肝纤维化是慢性肝损伤的伤口愈合反应, 肝炎病毒感染、胆汁淤积、自身免疫、药物、毒素、酒精和非酒精性脂肪肝炎(nonalcoholic steatohepatitis, NASH) 等都可引起肝纤维化, 终末期可发展为肝硬化[1-4]。这种伤口愈合反应会减轻炎症组织的破坏, 但也会导致疤痕组织的形成, 其特征是肝细胞外基质(extracellular matrix, ECM) 大量沉积[5, 6]。目前, 肝纤维化的治疗手段仍然有限[7]。
肝星状细胞(hepatic stellate cell, HSC) 的活化是肝纤维化发生的关键步骤[8]。在肝纤维化进程中, 转化生长因子β1 (transforming growth factor-β1, TGF-β1) 被认为是迄今已知的最重要的促纤维化细胞因子之一[9, 10]。TGF-β1信号通路在启动并促进HSC活化过程中发挥重要作用, 可促进胶原(collagen) 的合成以及α-平滑肌肌动蛋白(α-smooth muscle actin, α-SMA) 组成以及应力纤维形成[11, 12]。当肝星状细胞被激活后, TGF-β与Ⅰ型和Ⅱ型跨膜受体的异质复合体结合, 磷酸化并激活受体调节的R-Smads[11, 13], 磷酸化的Smad2/3进一步激活TGF-β靶基因[如Ⅰ型胶原α1 (collagen type Ⅰ α 1 chain, COL1A1)] 的表达[14-16]。因此, TGF-β/Smad通路对于维持肝星状细胞激活状态具有重要作用[9, 17, 18]。此外, Hippo信号通路被公认为是肝脏发育的重要调节通路, 其下游主要的效应分子yes相关蛋白(yes-associated protein, YAP) 在肝星状细胞活化早期就会被激活[19]。最近的研究表明, YAP激活是HSC活化以及肝纤维化进展中的关键驱动因素, 阻断YAP可以阻止静息的HSC活化为肌成纤维细胞[20]。有研究发现, TGF-β与Hippo-YAP的相互作用对调控Smads活性起着关键作用。在缺乏YAP的情况下, Smads不能进一步促进TGF-β的表达[21]。
苦蘵(Physalis angulata L.) 为茄科酸浆属植物, 其功能主治清热、利尿、解毒, 治感冒、肺热咳嗽、咽喉肿痛、龈肿、湿热黄疸、痢疾、水肿、热淋、天疱疮和疔疮[22]。临床多用于治疗黄疸、慢性气管炎和细菌性痢疾, 其中睡茄内酯类化合物为该植物主要活性成分。近期的研究表明, 睡茄内酯类化合物在治疗肝脏疾病方面有较大潜力, 其中单体化合物如physalin A具有潜在的抗肝癌活性, physalin D有较好的抗肝纤维化活性, physalin B则可有效缓解NASH和肝纤维化[23-27]。本课题组从苦蘵中提取并富集得到了睡茄内酯部位(the withanolide extract of Physalis angulata, WEP), 经质谱和HPLC-DAD (high performance liquid chromatography-diode array detector) 检测得到16个化合物峰, 其中10个峰显示睡茄内酯类化合物, 包括physalin B、D、F、I和H 5个physalin类睡茄内酯。随后测定各个化合物的相对含量, 分别为physalin B: 16.50%、physalin D: 10.95%、physalin F: 7.85%、physalin I: 8.08%和physalin H: 8.20%。为了研究WEP对肝纤维化的作用, 本研究构建了四氯化碳(CCl4) 和胆总管结扎(bile duct ligation, BDL) 诱导的两种小鼠肝纤维化动物模型, 研究WEP的体内外抗肝纤维化作用, 同时探讨了WEP抑制肝星状细胞激活改善肝纤维化的机制。结果表明, WEP可有效缓解肝星状细胞激活和肝纤维化, 为中药苦蘵应用于肝纤维化的临床治疗提供实验基础和理论依据。
材料与方法动物 雄性C57 BL/6J小鼠(5周, 体重18~22 g), 购自杭州子源实验动物科技有限公司[SPF级, 合格证号: SCXK (浙) 2019-0004], 饲养于中国药科大学药学动物实验中心。所有动物实验都获得中国药科大学伦理学委员会批准。实验动物在明暗交替(12 h∶12 h) 的SPF级动物房中饲养, 自由进食饮水, 预适应环境7天。
药品与试剂 WEP来源于本实验室, 从苦蘵中分离得到: 苦蘵药材粉碎后95%乙醇超声提3次, 过滤合并滤液, 回收溶剂至干, 得到浸膏, 浸膏拌样上大孔, 梯度冲洗(40%、80%乙醇), 收集80%梯度冲洗下来的馏分, 回收溶剂至干, 得到富集部位, 以50 μg·mL-1储备浓度溶于DMSO中。α-SMA抗体、COL1A1抗体(Abcam公司); Smad2/3、p-Smad2 (Ser465/467)、YAP抗体(Cell Signaling Technology公司); 二抗(上海翊圣生物技术有限公司); β-actin、Smad7抗体(Proteintech公司); 谷丙转氨酶(alanine transaminase, ALT)、谷草转氨酶(aspartate transaminase, AST) 试剂盒(南京建成生物工程研究所有限公司); RIPA裂解液(上海翊圣生物技术有限公司); ECL超敏发光液(南京诺唯赞生物技术有限公司)。
