/fimmu.2019.01068 |
| [67] |
Laubli H, Borsig L. Heparins attenuate cancer metastasis: are selectins the link[J]. Cancer Invest, 2010, 27: 474-481. |
| [68] |
Rzeniewicz K, Newe A, Rey Gallardo A, et al. L-selectin shedding is activated specifically within transmigrating pseudopods of monocytes to regulate cell polarity in vitro[J]. Proc Natl Acad Sci U S A, 2015, 112: 1461-1470. DOI:10.1073/pnas.1417100112 |
| [69] |
Zuchtriegel G, Uhl B, Puhr-Westerheide D, et al. Platelets guide leukocytes to their sites of extravasation[J]. PLoS Biol, 2016, 14: 1-28. |
| [70] |
Dinkla S, Van Cranenbroek B, Van DH, et al. Platelet microparticles inhibit IL-17 production by regulatory T cells through P-selectin[J]. Blood, 2016, 127: 1976-1986. DOI:10.1182/blood-2015-04-640300 |
| [71] |
O'Reilly MK, Paulson JC. Siglecs as targets for therapy in immune-cell-mediated disease[J]. Trends Pharmacol Sci, 2009, 30: 240-248. DOI:10.1016/j.tips.2009.02.005 |
| [72] |
Delputte PL, Van Gorp H, Favoreel HW, et al. Porcine sialoadhesin (CD169/Siglec-1) is an endocytic receptor that allows targeted delivery of toxins and antigens to macrophages[J]. PLoS One, 2011, 6: e16827. DOI:10.1371/journal.pone.0016827 |
| [73] |
Kawasaki N, Vela JL, Nycholat CM, et al. Targeted delivery of lipid antigen to macrophages via the CD169/sialoadhesin endocytic pathway induces robust invariant natural killer T cell activation[J]. Proc Natl Acad Sci U S A, 2013, 110: 7826-7831. DOI:10.1073/pnas.1219888110 |
| [74] |
Chen WC, Kawasaki N, Nycholat CM, et al. Antigen delivery to macrophages using liposomal nanoparticles targeting sialoadhesin/CD169[J]. PLoS One, 2012, 7: e39039. DOI:10.1371/journal.pone.0039039 |
| [75] |
Jabbour E, O'brien S, Ravandi F, et al. Monoclonal antibodies in acute lymphoblastic leukemia[J]. Blood, 2015, 125: 4010-4016. DOI:10.1182/blood-2014-08-596403 |
| [76] |
Norsworthy KJ, Ko CW, Lee JE, et al. FDA approval summary: mylotarg for treatment of patients with relapsed or refractory CD33‐positive acute myeloid leukemia[J]. Oncologist, 2018, 23: 1103-1108. DOI:10.1634/theoncologist.2017-0604 |
| [77] |
Li JG, Luan SR, Zhou XB. A new ADC drug for the treatment of acute lymphoblastic leukemia——BESPONSA[J]. Clin Med J (临床药物治疗杂志), 2017, 15: 9-14. |
| [78] |
Sohita D. Moxetumomab pasudotox: first global approval[J]. Drugs, 2018, 78: 1763-1767. DOI:10.1007/s40265-018-1000-9 |
| [79] |
Sullivan-Chang L, O'donnell RT, Tuscano JM. Targeting CD22 in B-cell malignancies: current status and clinical outlook[J]. BioDrugs, 2013, 27: 293-304. DOI:10.1007/s40259-013-0016-7 |
| [80] |
Laszlo GS, Estey EH, Walter RB. The past and future of CD33 as therapeutic target in acute myeloid leukemia[J]. Blood Rev, 2014, 28: 143-153. DOI:10.1016/j.blre.2014.04.001 |
| [81] |
Kawasaki N, Rillahan CD, Cheng TY, et al. Targeted delivery of mycobacterial antigens to human dendritic cells via Siglec-7 induces robust T cell activation[J]. J Immunol, 2014, 193: 1560-1566. DOI:10.4049/jimmunol.1303278 |
| [82] |
Quentin H, Kayluz FB, Camilla J, et al. Siglec-9 regulates an effector memory CD8+ T-cell subset that congregates in the melanoma tumor microenvironment[J]. Cancer Immunol Res, 2019, 7: 707-718. DOI:10.1158/2326-6066.CIR-18-0505 |
