2. 江苏省中医药研究院, 中药组分与微生态研究中心, 江苏 南京 210028
2. Multi-component of Traditional Chinese Medicine and Microecology Research Center, Jiangsu Provincial Academy of Chinese Medicine, Nanjing 210028, China
癌症是全球第二大死因, 对人类健康造成严重威胁[1]。癌症初期, 肿瘤组织可以得到不间断的营养和氧气供应。但当肿瘤生长到约2~3 mm3时将会出现血液供应不足, 导致肿瘤细胞缺氧[2, 3], 进而引起缺氧诱导因子-1α (hypoxia-inducible factor 1 alpha, HIF-1α) 的过度表达。HIF-1α是HIF-1的功能亚基, 稳定的HIF-1α进入细胞核与缺氧诱导因子-1β (hypoxia-inducible factor-1β, HIF-1β) 结合, 形成HIF-1复合物。随后, HIF-1与缺氧反应元件(hypoxia response element, HRE) 结合, 激活靶基因的转录, 从而调控恶性肿瘤的细胞增殖、血管生成、糖酵解、治疗抵抗、侵袭和转移[4]。临床研究表明, HIF-1α的过度表达是导致多种类型的癌症患者不良预后的重要因素[2]。因此, 抑制HIF-1α的表达是治疗缺氧肿瘤的有效策略。
至今, 已经报道可用于直接或间接阻断HIF-1α激活的药物有PX-478、EZN-2968、echinomycin、YC-1、topotecan和digoxin等[5-7]。然而, 目前大部分抑制剂的临床效果受到高毒性和低效性的限制[5]。因此, 针对HIF-1α的过度表达开发安全强效的新型抗肿瘤药物至关重要。近年来, 从中药中发现有活性的天然产物已成为新药研发的热点。国内外大量研究发现, 黄芩素、大黄素、雷公藤红素、小檗碱和姜黄素等多种中药化学成分可通过直接或间接抑制HIF-1α的表达发挥抗肿瘤细胞增殖、抗肿瘤血管生成、逆转糖酵解、治疗增敏、抗肿瘤侵袭和转移的作用。本综述介绍了HIF-1α与肿瘤的关系以及HIF-1的结构特点、HIF-1α表达的调控, 并汇总了近年来国内外对抑制HIF-1α表达的中药抗肿瘤活性成分的研究进展, 对它们的结构类型和调控机制进行了系统归纳、总结和讨论, 以期为未来研发抑制HIF-1α表达的新型药物提供参考。
1 HIF-1α与肿瘤进展之间的关系大量数据显示, HIF-1α在大多数(> 70%) 类型的人类原发性癌症以及区域和远处转移中过度表达[8]。已有临床研究证实, 在乳腺癌[9]、非小细胞肺癌[10]、前列腺癌[11]、脑部肿瘤[12]、头颈部肿瘤[13]、宫颈癌[14]、结肠癌[15]、胰腺癌[16]、肝细胞癌[17]、皮肤癌[18]、胃癌[19]、食管鳞状细胞癌[20]和卵巢上皮癌[21]等多种癌症患者的肿瘤组织中, HIF-1α表达水平显著升高。这些临床研究还表明, 肿瘤组织中HIF-1α的过度表达是导致癌症患者不良预后、不良临床结果和死亡率增加的独立因素[22]。此外, HIF-1α在癌前病变部位(如结肠腺瘤、乳腺导管原位癌和前列腺上皮内瘤变) 中也存在过度表达, 可能作为临床监测或治疗干预的癌前病变的新型生物标志物。
毫无疑问, HIF-1α信号通路与肿瘤之间存在着密切的关系(图 1)。第一, HIF-1α通过上调血管内皮生长因子(vascular endothelial growth factor, VEGF)、干细胞因子(stem cell factor, SCF) 和血管生成素(angiopoietin, ANGPT) 等促血管生成因子的表达刺激了肿瘤血管的异常增生[23], 进而为肿瘤生长提供充足的血液营养物质。第二, HIF-1α的过表达促进了肿瘤细胞的侵袭和转移, 其机制在于: ①HIF-1α诱导生成的肿瘤血管具有高渗漏、高通透性和无基膜的特点, 为肿瘤细胞侵入血管并向远端器官转移提供了捷径[2]; ②HIF-1α的过表达上调了TWIST、Snail、基质金属蛋白酶(matrix metalloproteinases, MMPs) 等因子的表达, 进而促进肿瘤细胞上皮-间充质转化(epithelial-mesenchymal transition, EMT), 使其获得可塑性和移动性表型; ③HIF-1α还可通过激活Hedgehog、赖氨酰氧化酶(lysyl oxidase, LOX)、核因子кB (nuclear factor кB, NF-кB) 等信号通路间接促进肿瘤的侵袭和转移[23]。第三, HIF-1α通过激活己糖激酶(hexokinase, HK) 1、HK2、葡萄糖转运蛋白(glucose transporter, GLUT) 1、GLUT3、3-磷酸肌醇依赖性蛋白激酶1 (3-phosphoinositide-dependent protein kinase-1, PDK1) 和乳酸脱氢酶A (lactate dehydrogenase A, LDHA) 等基因的表达对肿瘤细胞的葡萄糖代谢进行重新编程, 促进肿瘤细胞的糖酵解作用和“沃伯格效应” (Warburg effect), 保证在缺氧状态下产生足够的能量[24]。第四, HIF-1α的过度激活通过上调促红细胞生成素(erythropoietin, EPO)、胰岛素样因子-2 (insulin-like factor-2, IGF2) 和血小板衍生生长因子(platelet derived growth factor, PDGF) 等因子的表达, 促进了肿瘤细胞增殖。第五, HIF-1α降低了放疗、化疗和免疫治疗等抗肿瘤治疗的有效性。