动物模型
CCl4模型的建立 采用CCl4诱导小鼠肝纤维化, 将小鼠随机分为5组, 分别为橄榄油对照组、CCl4模型组及CCl4模型给药(WEP) 组, 每组7只。采用腹腔注射溶于橄榄油的CCl4 (0.25 mL·kg-1) 溶液, 每周3次, 持续4周造成肝纤维化。CCl4模型给药(WEP) 组给药剂量分别为2.5、5和10 mg·kg-1, 在注射CCl4造模的同时伴随每隔一天给药一次[25, 26], 对照组仅注射相同体积的橄榄油。最后一次CCl4注射48 h后麻醉小鼠, 眼眶取血, 采集血样后脱臼处死并收集肝小叶, 部分肝组织样品用4%多聚甲醛固定, 以备随后的组织学检查, 另一部分保存在-80 ℃中, 待进一步分析。
BDL模型的建立 小鼠首先被随机分为两组, 其中一组7只为假手术组, 另一组21只为BDL组。在异氟烷吸入麻醉期间, 通过2 mm中线切口剖腹术打开腹膜腔, 分离胆总管, 外科缝合线结扎胆总管, 术后24 h, BDL组随机分为3组, 其中两组作为BDL-WEP组, 每隔一天分别腹腔注射5和10 mg·kg-1 WEP。术后14天结束实验, 禁食一夜, 使用异氟醚将小鼠麻醉, 从眼眶后静脉丛中采集血样, 采集血样后脱臼处死, 取肝小叶。取部分肝组织样本用4%多聚甲醛固定, 用于随后的组织学检查, 其他的保存在-80 ℃, 用于随后的分析。
体内急性毒性实验 小鼠被随机分为两组, 分别为橄榄油对照组和给药(WEP) 组, 每组5只。给药(WEP) 组腹腔注射100 mg·kg-1 WEP, 对照组仅注射相同体积的橄榄油, 一次给药后观察10天。实验结束时, 禁食一夜, 在异氟醚麻醉后脱臼处死, 取心、肝、脾、肺和肾, 固定于4%的多聚甲醛中, 用于随后的组织学分析。
细胞培养与处理 LX-2细胞来自于Scott Friedman (Mount Sinai School of Medicine, New York, USA) 馈赠。细胞系常规检测支原体。LX-2细胞用DMEM培养基(含10%胎牛血清和1%双抗) 培养于37 ℃、5% CO2的培养箱中。LX-2细胞在无血清培养基中孵育4 h, 分别给予或不给予2 ng·mL-1 TGF-β1刺激6 h, 再给予WEP治疗24 h。
血清生化指标检测 将全血室温静置2 h后进行离心, 常规分离血清, 血清ALT和AST根据说明书按照标准操作程序测定。
肝脏病理切片 取小鼠肝脏固定于4%多聚甲醛24 h以上, 石蜡切片脱蜡水化, 进行苏木素-伊红染色(苏木素染细胞核, 伊红染细胞质), 脱水封片, 显微镜镜检, 图像采集分析。
Sirius red染色 石蜡切片脱蜡水化, 将切片放入饱和苦味酸天狼猩红染色液内染色8 min。切片放入无水酒精漂洗数分钟, 显微镜下观察满意为止。切片于60 ℃烤箱烤干后于二甲苯透明5 min, 中性树胶封片, 显微镜镜检, 图像采集分析。
Masson染色 石蜡切片脱蜡水化, 苏木素染细胞核, 丽春红染色, 磷钼酸处理, 苯胺蓝染色, 分化, 脱水, 中性树胶封片, 显微镜镜检, 图像采集分析。
Western blot 小鼠安乐处死, 分离并取出肝脏, 总蛋白提取: 液氮速冻后加入含有蛋白酶抑制剂的RIPA裂解液超声破碎, 离心取上清液, BCA法蛋白定量, 加入上样缓冲液, 沸水煮15 min, -20 ℃保存。蛋白上样量为40 μg, 具体步骤: 配制聚丙烯酰胺凝胶, 电泳, 将蛋白转印至PVDF膜, 用封闭液3% BSA封闭2 h, 一抗(α-SMA、COL1A1、YAP、Smad2/3、p-Smad2、Smad7、β-actin) 4 ℃孵育过夜, TBST漂洗10 min×3次, 二抗室温孵育2 h, TBST漂洗10 min×3次, 加入ECL超敏发光液显影。使用Bio-Rad ChemiDoc XRS+成像系统进行分析。
Real-time PCR分析 小鼠安乐处死, 分离并取出肝脏, 采用RNA提取试剂盒提取肝脏组织中总RNA, 总RNA经纯度分析及含量计算后逆转录得到cDNA, 获得的cDNA保存于-20 ℃。实时荧光定量PCR检测, 检测指标ACTA2 (actin alpha 2, smooth muscle, 表达α-SMA的基因)、COL1A1以及组织金属蛋白酶抑制剂1 (tissue inhibitor of metalloproteinase 1, TIMP1)。引物序列见表 1。
| Table 1 PCR primers. ACTA2: Actin alpha 2, smooth muscle; COL1A1: Collagen type Ⅰ α 1 chain; TIMP1: Tissue inhibitor of metalloproteinase 1; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase |
统计学分析 实验数据采用