| [83] |
Vivier E, Ugolini S, Blaise D, et al. Targeting natural killer cells and natural killer T cells in cancer[J]. Nat Rev Immunol, 2012, 12: 239-252. DOI:10.1038/nri3174 |
| [84] |
Choi J, Gyamfi J, Jang H, et al. The role of tumor-associated macrophage in breast cancer biology[J]. Histol Histopathol, 2018, 33: 133-145. |
| [85] |
Büll C, Heise T, Adema GJ, et al. Sialic acid mimetics to target the sialic acid–Siglec axis[J]. Trends Biochem Sci, 2016, 41: 519-531. DOI:10.1016/j.tibs.2016.03.007 |
| [86] |
Attrill H, Takazawa H, Witt S, et al. The structure of Siglec-7 in complex with sialosides: leads for rational structure-based inhibitor design[J]. Biochem J, 2006, 397: 271-278. DOI:10.1042/BJ20060103 |
| [87] |
Kelm SR, Madge P, Islam T, et al. C-4 modified sialosides enhance binding to Siglec-2 (CD22): towards potent Siglec inhibitors for immunoglycotherapy[J]. Angew Chem Int Ed Engl, 2013, 52: 3616-3620. DOI:10.1002/anie.201207267 |
| [88] |
Madge PD, Maggioni A, Pascolutti M, et al. Structural characterisation of high affinity Siglec-2 (CD22) ligands in complex with whole Burkitt's lymphoma (BL) Daudi cells by NMR spectroscopy[J]. Sci Rep, 2016, 6: 36012. DOI:10.1038/srep36012 |
| [89] |
Gonzálezamaro R, Sánchezmadrid F. Cell adhesion molecules: selectins and integrins[J]. Crit Rev Immunol, 1999, 19: 389-429. |
| [90] |
Hirsh J. Low molecular weight heparin[J]. Harefuah, 1993, 70: 204-207. |
| [91] |
Lubor B. Selectins facilitate carcinoma metastasis and heparin can prevent them[J]. Physiology, 2004, 19: 16-21. DOI:10.1152/nips.01450.2003 |
| [92] |
Kevane B, Egan K, Allen S, et al. Endothelial barrier protective properties of low molecular weight heparin: a novel potential tool in the prevention of cancer metastasis[J]. Res Pract Thromb Haemost, 2017, 1: 23-32. DOI:10.1002/rth2.12011 |
| [93] |
Alam F, Al-Hilal TA, Park J, et al. Multi-stage inhibition in breast cancer metastasis by orally active triple conjugate, LHTD4 (low molecular weight heparin-taurocholate-tetrameric deoxycholate)[J]. Biomaterials, 2016, 86: 56-67. DOI:10.1016/j.biomaterials.2016.01.058 |
| [94] |
Koenig A, Norgard-Sumnicht K, Linhardt R, et al. Differential interactions of heparin and heparan sulfate glycosaminoglycans with the selectins. Implications for the use of unfractionated and low molecular weight heparins as therapeutic agents[J]. J Clin Invest, 1998, 101: 877-889. DOI:10.1172/JCI1509 |
| [95] |
Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D)[J]. J Clin Oncol, 2018, 36: 2017-2023. DOI:10.1200/JCO.2018.78.8034 |
| [96] |
Stevenson JL, Varki A, Borsig L. Heparin attenuates metastasis mainly due to inhibition of P-and L-selectin, but non-anticoagulant heparins can have additional effects[J]. Thromb Res, 2007, 120: S107-S111. DOI:10.1016/S0049-3848(07)70138-X |
| [97] |
Kragh M, Binderup L, Vig Hjarnaa PJ, et al. Non-anti-coagulant heparin inhibits metastasis but not primary tumor growth[J]. Oncol Rep, 2005, 14: 99-104. |
| [98] |