辐射或化疗药物诱导或产生的活性氧(reactive oxygen species, ROS) 可通过抑制HIF-1α的泛素化降解从而导致HIF-1α的蛋白积聚。而高表达的HIF-1α通过上调VEGF和B淋巴细胞瘤/白血病-2 (B cell lymphoma/lewkmia-2, Bcl-2) 基因的表达, 下调Bcl-2相关X蛋白(Bcl-2 associated X protein, BAX) 和caspase-3的表达发挥抗细胞凋亡和保护肿瘤血管免受辐射损伤的作用[25]。此外, HIF-1α介导了DNA损伤抑制和P-糖蛋白(P-glycoprotein, P-gp) 引起的药物外排, 并激活了多药耐药基因1 (multi-drug resistance gene 1, MDR1) 等多药耐药表型的表达。最近的研究表明, HIF-1α通路的激活可增加程序性死亡配体1 (programmed cell death-ligand 1, PD-L1) 和细胞毒性T淋巴细胞相关蛋白4 (cytotoxic T-lymphocyte-associated protein 4, CTLA-4) 等免疫抑制分子的表达, 进而降低免疫治疗的有效性[26, 27]。

|
Figure 1 HIF-1α in cancer progression. HIF-1α: Hypoxia inducible factor-1α; VEGF: Vascular endothelial growth factor; NOS: Nitric oxide synthase; SDF1: Stromal cell derived factor 1; ADM: Adrenomedullin; SCF: Stem cell factor; ANGPT1: Angiopoietin 1; ANGPT2: Angiopoietin 2; Sema4D: Semaphorin 4D; ROS: Reactive oxygen species; AMPK: Adenosine 5'-monophosphate (AMP)-activated protein kinase; MDR1: Multi-drug resistance gene 1; P-gp: P-glycoprotein; CTLA-4: Cytotoxic T-lymphocyte-associated protein 4; Bcl-2: B-cell lymphoma-2; BAX: Bcl-2 associated X protein; PD-L1: Programmed cell death-ligand 1; TGF-β: Transforming growth factor-β; MMPs: Matrix metalloproteinases; EMT: Epithelial-mesenchymal transition; NF-кB: Nuclear factor кB; EPO: Erythropoietin; IGF2: Insulin like growth factor 2; TGF-α: Transforming growth factor-α; PAI-1: Plasminogen activator inhibitor-1; CTGF: Connective tissue growth factor; PDGF: Platelet derived growth factor; HK1: Hexokinase 1; HK2: Hexokinase 2; GLUT1: Glucose transporter 1; GLUT3: Glucose transporter 3; SLC2A1: Solute carrier family 2 member 1; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; PGK1: Phosphoglycerate kinase 1; PKM2: M2-type pyruvate kinase; PDK1: 3-Phosphoinositide-dependent protein kinase-1 |
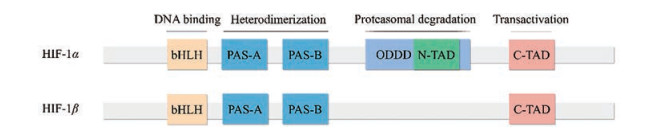
HIF-1是一种异源二聚体, 主要由HIF-1α (120 kD) 和HIF-1β (91~94 kD) 两个亚基构成(图 2)。HIF-1α基因定位于人类14号染色体q21~24区, 受缺氧信号的调控, 是HIF-1的活性亚基[28]。HIF-1β亚基也称芳香烃受体核转运子(aryl hydrocarbon receptor nuclear translocator, ARNT), 基因定位于人类1号染色体q21区, 在细胞内稳定表达, 起结构性作用。二者的氨基端均含PER-ARNT-SIM (PAS) 结构和碱性的螺旋-环-螺旋(basic-helix-loop-helix, bHLH) 构型[29], PAS基序对HIF-1 DNA亚基异源二聚体的形成起关键作用, bHLH域则有助于异源二聚体与靶基因上HRE的5'-AGCGTG-3'序列的结合。作为活性亚基的HIF-1α由826个氨基酸构成, 其两个末端是感受缺氧信号的活性调控区域, C末端有一个富含脯氨酸-丝氨酸-苏氨酸(Pro/Ser/Thr) 的氧依赖降解结构域(oxygen-dependent degradation domain, ODDD) 和反式激活结构域(transactivation domain, TAD), 即C-TAD (786~826位氨基酸序列), N末端含有N-TAD (531~575位氨基酸序列)。这些结构域是缺氧诱导蛋白稳定、核定位和转录激活的调节域, 其中C-TAD发挥精细调整作用, N-TAD为激活转录所必需。它们与辅活化因子CBP和p300一起调控HIF-1α靶基因的转录, 并阻止HIF-1α的降解。稳定的HIF-1α进入细胞核并与HIF-1β结合, 形成有效的转录因子HIF-1, 随后结合到靶基因的HRE上, 从而触发细胞对多种靶基因表达的调节。