本研究在TGF-β1诱导的LX-2细胞中考察WEP对肝星状细胞活化的影响。在无血清条件下饥饿LX-2细胞后用TGF-β1刺激, 使LX-2细胞在体外显示其激活表型。如图 1A所示, WEP显著抑制了LX-2细胞中肝纤维化标志基因ACTA2和COL1A1的表达, 但是TIMP1的mRNA表达量没有显著变化。WEP处理LX-2细胞后, COL1A1和α-SMA的蛋白表达水平也相应下降了(图 1B)。进一步采用CCK8法检测了WEP对LX-2细胞的影响。结果表明, 5和10 μg·mL-1 WEP对LX-2细胞无明显的细胞毒性(图 1C)。以上结果表明, WEP能够显著抑制TGF-β1诱导的肝星状细胞激活, 而无明显的细胞毒性作用。
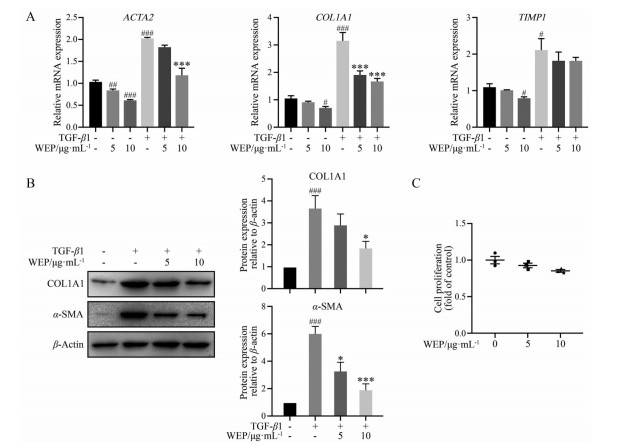
|
Figure 1 The withanolide extract of Physalis angulata (WEP) inhibits the activation of hepatic stellate cells (HSCs). LX-2 cells were treated with 2 ng·mL-1 transforming growth factor-β1 (TGF-β1) with or without different concentrations of WEP for 24 h after no fetal bovine serum (FBS) starvation. A: WEP repressed the mRNA expressions of COL1A1 and ACTA2 in a dose-dependent manner in LX-2 cells. GAPDH served as a loading control; B: WEP inhibited the protein levels of COL1A1 and α-smooth muscle actin (α-SMA) in LX-2 cells; C: Cell proliferation was determined by CCK8 assay. n = 3, |
在进行正式的动物实验之前, 出于药物安全性的考虑, 本研究首先在小鼠体内验证了WEP的毒性。小鼠一次给药后连续观察10天, 观察期间给药组与对照组小鼠无明显行为差异, 随后经H & E染色法考察了WEP给药后对小鼠心、肝、脾、肺和肾的组织结构的影响, 如图 2所示, 给药组相较于对照组, 各器官的组织结构均无明显变化, 由此可说明WEP对小鼠无明显的毒副作用。
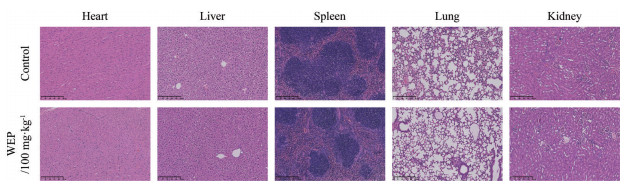
|
Figure 2 WEP has no obvious toxicity in vivo. The histopathology of heart, liver, spleen, lung and kidney was observed by hematoxylin-eosin staining (scale bars: 250 μm) |
本研究进一步在CCl4诱导的小鼠肝纤维化模型中验证WEP的抗肝纤维化作用。采用H & E染色法考察了WEP给药治疗后肝组织病理结构的变化。同时, 采用ALT和AST试剂盒检测血清中谷丙转氨酶和谷草转氨酶的含量, 考察WEP给药治疗后对CCl4诱导的肝损伤的影响。H & E染色结果显示, 相比于CCl4模型组, WEP处理组可明显减轻炎症和小叶中心坏死, 可保护肝细胞组织结构(图 3A)。生化指标检测结果显示, 相较于正常组, 模型组小鼠血清中肝损伤指标ALT和AST显著升高, WEP治疗可降低小鼠血清中ALT和AST水平(图 3B)。接下来, 对肝组织切片进行了Masson染色和Sirius red染色。结果显示, 对照组小鼠肝组织中几乎没有胶原纤维沉积, 而CCl4诱导的模型组小鼠肝中出现大量胶原沉积, 主要分布在汇管区和坏死区域。WEP治疗组中胶原染色面积明显下降, 且高剂量WEP组的改善情况更为显著(图 3C、D)。活化的HSC细胞是ECM主要的来源, 而α-SMA是HSC细胞活化的标记物。如图 3E所示, 与CCl4组相比, WEP治疗组可显著降低肝脏中α-SMA蛋白的表达水平, 证明WEP处理可抑制HSC细胞的活化。以上结果表明WEP治疗后对CCl4诱导的肝损伤以及肝纤维化具有保护作用。
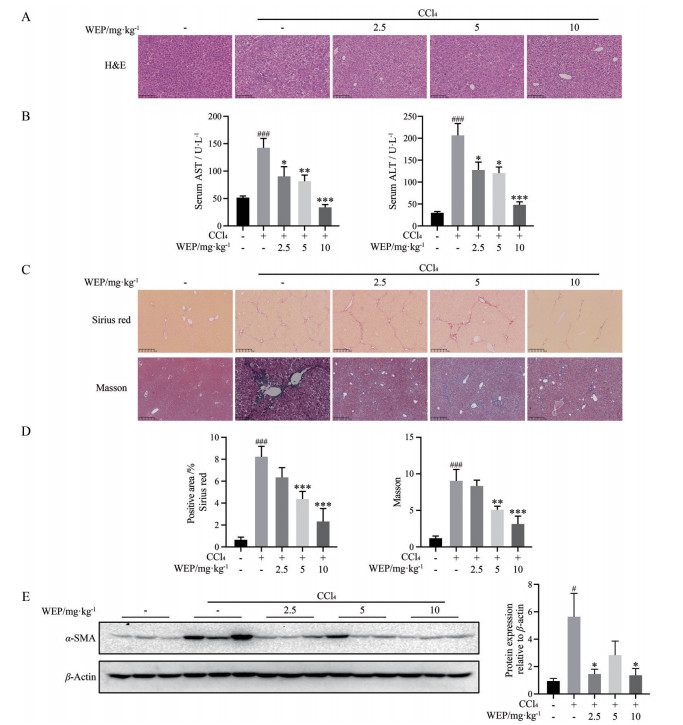
|