Kragh M, Loechel F. Non-anti-coagulant heparins: a promising approach for prevention of tumor metastasis[J]. Int J Oncol, 2005, 27: 1159-1167. |
| [99] |
Fritzsche J, Alban S, Ludwig RJ, et al. The influence of various structural parameters of semisynthetic sulfated polysaccharides on the P-selectin inhibitory capacity[J]. Biochem Pharmacol, 2006, 72: 474-485. DOI:10.1016/j.bcp.2006.05.006 |
| [100] |
Bachelet L, Bertholon I, Lavigne D, et al. Affinity of low molecular weight fucoidan for P-selectin triggers its binding to activated human platelets[J]. Biochim Biophy Acta, 2009, 1790: 141-146. DOI:10.1016/j.bbagen.2008.10.008 |
| [101] |
Fuster M, Brown JR, Wang L, et al. A disaccharide precursor of sialyl Lewis X inhibits metastatic potential of tumor cells[J]. Cancer Res, 2003, 63: 2775-2781. |
| [102] |
Marathe DD, Buffone AJ, Chandrasekaran EV, et al. Fluorinated per-acetylated GalNAc metabolically alters glycan structures on leukocyte PSGL-1 and reduces cell binding to selectins[J]. Blood, 2010, 115: 1303-1312. DOI:10.1182/blood-2009-07-231480 |
| [103] |
Azab AK, Quang P, Azab F, et al. P-selectin glycoprotein ligand regulates the interaction of multiple myeloma cells with the bone marrow microenvironment[J]. Blood, 2012, 119: 1468-1478. DOI:10.1182/blood-2011-07-368050 |
| [104] |
Dorian C, Misha D, Angela H, et al. E-selectin inhibition with GMI-1271 decreases venous thrombosis without profoundly affecting tail vein bleeding in a mouse model[J]. Thromb Haemost, 2017, 117: 1171-1181. DOI:10.1160/TH16-04-0323 |
| [105] |
Steele MM, Radhakrishnan P, Magnani JL, et al. Abstract 4503: a small molecule glycomimetic antagonist of E-selectin (GMI-1271) prevents pancreatic tumor metastasis and offers a novel treatment for improved efficacy of chemotherapy[J]. Cancer Res, 2014, 74: 4503. |
| [106] |
Steele MM, Fogler WE, Magnani JL, et al. Abstract 425: a small molecule glycomimetic antagonist of E-selectin and CXCR4 (GMI-1359) prevents pancreatic tumor metastasis and improves chemotherapy[J]. Cancer Res, 2015, 75: 425. |
| [107] |
Shamay Y, Raviv L, Golan M, et al. Inhibition of primary and metastatic tumors in mice by E-selectin-targeted polymer-drug conjugates[J]. J Control Release, 2015, 217: 102-112. DOI:10.1016/j.jconrel.2015.08.029 |
| [108] |
Shamay Y, Elkabets M, Li H, et al. P-selectin is a nanotherapeutic delivery target in the tumor microenvironment[J]. SciTransl Med, 2016, 8: 345ra87. |
| [109] |
Chu PY, Tsai SC, Ko HY, et al. Co-delivery of natural compounds with a dual-targeted nanoparticle delivery system for improving synergistic therapy in an orthotopic tumor model[J]. ACS Appl Mater Interfaces, 2019, 11: 23880-23892. DOI:10.1021/acsami.9b06155 |
| [110] |
Wayne EC, Chandrasekaran S, Mitchell MJ, et al. TRAIL-coated leukocytes that prevent the bloodborne metastasis of prostate cancer[J]. J Control Release, 2016, 223: 215-223. DOI:10.1016/j.jconrel.2015.12.048 |
| [111] |
Wilhelm S, Tavares AJ, Qin D, et al. Analysis of nanoparticle delivery to tumours[J]. Nat Rev Mater, 2016, 1: 16014. DOI:10.1038/natrevmats.2016.14 |
| [112] |
Chen G, Roy I, Yang C, et al. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy[J]. Chem Rev, 2016, 116: 2826-2885. DOI:10.1021/acs.chemrev.5b00148 |