|
Figure 2 Structures of HIF-1α and HIF-1β. HIF-1β: Hypoxia inducible factor-1β; bHLH: Basic-helix-loop-helix; PAS-A: PER-ARNT-SIM-A; PAS-B: PER-ARNT-SIM-B; ODDD: Oxygen-dependent degradation domain; N-TAD: N-transactivation domain; C-TAD: C-transactivation domain |
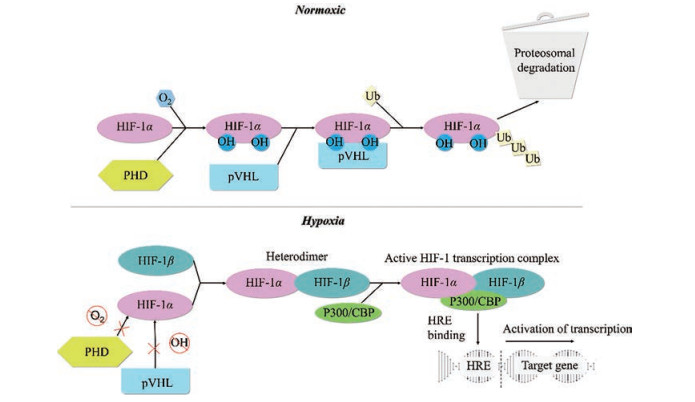
氧依赖的羟基化降解是调控HIF-1α的最关键手段, 其主要通过脯氨酰羟化酶(prolyl hydroxylases, PHD)/肿瘤抑制蛋白希佩尔林道蛋白(Von Hippel Lindau protein, pVHL) 信号通路来进行(图 3)。在常氧状态下, HIF-1α ODDD中的脯氨酸残基(P402和P564) 会被PHD羟基化[6]。这种羟基化作用是通过将两个氧分子分别插入到脯氨酸和α-酮戊二酸中, 将其分解为琥珀酸和二氧化碳来实现的。随后, pVHL与二羟基化的HIF-1α相结合[30], 募集泛素蛋白, 导致HIF-1α亚基泛素化和随后的蛋白酶体途径降解。在某些情况下, ARD1乙酰基转移酶介导的ODDD中的K532乙酰化可促进pVHL依赖的HIF-1α降解, 而K709和K674的乙酰化则可通过稳定HIF-1α蛋白导致HIF-1α通路的上调[31]。HIF-1α还受到HIF抑制因子(factors inhibiting HIFs, FIHs) 的调节, 其可利用氧气和α-酮戊二酸进行反应, 羟化HIF-1α C-TAD中的天冬氨酸残基, 阻断HIF-1α与转录共激活子CBP/p300之间的相互作用。在缺氧条件下, HIF-1α的脯氨酸残基不能发生羟基化[32]。因此, pVHL无法与HIF-1α结合并将其作为26S蛋白酶体降解的靶点[33]。相似的, FIHs介导的羟基化作用也会减少, 进而使HIF与转录共激活因子CBP/p300结合蛋白发生反应, 导致下游基因的表达。除氧分子外, PHD和FIH还需要Fe2+和2-氧代戊二酸酯辅助因子才能发挥活性。而ROS的产生可通过将Fe2+氧化为Fe3+来抑制HIF-1α的羟基化, 进而提高HIF-1α的蛋白稳定性[5]。
|
Figure 3 HIF-1α degradation, stability, and activation. PHD: Prolyl hydroxylases; pVHL: Von Hippel Lindau protein; Ub: Ubiquitin chains; HRE: Hypoxia response element |
除缺氧诱导的HIF-1α激活外, 还有其他信号途径参与调节HIF-1α蛋白的合成、稳定性和转录活性, 主要有热休克蛋白(heat shock proteins, HSP) 信号通路、磷脂酰肌醇3-激酶(phosphatidylinositol 3-hydroxykinase, PI3K)/蛋白激酶B (protein kinase B, PKB/AKT)/哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin, mTOR) 信号通路、细胞外信号调节激酶(extracellular signal-regulated protein kinase, ERK)/丝裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK) 信号通路等。
3.2.1 HSP信号通路研究表明, 有两种HSP (HSP90和HSP70) 参与了HIF-1α的调控。HSP90可通过与PAS结构域结合来稳定HIF-1α, 然后被HIF-1β取代, 进而与共激活因子结合。而受体激活C激酶1 (activated C-kinase 1, RACK1) 会与HSP90竞争PAS-A结构域的结合位点, 并促进HIF-1α的泛素化降解[29]。与HSP90的调控作用相反, HSP70可募集泛素连接酶, 通过20S和26S蛋白酶体途径促进HIF-1α蛋白降解[34]。Bcl-2相关性抗凋亡基因3 (Bcl-2 associated athanogene 3, BAG3) 可通过与HSP70形成蛋白酶体复合物, 抑制HSP70蛋白对HIF-1α的降解作用, 促进HIF-1α表达。
3.2.2 PI3K/AKT/mTOR信号通路缺氧环境会诱导PI3K/AKT/mTOR信号通路的激活, 并影响不同细胞类型中的HIF-1α的表达[35, 36]。研究表明, 活化的PI3K/AKT/mTOR信号通路可通过两条途径来提高HIF-1α mRNA的翻译速率, 进而增加HIF-1α的蛋白积累: ①促进真核翻译起始因子4E (eukaryotic translation initiation factor 4E, eIF-4E) 的磷酸化激活, 增强其与HIF-1α mRNA 5'端寡聚嘧啶核苷酸序列的结合力; ②促进p70核糖体蛋白S6激酶(p70 ribosomal protein S6 kinase, p70S6K) 的磷酸化, 进而激活核糖体蛋白S6 (ribosomal protein S6, rpS6)[29]。人第10号染色体缺失的磷酸酶及张力蛋白同源(phosphatase and tensin homolog deleted on chromosome ten, PTEN) 的基因对AKT信号通路的激活具有拮抗作用。研究发现, PTEN的缺失增强了HIF-1α介导的基因表达, 而PTEN的恢复可以抑制HIF-1α的表达[37]。