Figure 3 WEP ameliorated CCl4-induced liver injury and liver fibrosis. A: Liver histopathology was observed by H & E staining (scale bars: 250 μm); B: Serum aspartate transaminase (AST) and alanine transaminase (ALT) assays demonstrated that WEP reduced liver injury in CCl4-induced mice; C: The obtained liver sections were subjected to Sirius red staining (scale bars: 100 μm, up panel), and Masson trichrome staining (scale bars: 100 μm, down panel); D: The positive areas of Sirius red staining and Masson trichrome staining were normalized against the control group; E: Western blot analysis of α-SMA expression. n = 7, |
本研究构建了BDL诱导的小鼠肝纤维化模型, 进一步考察WEP在BDL诱导的肝纤维化进展中的作用。小鼠肝组织H & E染色结果显示, 正常小鼠肝小叶结构清晰, 肝细胞形态完整; BDL诱导的模型组小鼠肝组织可见大量炎性细胞浸润、空泡样病变和肝细胞坏死; WEP治疗可保护肝细胞组织结构, 缓解BDL导致的小鼠肝组织肿胀、点状坏死、融合性坏死和大量炎性细胞浸润的现象(图 4A)。AST和ALT测定结果表明, 模型组小鼠的肝功能明显受到损伤, WEP治疗可降低血清中AST和ALT水平(图 4B)。进一步采用Masson染色和Sirius red染色评价肝组织中胶原沉积情况。结果显示, BDL组胶原纤维不仅延伸到门静脉区, 也延伸到肝实质, 而WEP治疗组可显著减少肝脏胶原沉积(图 4C、D)。同时, WEP治疗也能降低肝脏组织中α-SMA蛋白的表达水平, 抑制HSC细胞的活化(图 4E)。综上, WEP能够改善BDL诱导的小鼠肝损伤, 减轻肝纤维化水平。

|
Figure 4 WEP ameliorated bile duct ligation (BDL)-induced liver injury and liver fibrosis. A: Liver histopathology was observed by H & E staining (scale bars: 250 μm); B: Serum AST and ALT assays demonstrated that WEP reduced liver injury in BDL-induced mice; C: The obtained liver sections were subjected to Sirius red staining (scale bars: 100 μm, up panel), and Masson trichrome staining (scale bars: 100 μm, down panel); D: The positive areas of Sirius red staining and Masson trichrome staining were normalized against the sham group; E: Western blot analysis of α-SMA expression. n = 7, |
肝纤维化发生过程中, TGF-β1被认为是肝纤维化过程中的关键介质。慢性肝损伤诱导高水平TGF-β1, 从而导致星状细胞活化, 最终促进肝纤维化和肝硬化的发生发展。有研究表明, Hippo通路的关键转录调控因子YAP水平升高与星状细胞活化和肝细胞癌发生密切相关。YAP还可与TGF-β诱导的Smad2/3相互作用, 这表明YAP与Smad2/3复合物协同调节TGF-β信号通路, 从而抑制下游Smad复合物的活性[20, 28]。在LX-2细胞中, WEP处理能够显著抑制TGF-β1诱导的YAP及p-Smad2蛋白表达水平, 但Smad7没有显著变化(图 5A)。此外, 在两种动物模型肝组织中, WEP治疗也能够抑制YAP和p-Smad2的表达(图 5B、C)。以上结果表明, WEP可以通过下调YAP和TGF-β/Smad通路抑制肝星状细胞激活。
讨论HSC持续激活是导致ECM过度积累和肝纤维化的主要驱动力, 因此抑制HSC的激活是逆转肝纤维化的核心[29, 30]。近年来, 已有较多的天然药物活性提取物和单体活性成分被报道具有良好的抗肝纤维化作用。苦蘵为茄科酸浆属植物, 具有清热、利尿、解毒功效。睡茄内酯是其主要活性成分, 它是一类变型甾体化合物, 具有抗肿瘤、抗炎和免疫调节等活性[31-33]。值得注意的是, 目前胆汁淤积性肝病临床用药奥贝胆酸也是一个甾体类化合物, 具有显著的抗炎和抗肝纤维化作用。这启发作者从天然甾体化合物中发现具有抗肝纤维化的活性成分是研发抗肝纤维化药物的有效途径。本研究在体内肝纤维化动物模型和体外肝星状细胞中阐明了苦蘵睡茄内酯提取物(WEP) 缓解肝纤维化的作用和潜在机制。本研究发现: ① WEP治疗可显著改善BDL和CCl4诱导的小鼠肝损伤和纤维化; ② WEP治疗后肝纤维化标志物mRNA和蛋白水平在体内外均显著下调; ③ WEP阻断了TGF-β1和YAP信号通路, 从而抑制HSC激活和肝纤维化。