| [113] |
Zheng HL, Song YZ, Deng YH. A review for phagocyte system: the executors of nanoparticles clearance[J]. J Shenyang Pharm Univ (沈阳药科大学学报), 2019, 36: 91-102. |
| [114] |
Biffi S, Voltan R, Bortot B, et al. Actively targeted nanocarriers for drug delivery to cancer cells[J]. Expert Opin Drug Deliv, 2019, 16: 481-496. DOI:10.1080/17425247.2019.1604679 |
| [115] |
Hua Q, Qiang Z, Chu M, et al. Polymeric drug delivery system with actively targeted cell penetration and nuclear targeting for cancer therapy[J]. ACS Appl Biol Mater, 2019, 2: 1724-1731. DOI:10.1021/acsabm.9b00097 |
| [116] |
Varki A. Are humans prone to autoimmunity? Implications from evolutionary changes in hominin sialic acid biology[J]. J Autoimmun, 2017, 83: 134-142. DOI:10.1016/j.jaut.2017.07.011 |
| [117] |
Qiu QJ, Lu M, Li C, et al. Novel self-assembled ibrutinib-phospholipid complex for potently peroral delivery of poorly soluble drugs with pH-dependent solubility[J]. AAPS PharmSciTech, 2018, 19: 3571-3583. DOI:10.1208/s12249-018-1147-4 |
| [118] |
Zheng H, Li J, Luo X, et al. Murine RAW264.7 cells as cellular drug delivery carriers for tumor therapy: a good idea[J]. Cancer Chemoth Pharm, 2019, 83: 361-374. DOI:10.1007/s00280-018-3735-0 |
| [119] |
Luo X, Hu L, Zheng H, et al. Neutrophil-mediated delivery of pixantrone-loaded liposomes decorated with poly(sialic acid)-octadecylamine conjugate for lung cancer treatment[J]. Drug Deliv, 2018, 25: 1200-1212. DOI:10.1080/10717544.2018.1474973 |
| [120] |
Deninno MP. The synthesis and glycosidation of N-acetylneuraminic acid[J]. Synthesis, 1991, 23: 583-593. |
| [121] |
Allen TM, Chonn A. Large unilamellar liposomes with low uptake into the reticuloendothelial system[J]. FEBS Lett, 1987, 223: 42-46. DOI:10.1016/0014-5793(87)80506-9 |
| [122] |
Gregoriadis G, Mccormack B, Wang Z, et al. Polysialic acids: potential in drug delivery[J]. FEBS Lett, 1993, 315: 271-276. DOI:10.1016/0014-5793(93)81177-2 |
| [123] |
Jung B, Shim MK, Park MJ, et al. Hydrophobically modified polysaccharide-based on polysialic acid nanoparticles as carriers for anticancer drugs[J]. Int J Pharm, 2017, 520: 111-118. DOI:10.1016/j.ijpharm.2017.01.055 |
| [124] |
Lehti TA, Pajunen MI, Skog MS, et al. Internalization of a polysialic acid-binding Escherichia coli bacteriophage into eukaryotic neuroblastoma cells[J]. Nat Commun, 2017, 8: 1915. DOI:10.1038/s41467-017-02057-3 |
| [125] |
Zhang R, Jain S, Rowland M, et al. Advances in insulin pen technologies: development and testing of solid dose formulations containing polysialic acid insulin conjugate: next generation of long-acting insulin[J]. J Diabetes Sci Technol, 2010, 4: 532. DOI:10.1177/193229681000400305 |
| [126] |
Kontermann RE. Strategies for extended serum half-life of protein therapeutics[J]. Curr Opin Biotech, 2011, 22: 868-876. DOI:10.1016/j.copbio.2011.06.012 |
| [127] |
Hu JB, Kang XQ, Jing L, et al. E-selectin-targeted sialic acid-PEG-dexamethasone micelles for enhanced anti-inflammatory efficacy for acute kidney injury[J]. Theranostics, 2017, 7: 2204-2219. DOI:10.7150/thno.19571 |
| [128] |