3.2.3 ERK/MAPK信号通路MAPK级联是调节增殖、分化、凋亡和应激反应等多种细胞过程的关键信号通路。而Ras/Raf/MAPK (MEK)/ERK通路是MAPK信号转导通路中最重要的信号级联, 在肿瘤细胞的生存和发展中起着至关重要的作用[38]。近年来研究表明, HIF-1α是ERK/MAPK信号通路的重要调控靶点之一。ERK包含p42和p44两个结构激酶, 均为MAPK信号通路的激酶。ERK可通过磷酸化HIF-1α的C-TAD增加其转录活性, 但对其稳定性无明显影响。此外, p42和p44 MAPK信号通路可通过磷酸化641和643位丝氨酸残基来促进HIF-1α的核积聚[5]。Ras/MAPK通路的激活还可以上调eIF4E的活性, 进而增加HIF-1α mRNA的翻译速率[39]。反之, 抑制ERK/MAPK信号通路的激活可加速HIF-1α的泛素化降解, 阻碍HIF-1α向细胞核的易位。
3.2.4 HIF-1α的其他调控通路除上述信号通路外, HIF-1α的表达还受其他因子的调控。研究表明, 多种基因可对HIF-1α的氨基酸残基进行磷酸化, 干预其稳定性或转录活性[5]。如HIF-1α N-TAD中的551、555和589位丝氨酸残基可被糖原合成酶激酶-3 (glycogen synthase kinase-3, GSK-3) 磷酸化, 随后招募泛素连接酶Fbw7和USP28, 介导HIF-1α的泛素化和pVHL非依赖性蛋白酶体降解。再如, HIF-1α的PAS-B结构域的Ser-247可被蛋白激酶CK1磷酸化, 继而破坏HIF-1α/ 1β复合物的稳定性, 降低其转录活性。HIF-1α的稳定性或转录活性也受到非磷酸化方式的调控。比如E3泛素蛋白连接酶Mdm2可被肿瘤抑制基因p53募集以降解HIF-1α[40]。此外, 铜离子代谢结构域包含体1 [copper metabolism (murr1) domain-containing 1, COMMD1] 可通过与HIF-1α的N末端结构域进行结合与HIF-1β产生竞争作用, 随后降低HIF-1复合物的DNA结合和转录活性[41]。
4 HIF-1α抑制剂的研究现状HIF-1α是治疗缺氧肿瘤的有效靶点和评估癌症患者生物学行为和预后的重要因素, 阻断HIF-1α激活的有效策略总结如下[5]: ①降低HIF-1α的mRNA水平; ②抑制HIF-1α蛋白的合成; ③促进HIF-1α蛋白的降解; ④抑制HIF-1α蛋白的核易位过程; ⑤抑制HIF-1α与HIF-1β的异源二聚体化; ⑥减少HIF-1与靶基因HRE的结合, 降低HIF-1α的转录活性。目前, 已开发的HIF-1α抑制剂主要分为化学合成/半合成药物、抗生素类药物和抗体类药物。其中, PX-478、EZN-2968、YC-1及其衍生物、acriflavin、echinomycin等一些化合物可特异性抑制HIF-1α的表达(表 1)。PX-478是临床第一个用于实体瘤治疗的HIF-1α抑制剂, 可在转录和翻译等多个水平下调HIF-1α的表达。EZN-2968是一种靶向HIF-1α的反义寡脱氧核苷酸, 可选择性阻断HIF-1α的mRNA表达。此外, 一些非直接靶向HIF-1α的药物也被报道具有抑制HIF-1α表达的作用, 被称为HIF-1α间接抑制剂[42], 包括HSP90拮抗剂(如geldanamcin)、DNA拓扑异构酶1抑制剂(如topotecan)、微管靶向药物(如2-methoxyestradiol, 2ME2)、PI3K/AKT/mTOR抑制剂(如temsirolimus)、蛋白酶体抑制剂(如bortezomib)、HDAC抑制剂(如romidepsin)、硫氧还蛋白抑制剂(如PX-12)、酪氨酸激酶抑制剂(如semaxanib)、蒽环类药物(如doxorubicin)、强心苷类药物(如digoxin) 等(表 1)[4-7, 22, 42, 43]。此外, 已经有几种间接干预HIF-1α活性的药物被批准用于现有癌症治疗的辅助治疗[4]。
| Table 1 Structures and mechanisms of some existing direct or indirect inhibitors of HIF-1α. mRNA: Messenger ribonucleic acid; FIH: Factors inhibiting HIFs; AKT: Protein kinase B; p70S6K: P70 ribosomal protein S6 kinase; S6RP: S6 ribosomal protein; MAPK: Mitogen-activated protein kinase; PI3K: Phosphatidylinositol 3-hydroxykinase; mTOR: Mammalian target of rapamycin; HSP90: Heat shock protein 90; DNA: Deoxyribonucleic acid; HDAC: Histone deacetylase; Nrf2: Nuclear factor erythroid-related factor 2; SSAT1: Spermidine/spermine-N1-acetyltransferase 1 |