TGF-β超家族含有众多成员, 如TGF-β、骨形态发生蛋白(bone morphogenetic proteins, BMPs)、激活素、抑制素、生长分化因子(growth differentiation factors, GDFs) 和胶质细胞源性神经营养因子(glial cell line-derived neurotrophic factors, GDNFs) 等, 它们在细胞生命活动中有着不可或缺的作用。多种细胞因子参与了慢性肝损伤进展为肝纤维化的过程, 而TGF-β1是HSC激活的主要调节因子, 在肝损伤、炎症, 到肝纤维化、肝硬化直至最后的肝癌进程中发挥重要作用[10]。肝脏受到损伤后, 坏死的肝细胞分泌TGF-β1将HSC激活, 使其向肌成纤维细胞分化, HSC在激活后会分泌大量的TGF-β1, 又会继续促进HSC活化, TGF-β1在将HSC激活的同时还会通过血小板衍生生长因子受体β (platelet-derived growth factor receptor β, PDGFRβ)、氧化应激和鸟苷三磷酸(Rho GTPase) 等介导HSC的增殖和迁移并维持肌成纤维细胞的表型。在TGF-β1的经典通路中, TGF-β1与TGF-βRⅡ结合, 将TGF-βRⅠ磷酸化并激活, 激活的TGF-βRⅠ进而磷酸化Smad2/3, p-Smad2/3与Smad4结合后进入细胞核发挥作用。TGF-β1还可以通过NADPH氧化酶4 (NADPH oxidase 4, NOX4)、YAP以及丝裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK) 等信号通路发挥促纤维化作用。此外, TGF-β1干扰基质降解蛋白酶的产生, 上调蛋白酶抑制剂, 如组织金属蛋白酶抑制剂(TIMP) 和纤溶酶原激活物抑制剂, 促进ECM的产生并抑制其分解[11, 34-38]。因此, 开发基于TGF-β信号通路的抑制剂有广泛的应用前景, TGF-β靶向化合物包括反义寡核苷酸(AONs)、抑制配体-受体相互作用的中和抗体、配体陷阱以及受体激酶抑制剂。目前, trabedersen (一种AON药物)、fresolimumab (一种泛TGF-β抗体) 以及galunisertib (一种TGF-βRI激酶抑制剂) 等已经进入临床试验[39-41]。本研究表明, 苦蘵睡茄内酯提取物WEP可抑制TGF-β1诱导的Smad2磷酸化, 从而抑制肝星状细胞中纤维生成基因的表达。因此, 作者认为WEP对肝纤维化的抑制作用可能部分依赖于抑制TGF-β/Smad信号。
有研究表明, Hippo通路及其下游效应YAP是星状细胞活化的关键调控因子[42, 43]。HSC/肌成纤维细胞中YAP的上调延长了它们的活化, 促进了ECM的合成, 从而导致更严重的纤维化。此外, 有研究显示YAP靶基因结缔组织因子(connective tissue growth factor, CTGF) 在肝纤维化组织和活化的肝星状细胞中过表达。CTGF可诱导ECM蛋白的合成和分泌, 特别是纤维性胶原, 而纤维性胶原是纤维沉积的主要成分[44]。本研究的另一个重要发现是, WEP显著下调LX-2细胞中YAP的表达。WEP治疗显著降低了CCl4及BDL诱导的肝纤维化组织中YAP的表达。这些结果表明, WEP处理导致的YAP下调可抑制星状细胞活化和肝纤维化。YAP/Smad转录复合物与转录增强相关结构蛋白(transcriptional enhanced associate domain, TEAD) 联合有助于致瘤表型, 这表明YAP可促进并维持TGF-β1通路的致瘤活性。YAP/TEAD与Smad转录因子结合, 促进肾纤维化[45, 46]。在肝纤维化进展过程中, 肝星状细胞激活会导致ECM沉积和TGF-β1增多, 进一步向肝星状细胞发出信号, 以正反馈回路进一步激活YAP[42]。因此, 同时干预YAP和TGF-β/Smad信号通路可能是一种有前途的抗纤维化治疗策略。本研究发现, WEP可以抑制TGF-β/Smad信号, 降低YAP表达, 从而发挥抗纤维化作用。
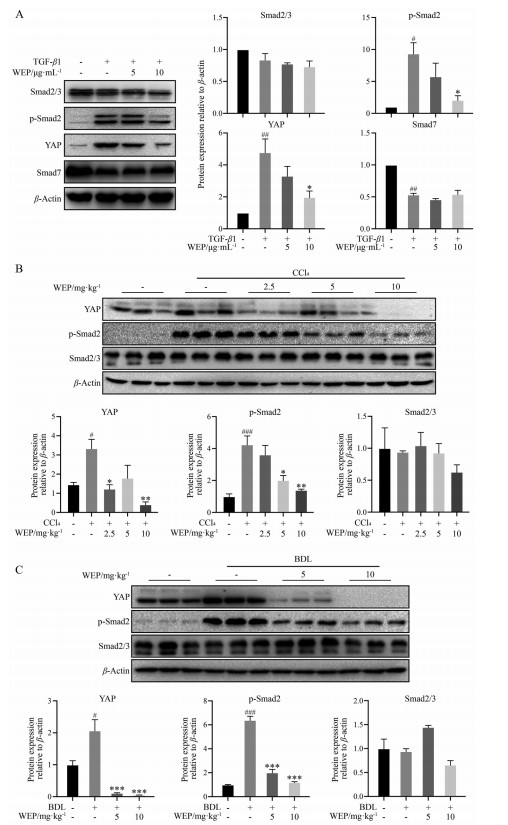
|
Figure 5 WEP inhibits liver fibrosis through regulation of yes-associated protein (YAP) and TGF-β1/Smad pathway. A: LX-2 cells were treated with 2 ng·mL-1 TGF-β1 with or without different concentrations of WEP for 24 h after no FBS starvation. WEP down-regulated the protein expressions of YAP and p-Smad2 in LX-2 cells; B, C: WEP down-regulated the protein expressions of YAP and p-Smad2 in CCl4-treated and BDL-treated livers. n = 3 (A), n = 7 (B, C), |