Huang Z, Zhang Z, Jiang Y, et al. Targeted delivery of oligonucleotides into tumor-associated macrophages for cancer immunotherapy[J]. J Control Release, 2012, 158: 286-292. DOI:10.1016/j.jconrel.2011.11.013 |
| [129] |
Zhang T, Zhou S, Hu L, et al. Polysialic acid-polyethylene glycol conjugate-modified liposomes as a targeted drug delivery system for epirubicin to enhance anticancer efficiency[J]. Drug Deliv Transl Res, 2018, 8: 602-616. DOI:10.1007/s13346-018-0496-6 |
| [130] |
Xiong Y, Li M, Lu Q, et al. Sialic acid-targeted biointerface materials and bio-applications[J]. Polymers, 2017, 9: 249. DOI:10.3390/polym9070249 |
| [131] |
Jayant S, Khandare JJ, Wang Y, et al. Targeted sialic acid-doxorubicin prodrugs for intracellular delivery and cancer treatment[J]. Pharm Res, 2007, 24: 2120-2130. DOI:10.1007/s11095-007-9406-1 |
| [132] |
Vodovozova EL, Moiseeva EV, Grechko GK, et al. Antitumour activity of cytotoxic liposomes equipped with selectin ligand SiaLe(X), in a mouse mammary adenocarcinoma model[J]. Eur J Cancer, 2000, 36: 942-949. DOI:10.1016/S0959-8049(00)00029-0 |
| [133] |
Vodovozova EL, Gaenko GP, Bobrikova ES, et al. A diglyceride derivative of methotrexate: synthesis and cytotoxic activity in addressed liposomes[J]. Pharm Chem J, 2008, 41: 297-301. |
| [134] |
Hirai M, Minematsu H, Hiramatsu Y, et al. Novel and simple loading procedure of cisplatin into liposomes and targeting tumor endothelial cells[J]. Int J Pharm, 2010, 391: 274-283. DOI:10.1016/j.ijpharm.2010.02.030 |
| [135] |
Xu XL, Lu KJ, Zhu ML, et al. Sialic acid-functionalized pH-triggered micelles for enhanced tumor tissue accumulation and active cellular internalization of orthotopic hepatocarcinoma[J]. ACS Appl Mater Interfaces, 2018, 10: 31903-31914. DOI:10.1021/acsami.8b09498 |
| [136] |
Zheng JS, Zheng SY, Zhang YB, et al. Sialic acid surface decoration enhances cellular uptake and apoptosis-inducing activity of selenium nanoparticles[J]. Colloid Surface B, 2011, 83: 183-187. DOI:10.1016/j.colsurfb.2010.11.023 |
| [137] |
Zeisig R, Stahn R, Wenzel K, et al. Effect of sialyl Lewis X-glycoliposomes on the inhibition of E-selectin-mediated tumour cell adhesion in vitro[J]. Biochim Biophys Acta, 2004, 1660: 31-40. DOI:10.1016/j.bbamem.2003.10.014 |
| [138] |
Saiki I, Koike C, Obata A, et al. Functional role of sialyl Lewis X and fibronectin-derived RGDS peptide analogue on tumor-cell arrest in lungs followed by extravasation[J]. Int J Cancer, 1996, 65: 833-839. DOI:10.1002/(SICI)1097-0215(19960315)65:6<833::AID-IJC21>3.0.CO;2-3 |
| [139] |
Keil C, Zeisig R, Fichtner I. Effect of surface modified liposomes on the aggregation of platelets and tumor cells[J]. Thromb Haemost, 2005, 94: 404-411. |
| [140] |
She ZN, Zhang T, Wang XL, et al. The anticancer efficacy of pixantrone-loaded liposomes decorated with sialic acid-octadecylamine conjugate[J]. Biomaterials, 2014, 35: 5216-5225. DOI:10.1016/j.biomaterials.2014.03.022 |
| [141] |
Zhou SL, Zhang T, Peng B, et al. Targeted delivery of epirubicin to tumor-associated macrophages by sialic acid-cholesterol conjugate modified liposomes with improved antitumor activity[J]. Int J Pharm, 2017, 523: 203-216. DOI:10.1016/j.ijpharm.2017.03.034 |