然而, 目前开发的大部分HIF-1α抑制剂的临床效果受到毒性过高和/或有效性不足的限制[5], 同时缺乏特异性。如2ME2纳米晶分散体在治疗去势抵抗性前列腺癌和转移性肾癌II期临床研究中, 由于缺乏客观效果和高毒性而被终止试验[5]。类似的, geldanamcin因在动物模型中的药理活性差和肝毒性而未能应用于临床[5]。因此, 研发下调HIF-1α表达的强效低毒的新型抗肿瘤药物至关重要。
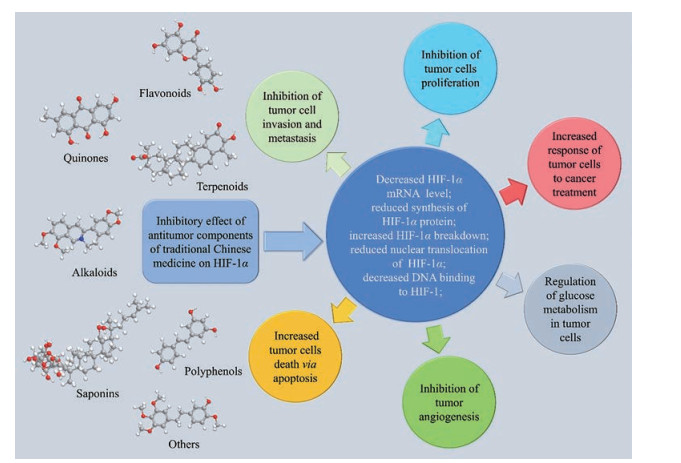
5 抑制HIF-1α表达的中药抗肿瘤活性成分研究中药具有悠久的历史和独特的优势, 其安全性和有效性在千百年临床实践中已得到反复验证和深入的了解。此外, 中药单体的来源更为广泛, 大大降低了新药开发的成本。近年来, 越来越多抑制HIF-1α表达的中药抗肿瘤活性成分被发掘和研究(图 4、5)。这些成分按照其结构不同可分为黄酮类、醌类、萜类、生物碱类、多酚类、皂苷类和其他类。一些中药活性成分可直接靶向HIF-1α。如海绵中分离得到的discorhabdin B、3-dihydrodiscorhabdin C等多种吡咯烷酮生物碱可阻断HIF-1α与共激活因子p300的结合[44], 宝藿苷[45]、穿心莲内酯[46]等可促进HIF-1α的蛋白降解, β-榄香烯[47]、三氧化二砷[48]等可抑制HIF-1α的mRNA表达。一些中药活性成分则通过干预PHD/VHL、HSP90、PI3K/AKT/mTOR、ERK/MAPK等上游信号通路间接影响HIF-1α的表达, 如大黄酚[49]通过阻断PI3K/AKT通路的磷酸化激活, 下调结肠癌细胞中HIF-1α的蛋白水平。此外, 槲皮素[50, 51]、姜黄素[52, 53]等多种中药单体既可直接抑制HIF-1α的活性, 又可阻断其上游激活通路, 多途径下调肿瘤细胞中HIF-1α的表达。
|
Figure 4 Inhibitory effect of antitumor components of traditional Chinese medicine on HIF-1α |

|
Figure 5 Anti-tumor components from traditional Chinese medicine inhibit the activation of HIF-1α in cancer treatment through different pathway. IDO: 2, 3-Dioxygenase; STAT3: Signal transducer and activator of transcription 3; PTEN: Phosphatase and tensin homolog deleted on chromosome ten; SPHK1: Sphingosine kinase-1; MAOA: Monoamine oxidase; IGF-1: Insulin-like growth factor-1; ID-1: Inhibitor of differentiation/DNA binding-1; rpS6: Ribosomal protein S6; 4E-BP1: Eukaryotic translation initiation factor 4E-bingding protein 1; eIF-4E: Eukaryotic translation initiation factor 4E; PAK1: Recombinant p21 protein activated kinase 1; ERK: Extracellular signal-regulated kinase; MNK1/2: Mitogen-activated protein kinase (MAPK)-interacting kinases 1 and 2; EIF2α: Eukaryotic translation initiation factor 2α; UBE2S: Ubiquitin E2S ligase; TRAF6: Tumor necrosis factor receptor associated factor 6; YAP: Yes-associated protein; SIRT3: Silent mating type information regulation2 homolog-3; DNMT3A: DNA methyltransferase 3A; MAT1: Menage a trois 1; APE1: Apurinic/aprimidinic endonuclease 1; Ref-1: Redox factor-1 |
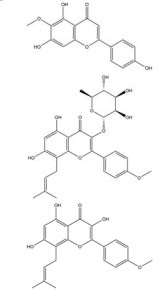
黄酮类化合物是一类广泛存在于中药中的具有2-苯基色原酮结构的活性成分, 具有丰富的药用价值。一些黄酮类物质可下调HIF-1α的表达, 对抗缺氧诱导的恶性肿瘤生长、耐药、血管生成、侵袭和转移(表 2)[45, 50, 51, 54-70]。这里主要介绍槲皮素、木犀草素、染料木素、黄芩素及其类似物。
| 表 2 Anti-tumor components of flavonoids of traditional Chinese medicine inhibiting the expression of HIF-1α. DOX: Doxorubicin; AP-1: Activator protein-1; Pin1: Peptidyl-prolyl isomerase NIMA-interacting 1; 5-FU: 5-Fluorouracil; HDM2: Human double minute 2; uPAR: Urokinase plasminogen activator receptor; MMP2: Matrix metalloproteinase 2; PTEN: Phosphatase and tensin homolog deleted on chromosome ten; EMT: Epithelial-mesenchymal transition |