综上所述, WEP在体内外可抑制肝星状细胞激活和肝纤维化, WEP可下调YAP和TGF-β/Smad信号通路, 从而改善CCl4和BDL诱导的小鼠肝纤维化。本研究为中药苦蘵在临床中治疗肝纤维化提供了实验依据。
作者贡献: 杨挺负责细胞实验和动物实验; 陈馨霖负责苦蘵睡茄内酯提取物的分析和制备; 祝小云和项德娟参与动物实验; 罗建光和张浩负责课题设计、指导和论文审阅。
利益冲突: 所有作者均声明不存在利益冲突。
| [1] |
Bataller R, Brenner DA. Liver fibrosis[J]. J Clin Invest, 2005, 115: 209-218. DOI:10.1172/JCI24282 |
| [2] |
Ge MX, Liu HT, Zhang N, et al. Costunolide represses hepatic fibrosis through WW domain-containing protein 2-mediated Notch3 degradation[J]. Br J Pharmacol, 2020, 177: 372-387. DOI:10.1111/bph.14873 |
| [3] |
Puche JE, Saiman Y, Friedman SL. Hepatic stellate cells and liver fibrosis[J]. Compr Physiol, 2013, 3: 1473-1492. |
| [4] |
Chung SI, Moon H, Ju HL, et al. Hepatic expression of Sonic Hedgehog induces liver fibrosis and promotes hepatocarcinogenesis in a transgenic mouse model[J]. J Hepatol, 2016, 64: 618-627. DOI:10.1016/j.jhep.2015.10.007 |
| [5] |
Hardy T, Mann DA. Epigenetics in liver disease: from biology to therapeutics[J]. Gut, 2016, 65: 1895-1905. DOI:10.1136/gutjnl-2015-311292 |
| [6] |
Friedman SL, Rockey DC, Bissell DM. Hepatic fibrosis 2006: report of the Third AASLD Single Topic Conference[J]. Hepatology, 2007, 45: 242-249. DOI:10.1002/hep.21459 |
| [7] |
Zhang WD, Wang RF, Wu HM, et al. Research progress in drugs for anti-hepatic fibrosis[J]. Acta Pharm Sin (药学学报), 2018, 53: 667-675. |
| [8] |
Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression[J]. Nat Rev Gastroenterol Hepatol, 2021, 18: 151-166. DOI:10.1038/s41575-020-00372-7 |
| [9] |
Dooley S, Hamzavi J, Ciuclan L, et al. Hepatocyte-specific Smad7 expression attenuates TGF-beta-mediated fibrogenesis and protects against liver damage[J]. Gastroenterology, 2008, 135: 642-659. DOI:10.1053/j.gastro.2008.04.038 |
| [10] |
Zhao SS, Shao RG, He HW. Potential targets for anti-liver fibrosis[J]. Acta Pharm Sin (药学学报), 2014, 49: 1365-1371. |
| [11] |
Fabregat I, Moreno-Caceres J, Sanchez A, et al. TGF-beta signalling and liver disease[J]. FEBS J, 2016, 283: 2219-2232. DOI:10.1111/febs.13665 |
| [12] |
Wu X, Wu X, Ma Y, et al. CUG-binding protein 1 regulates HSC activation and liver fibrogenesis[J]. Nat Commun, 2016, 7: 13498. DOI:10.1038/ncomms13498 |
| [13] |