| [142] |
Qiu QJ, Li C, Song YZ, et al. Targeted delivery of ibrutinib to tumor-associated macrophages by sialic acid-stearic acid conjugate modified nanocomplexes for cancer immunotherapy[J]. Acta Biomater, 2019, 92: 184-195. DOI:10.1016/j.actbio.2019.05.030 |
| [143] |
Li C, Qiu QJ, Liu M, et al. Sialic acid-conjugate modified liposomes targeting neutrophils for improved tumour therapy[J]. Biomater Sci, 2020, 8: 2189-2201. DOI:10.1039/C9BM01732A |
| [144] |
Chen Z, Liu M, Li L, et al. Involvement of the Warburg effect in non-tumor diseases processes[J]. J Cell Physiol, 2018, 233: 2839-2849. DOI:10.1002/jcp.25998 |
| [145] |
Mandal C, Mandal C. Sialic acid binding lectins[J]. Experientia, 1990, 46: 433-441. DOI:10.1007/BF01954221 |
| [146] |
Zhang Z, Wuhrer M, Holst S. Serum sialylation changes in cancer[J]. Glycoconj J, 2018, 35: 139-160. DOI:10.1007/s10719-018-9820-0 |
| [147] |
Thing TS, Ogrodzinski MP, Christina R, et al. Sialic acid metabolism: a key player in breast cancer metastasis revealed by metabolomics[J]. Front Oncol, 2018, 8: 174. DOI:10.3389/fonc.2018.00174 |
| [148] |
Chiodelli P, Urbinati C, Paiardi G, et al. Sialic acid as a target for the development of novel antiangiogenic strategies[J]. Future Med Chem, 2018, 10: 2835-2854. DOI:10.4155/fmc-2018-0298 |
| [149] |
Moons SJ, Adema GJ, Derks MT, et al. Sialic acid glycoengineering using N-acetylmannosamine and sialic acid analogs[J]. Glycobiology, 2019, 29: 433-445. |
| [150] |
Kang SA, Blache CA, Bajana S, et al. The effect of soluble E-selectin on tumor progression and metastasis[J]. BMC Cancer, 2016, 16: 331. DOI:10.1186/s12885-016-2366-2 |
| [151] |
Ku AW, Muhitch JB, Powers CA, et al. Tumor-induced MDSC act via remote control to inhibit L-selectin-dependent adaptive immunity in lymph nodes[J]. eLife, 2016, 5: e17375. DOI:10.7554/eLife.17375 |
| [152] |
Grabowska J, Lopez-Venegas MA, Affandi AJ, et al. CD169+ macrophages capture and dendritic cells instruct: the interplay of the gatekeeper and the general of the immune system[J]. Front Immunol, 2018, 9: 2472. DOI:10.3389/fimmu.2018.02472 |
| [153] |
Korangath P, Barnett JD, Sharma A, et al. Nanoparticle interactions with immune cells dominate tumor retention and induce T cell-mediated tumor suppression in models of breast cancer[J]. Sci Adv, 2020, 6: eaay1601. DOI:10.1126/sciadv.aay1601 |
| [154] |
Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems[J]. Colloid Surface B, 2010, 75: 1-18. DOI:10.1016/j.colsurfb.2009.09.001 |
| [155] |
Witzigmann D, Uhl P, Sieber S, et al. Optimization-by-design of hepatotropic lipid nanoparticles targeting the sodium-taurocholate cotransporting polypeptide[J]. eLife, 2019, 8: e42276. DOI:10.7554/eLife.42276 |
| [156] |
Srinivasarao M, Galliford CV, Low PS. Principles in the design of ligand-targeted cancer therapeutics and imaging agents[J]. Nat Rev Drug Discov, 2015, 14: 203-219. DOI:10.1038/nrd4519 |
| [157] |
Zhang H, Ma Y, Sun XL. Recent developments in carbohydrate-decorated targeted drug/gene delivery[J]. Med Res Rev, 2011, 30: 270-289. |
 2021, Vol. 56
2021, Vol. 56