槲皮素是来源于银杏(Ginkgo biloba L.) 等药用植物中的一种多羟基黄酮类化合物。研究发现, 槲皮素可剂量依赖性地抑制多种肿瘤细胞中缺氧诱导的HIF-1α蛋白的生物合成和下游VEGF的基因表达, 其机制与槲皮素抑制真核细胞起始因子2α (eukaryotic translation initiation factor 2α, EIF2α) 亚基的磷酸化有关[54]。槲皮素还可通过抑制结直肠癌细胞中AMPK的激活显著抑制HIF-1α的转录活性, 进而降低VEGF和GLUT1的mRNA水平, 提高肿瘤细胞对顺铂和依托泊苷的敏感性和凋亡率[50]。此外, 槲皮素促进了乳腺癌细胞中HIF-1α的降解, 进而增强多柔比星(doxorubicin, DOX) 对肿瘤细胞的细胞毒性和促凋亡作用, 同时降低DOX引起的不良反应, 延缓肿瘤生长并延长小鼠的生存时间[51]。在另一份报道中, Oh等[55]通过报告基因分析和蛋白印迹分析证实槲皮素可阻断他莫昔芬(tamoxifen, TAM) 耐药的乳腺癌MCF-7细胞(TAMR-MCF-7) 中HIF-1α和c-Jun/AP-1的核水平增加, 这可能归因于其抑制了PI3K依赖的Pin表达, 进而抑制VEGF的分泌和血管异常增生。
木犀草素主要存在于十字花科、天南星科、桔梗科等植物, 具有抗肿瘤、抗氧化、免疫调节、抗炎等药理作用。木犀草素是半边莲(Lobelia chinensis Lour.) 中下调HIF-1α蛋白表达的主要成分, 可通过抑制AKT的磷酸化抑制HIF-1α/VEGF信号通路的激活, 从而抑制缺氧条件下内皮细胞的迁移和增殖, 降低肿瘤微血管密度[57]。最近的一份研究[56]发现木犀草素和槲皮素逆转宫颈癌EMT过程的机制可能与HIF-1α通路相关。进一步的实验表明, 这两种黄酮可能通过抑制E2S泛素连接酶(ubiquitin E2S ligase, UBE2S) 的表达增加pVHL/PHD途径对HIF-1α的降解作用。此外, Ansó等[58]发现木犀草素能够抑制肺癌细胞中缺氧诱导的HIF-1α的激活和STAT3的磷酸化, 进而发挥降低VEGF表达的作用。
染料木素为淡豆豉(Sojae Semen Praeparatum) 等中药中的异黄酮类有效成分。Mukund等[59]首次证明了染料木素可下调乳腺癌细胞中的HIF-1α蛋白水平, 分子对接结果显示Thr183、Ser184、Asp201、Gln203和Arg238残基是染料木素靶向HIF-1α蛋白的关键位点。染料木素对HIF-1α表达的下调作用在肝细胞癌细胞中也被证实, 同时使其下游的GLUT1和HK2失活, 从而抑制有氧糖酵解, 显著提高肿瘤细胞和荷瘤小鼠对索拉非尼的敏感性[60]。APE1/Ref-1为参与HIF-1α和NF-κB活化的一种关键蛋白, 与肿瘤放疗抵抗密切相关。而染料木素和另一种异黄酮类活性物质大豆苷元均可通过下调APE1/Ref-1的表达抑制HIF-1α和NF-κB的DNA结合活性, 阻断辐射诱导的HIF-1α和NF-κB的激活, 具有作为前列腺癌治疗的放射增敏剂的前景。有趣的是, 尽管大豆苷元的抑制作用弱于染料木素[61], 但单一使用染料木素将促进肿瘤细胞的淋巴结转移, 而与大豆苷元的联用则可抵抗这种作用。
黄芩素是中药黄芩(Scutellariae Radix) 中分离得到的天然黄酮类化合物, 其可通过促进PTEN蛋白的积聚来抑制缺氧诱导的AKT磷酸化, 进而降低HIF-1α和糖酵解相关基因HK2、LDHA和PDK1的表达, 提高胃癌AGS细胞对5-氟尿嘧啶(5-fuorouracil, 5-FU) 的敏感性[62]。此外, 有研究表明黄芩素和黄芩苷作用于卵巢癌OVCAR-3和CP-70细胞后, HIF-1α的mRNA表达水平显著降低, 且黄芩素表现出更强的抑制作用[63]。汉黄芩素与黄芩素结构相似, 但两者抑制HIF-1α表达的机制有所不同。Song等[64]的报道显示, 汉黄芩素可通过促进PHD/pVHL通路依赖的HIF-1α的脯氨酸羟基化和阻断HIF-1α的PAS结构域与HSP90的结合来增加HIF-1α蛋白的降解, 从而阻碍HIF-1α/VEGF轴的激活, 抑制多种恶性肿瘤的血管异常增生。
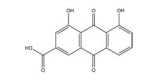
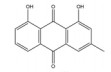
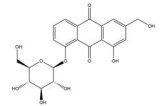
5.2 醌类及其糖苷醌类化合物是一类具有醌式结构的化学成分, 主要分布在蓼科、茜草科、豆科、唇形科、紫草科等植物中。具有抑制HIF-1α表达作用的醌类化合物主要有以大黄素为代表的蒽醌类成分和以丹参酮IIA为代表的菲醌类成分(表 3)[49, 71-81]。
| Table 3 Antitumor components of quinones of traditional Chinese medicine inhibiting the expression of HIF-1α. COX-2: Cyclooxygenase-2; GRB2: Growth factor receptor-bound protein 2; EGF: Epidermal growth factor; EMMPRIN: Extracellular matrix metalloproteinase; LDHA: Lactate dehydrogenase A |