Gressner AM, Weiskirchen R, Breitkopf K, et al. Roles of TGF-beta in hepatic fibrosis[J]. Front Biosci, 2002, 7: d793-d807. DOI:10.2741/gressner |
| [14] |
Song L, Chen TY, Zhao XJ, et al. Pterostilbene prevents hepatocyte epithelial-mesenchymal transition in fructose-induced liver fibrosis through suppressing miR-34a/Sirt1/p53 and TGF-beta1/Smads signalling[J]. Br J Pharmacol, 2019, 176: 1619-1634. DOI:10.1111/bph.14573 |
| [15] |
Liu J, Kong D, Qiu J, et al. Praziquantel ameliorates CCl4-induced liver fibrosis in mice by inhibiting TGF-beta/Smad signalling via up-regulating Smad7 in hepatic stellate cells[J]. Br J Pharmacol, 2019, 176: 4666-4680. DOI:10.1111/bph.14831 |
| [16] |
Cai X, Li Z, Zhang Q, et al. CXCL6-EGFR-induced Kupffer cells secrete TGF-beta1 promoting hepatic stellate cell activation via the SMAD2/BRD4/C-MYC/EZH2 pathway in liver fibrosis[J]. J Cell Mol Med, 2018, 22: 5050-5061. DOI:10.1111/jcmm.13787 |
| [17] |
Koo JH, Lee HJ, Kim W, et al. Endoplasmic reticulum stress in hepatic stellate cells promotes liver fibrosis via PERK-mediated degradation of HNRNPA1 and up-regulation of SMAD2[J]. Gastroenterology, 2016, 150: 181-193. DOI:10.1053/j.gastro.2015.09.039 |
| [18] |
Xu T, Ni MM, Xing L, et al. NLRC5 regulates TGF-beta1-induced proliferation and activation of hepatic stellate cells during hepatic fibrosis[J]. Int J Biochem Cell Biol, 2016, 70: 92-104. DOI:10.1016/j.biocel.2015.11.010 |
| [19] |
Grannas K, Arngarden L, Lonn P, et al. Crosstalk between Hippo and TGFbeta: subcellular localization of YAP/TAZ/Smad complexes[J]. J Mol Biol, 2015, 427: 3407-3415. DOI:10.1016/j.jmb.2015.04.015 |
| [20] |
Hiemer SE, Szymaniak AD, Varelas X. The transcriptional regulators TAZ and YAP direct transforming growth factor beta-induced tumorigenic phenotypes in breast cancer cells[J]. J Biol Chem, 2014, 289: 13461-13474. DOI:10.1074/jbc.M113.529115 |
| [21] |
Lee EH, Park KI, Kim KY, et al. Liquiritigenin inhibits hepatic fibrogenesis and TGF-beta1/Smad with Hippo/YAP signal[J]. Phytomedicine, 2019, 62: 152780. DOI:10.1016/j.phymed.2018.12.003 |
| [22] |
Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China 2010 Edition[S]. Beijing: Chemical Industry Press, 2010: 337-338.