大黄素主要来源于中药大黄(Rhei Radix Et Rhizoma) 和虎杖(Polygoni Cuspidati Rhizoma Et Radix)。大黄素可通过下调HIF-1α的基因和蛋白表达来调节肿瘤细胞的氧化还原状态, 有效增强鼻咽癌细胞和荷瘤动物对放射治疗的敏感性, 有望成为鼻咽癌患者的一类新型放射增敏药物[71]。大黄素还可抑制人神经母细胞瘤SH-SY5Y细胞的迁移和侵袭, 并具有剂量和时间依赖性。其机制与大黄素抑制HIF-1α、VEGF、MMPs、环氧合酶-2 (cyclooxygenase-2, COX-2) 等肿瘤转移和血管生成促进因子的表达相关[72]。此外, 大黄素可分别通过阻断肿瘤坏死因子受体相关因子6 (tumor necrosis factor receptor associated factor 6, TRAF6)/HIF-1α/VEGF和TRAF6/CD147/MMP9通路的激活抑制未分化甲状腺癌(anaplastic thyroid cancer, ATC) 裸鼠异种移植和肺转移模型中的肿瘤血管生成和转移[73]。大黄酸是另一种重要的蒽醌化合物, 其作用于结直肠癌细胞后可剂量依赖性地降低HIF-1α的mRNA和蛋白水平, 进而下调程序性死亡配体-1 (programmed death-ligand 1, PD-L1)、COX-2、加连蛋白-1、IL-10和转化生长因子-β1 (transforming growth factor-β1, TGF-β1) 等免疫抑制分子的mRNA表达水平, 改善肿瘤免疫微环境, 增强效应T淋巴细胞在缺氧条件下对肿瘤细胞的杀伤作用[75]。大黄酸还通过抑制缺氧诱导的PI3K/AKT/ERK通路激活进而下调HIF-1α的蛋白表达, 降低VEGF和表皮生长因子(epidermal growth factor, EGF) 的分泌水平, 是一种有前景的抗肿瘤新生血管形成药物[76]。此外, 大黄酸和大黄素均可抑制体内外人胰腺癌细胞系中HIF-1α的蛋白合成, 这种抑制作用强于PX-478等已知的HIF-1α抑制剂[74]。其他具有干扰HIF-1α信号通路表达的蒽醌类化合物还有大黄酚[49]、芦荟大黄素-8-O-β-葡萄糖苷[77]和大黄素甲醚[78]。
丹参酮IIA是从丹参(Salvia miltiorrhiza Bge.) 的干燥根及根茎中分离得到的一种重要的菲醌类化合物, 其可能通过抑制HIF-1α/TWIST的激活进而抑制低氧诱导的乳腺癌细胞EMT过程及对DOX的治疗抵抗作用[79]。另一份研究中[80]发现丹参提取物的氯仿部位可强烈抑制低氧诱导的报告基因表达。在进一步分离得到的化合物中, 轮叶婆婆纳对醌A和15, 16-双氢丹参酮Ⅰ剂量依赖性阻碍了胃癌AGS细胞和肝癌Hep3B细胞中的HIF-1α蛋白积累。此外, 轮叶婆婆纳对醌A可抑制VEGF的mRNA表达。隐丹参酮是丹参中的另一种代表性醌类活性成分, 最近的一份报道[81]显示其可调节裸鼠卵巢癌异种移植模型中信号转导和转录激活因子3 (signal transducer and activator of transcription 3, STAT3)/SIRT3/HIF-1α信号的表达, 显著降低HIF-1α的蛋白稳定性和GLUT1、LDHA和HK2的表达水平, 进而抑制糖酵解诱导的细胞生长和增殖, 具有发展为卵巢癌化疗药物的潜能。
5.3 萜类萜类中药有效成分大多存在于高等植物中, 由异戊二烯单元组成, 可分为单萜、倍半萜、二萜、三萜等。齐墩果酸、雷公藤红素等一些萜类中药单体可抑制HIF-1α通路的激活, 对抗缺氧诱导的肿瘤血管生成、糖酵解和远端转移(表 4)[25, 46, 47, 82-92]。
| 表 4 Anti-tumor components of terpenoids of traditional Chinese medicine inhibiting the expression of HIF-1α. NOX2: NADPH oxidase; PFK1: Phosphofructokinase 1; Ang2: Angiopoietin 2; IκB: Inhibitor of NF-κ |
齐墩果酸是夏枯草(Prunellae Spica) 等传统中药的三萜类活性成分之一。齐墩果酸可通过调控NADPH氧化酶2 (NADPH oxidase 2, NOX2)/ROS信号通路抑制HIF-1α表达, 诱导直肠癌细胞G1/S期阻滞, 抑制细胞增殖, 从而发挥其抗肿瘤作用[82]。这些结果表明, NOX2/ROS/HIF-1α轴可能是治疗直肠癌的新靶点。
此外, 齐墩果酸可通过抑制Yes相关蛋白信号通路的激活降低HIF-1α的表达和核丰度, 并抑制糖酵解限速酶HK2和磷酸果糖激酶-1 (phosphofructokinase 1, PFK1) 的表达和功能活性, 从而阻断HIF-1α介导的胃癌细胞有氧糖酵解和增殖[83]。
雷公藤红素为雷公藤(Tripterygium wilfordii Hook. f.) 根皮中的一种五环三萜, 具有广谱的抗肿瘤作用。雷公藤红素可以在多个水平上调控HIF-1α的表达, 发挥抗肿瘤血管生成和转移的活性。雷公藤红素可阻断mTOR/p70S6K/eIF4E和ERK信号通路的磷酸化, 在翻译水平上抑制肝癌细胞和宫颈癌细胞中HIF-1α蛋白的表达, 并下调VEGF和EPO的表达[84]。此外, 雷公藤红素在常氧和缺氧条件下均可降低肝癌细胞HIF-1α的表达水平, 抑制HIF-1α蛋白的核转位和核积聚。这与雷公藤红素抑制HSP90与HIF-1α的结合, 继而增加HIF-1α的降解有关[85]。雷公藤红素还可抑制PI3K/AKT/mTOR信号通路的激活, 降低HIF-1α、CD31、VEGFR2、ANG2和VEGFA的表达, 进而抑制胶质母细胞瘤中的血管生成和血管生成拟态的形成[86]。