|
| [23] |
Wu SJ, Ng LT, Chen CH, et al. Antihepatoma activity of Physalis angulata and P. peruviana extracts and their effects on apoptosis in human Hep G2 cells[J]. Life Sci, 2004, 74: 2061-2073. DOI:10.1016/j.lfs.2003.09.058 |
| [24] |
Shin JM, Lee KM, Lee HJ, et al. Physalin A regulates the Nrf2 pathway through ERK and p38 for induction of detoxifying enzymes[J]. BMC Complement Altern Med, 2019, 19: 101. DOI:10.1186/s12906-019-2511-y |
| [25] |
Xiang D, Zou J, Zhu X, et al. Physalin D attenuates hepatic stellate cell activation and liver fibrosis by blocking TGF-beta/Smad and YAP signaling[J]. Phytomedicine, 2020, 78: 153294. DOI:10.1016/j.phymed.2020.153294 |
| [26] |
Zhang MH, Li J, Zhu XY, et al. Physalin B ameliorates nonalcoholic steatohepatitis by stimulating autophagy and NRF2 activation mediated improvement in oxidative stress[J]. Free Radic Biol Med, 2021, 164: 1-12. DOI:10.1016/j.freeradbiomed.2020.12.020 |
| [27] |
Zhu X, Ye S, Yu D, et al. Physalin B attenuates liver fibrosis via suppressing LAP2alpha-HDAC1-mediated deacetylation of the transcription factor GLI1 and hepatic stellate cell activation[J]. Br J Pharmacol, 2021, 178: 3428-3447. DOI:10.1111/bph.15490 |
| [28] |
Noguchi S, Saito A, Nagase T. YAP/TAZ signaling as a molecular link between fibrosis and cancer[J]. Int J Mol Sci, 2018, 19: 3674. DOI:10.3390/ijms19113674 |
| [29] |
Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis[J]. Annu Rev Pathol, 2011, 6: 425-456. DOI:10.1146/annurev-pathol-011110-130246 |
| [30] |
Friedman SL, Sheppard D, Duffield JS, et al. Therapy for fibrotic diseases: nearing the starting line[J]. Sci Transl Med, 2013, 5: 167sr161. |
| [31] |
Yang YK, Xie SD, Xu WX, et al. Six new physalins from Physalis alkekengi var. franchetii and their cytotoxicity and antibacterial activity[J]. Fitoterapia, 2016, 112: 144-152. DOI:10.1016/j.fitote.2016.05.010 |
| [32] |
Helvaci S, Kokdil G, Kawai M, et al. Antimicrobial activity of the extracts and physalin D from Physalis alkekengi and evaluation of antioxidant potential of physalin D[J]. Pharm Biol, 2010, 48: 142-150. DOI:10.3109/13880200903062606 |
| [33] |
Ji L, Yuan Y, Luo L, et al. Physalins with anti-inflammatory activity are present in Physalis alkekengi var. franchetii and can function as Michael reaction acceptors[J]. Steroids, 2012, 77: 441-447. DOI:10.1016/j.steroids.2011.11.016 |
| [34] |
Xu F, Liu C, Zhou D, et al. TGF-beta/Smad pathway and its regulation in hepatic fibrosis[J]. J Histochem Cytochem, 2016, 64: 157-167. DOI:10.1369/0022155415627681 |
| [35] |
Dewidar B, Meyer C, Dooley S, et al. TGF-beta in hepatic stellate cell activation and liver fibrogenesis-updated 2019[J]. Cells, 2019, 8: 1419. DOI:10.3390/cells8111419 |
| [36] |
Hata A, Chen YG. TGF-beta signaling from receptors to Smads[J]. Cold Spring Harb Perspect Biol, 2016, 8: a022061. DOI:10.1101/cshperspect.a022061 |
| [37] |
Derynck R, Budi EH. Specificity, versatility, and control of TGF-beta family signaling[J]. Sci Signal, 2019, 12: eaav5183. DOI:10.1126/scisignal.aav5183 |
| [38] |
Zhang YE. Mechanistic insight into contextual TGF-beta signaling[J]. Curr Opin Cell Biol, 2018, 51: 1-7. DOI:10.1016/j.ceb.2017.10.001 |
| [39] |
Huynh LK, Hipolito CJ, Ten Dijke P. A perspective on the development of TGF-beta inhibitors for cancer treatment[J]. Biomolecules, 2019, 9: 743. DOI:10.3390/biom9110743 |
| [40] |
Colak S, Ten Dijke P. Targeting TGF-beta signaling in cancer[J]. Trends Cancer, 2017, 3: 56-71. DOI:10.1016/j.trecan.2016.11.008 |
| [41] |
Akhurst RJ, Hata A. Targeting the TGFbeta signalling pathway in disease[J]. Nat Rev Drug Discov, 2012, 11: 790-811. DOI:10.1038/nrd3810 |
| [42] |
Mannaerts I, Leite SB, Verhulst S, et al. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation[J]. J Hepatol, 2015, 63: 679-688. DOI:10.1016/j.jhep.2015.04.011 |
| [43] |
Swiderska-Syn M, Xie G, Michelotti GA, et al. Hedgehog regulates yes-associated protein 1 in regenerating mouse liver[J]. Hepatology, 2016, 64: 232-244. |
| [44] |
Williams EJ, Gaca MD, Brigstock DR, et al. Increased expression of connective tissue growth factor in fibrotic human liver and in activated hepatic stellate cells[J]. J Hepatol, 2000, 32: 754-761. DOI:10.1016/S0168-8278(00)80244-5 |
| [45] |
Pefani DE, Pankova D, Abraham AG, et al. TGF-beta targets the Hippo pathway scaffold RASSF1A to facilitate YAP/SMAD2 nuclear translocation[J]. Mol Cell, 2016, 63: 156-166. DOI:10.1016/j.molcel.2016.05.012 |
| [46] |
Fujii M, Toyoda T, Nakanishi H, et al. TGF-beta synergizes with defects in the Hippo pathway to stimulate human malignant mesothelioma growth[J]. J Exp Med, 2012, 209: 479-494. DOI:10.1084/jem.20111653 |
 2021, Vol. 56
2021, Vol. 56