5.4 生物碱类生物碱是一类含氮的碱性有机化合物, 在罂粟科、防己科、毛茛科、小檗科等植物中广泛分布。许多生物碱类中药成分被证实可显著抑制HIF-1α通路, 对抗实体瘤的恶性发展(表 5)[44, 93-108]。
| 表 5 Antitumor components of alkaloids of traditional Chinese medicine inhibiting the expression of HIF-1α. PEDF: Pigment epithelium-derived factor; ODC: Ornithine decarboxylase; OAZ1: ODC antizyme 1; GSK-3β: Glycogen synthase kinase-3β; BCRP: Breast cancer resistance protein; NF: Nuciferine |
小檗碱是从传统中药黄连(Coptidis Rhizoma) 中分离出的一种异喹啉类生物碱, 对肿瘤、炎症、糖尿病等疾病具有良好的治疗作用。小檗碱可通过多种机制干预HIF-1α通路的表达: ①小檗碱可通过阻断PI3K/AKT和Raf/MEK/ERK信号通路的激活, 抑制AKT和ERK蛋白的磷酸化, 从而下调HIF-1α的转录和翻译水平[93, 94]; ②小檗碱促进了26S蛋白酶体水解途径和赖氨酸乙酰化对HIF-1α蛋白的降解作用[95]; ③小檗碱通过
下调DNA结合分化抑制因子1 (inhibitor of differentiation/DNA binding-1, Id-1) 的启动子活性抑制了其转录水平, 继而降低Id-1对HIF-1α蛋白的稳定作用[96]。小檗碱对HIF-1α通路的抑制作用显著下调了下游VEGF、PEDF、P-gp等多种基因的表达, 从而有效抑制了肿瘤生长[97]和血管生成[93], 并明显提高了前列腺癌[98]、宫颈癌[94]、鼻咽癌[99]、乳腺癌[100]等多种肿瘤细胞系对化疗或放疗的敏感性(具体机制见表 5)。这些资料表明, 小檗碱是一种具有良好前景的抗肿瘤药物, 与化疗、放疗等传统肿瘤治疗手段联用可起到协同增效的作用。
血根碱是从博落回[Macleaya cordata (Willd.) R. Br.] 等罂粟科植物中发现的苯并菲啶类生物碱, 具有广谱的抗癌活性。血根碱在体内外均能抑制HIF-1α与p-STAT3-Tyr和p-STAT3-Ser的核共定位和相互作用, 破坏三者形成的转录复合体, 进而抑制VEGF等靶基因的激活和肿瘤生长[101]。缺氧可触发TGF-β和PI3K依赖的细胞外基质相关蛋白的表达或通过TGF-β的自分泌诱导EMT发生, 而TGF-β增强了HIF-1α的表达及其靶基因碳酸酐酶9 (carbonic anhydrase 9, CA9) 和VEGF的转录, 从而形成HIF-1α/TGF-β前馈通路。血根碱可显著抑制缺氧诱导的TGF-β的分泌, 阻断TGF-β诱导的HIF-1α表达增加, 有效抑制HIF-1α的核易位。此外, 血根碱还抑制了缺氧诱导的Snail易位、Smad和PI3K/AKT通路的激活, 从而抑制肿瘤生长、上皮间质转化和细胞迁移[102]。
5.5 多酚类多酚类化合物是一类复杂的具有多个酚羟基的中药活性成分, 具有抗肿瘤、抗氧化、清除自由基、降血脂等作用。其中姜黄素及其类似物、白藜芦醇等已被证明可通过多种机制抑制HIF-1α的过度激活(表 6)[52, 53, 109-130]。
| Table 6 Anti-tumor components of polyphenols of traditional Chinese medicine inhibiting the expression of HIF-1α. MCL-1: Myeloid cell leukemia 1; PARP: Poly(ADP-ribose) polymerase; CAF: Cancer-associated fibroblasts; CXCR4: C-X-C chemokine receptor type 4; IL-6: Interleukin-6; IUDR: 5-Iodo-2-deoxyuridine; CBR1: Carbonyl reductase 1; IL-1β: Interleukin-1β; SIRT1: Sirtuin 1; AR: Androgen receptor |
姜黄素是从姜黄(Curcuma longa L.) 的干燥根茎中提取的一种多酚类多功能分子, 其含有可与蛋白质发生共价结合的α, β-不饱和二酮基团[23]。姜黄素具有抗血管生成、抗肿瘤细胞增殖[109]、促进凋亡[109]、逆转耐药性[53]、抗侵袭和转移[53]等多种抗肿瘤活性, 其机制与姜黄素抑制HIF-1α的表达和激活caspase-3进而调控下游VEGF、P-gp、ROS、多聚腺苷二磷酸核糖聚合酶[poly(ADP-ribose) polymerase, PARP] 等的基因表达水平相关。姜黄素主要从以下途径来抑制HIF-1α的表达: ①姜黄素可在转录和翻译水平上抑制乳腺癌MDA-MB-231细胞和前列腺癌PC3细胞中HIF-1α的表达[110]; ②姜黄素和顺铂联合治疗可促进HIF-1α的蛋白酶体途径降解, 并激活caspase-3的表达[52]; ③姜黄素还可通过抑制单胺氧化酶(monoamine oxidase, MAOA)/mTOR通路的信号转导阻断肿瘤相关成纤维细胞(cancer-associated fibroblasts, CAF) 诱导的HIF-1α稳定性和活性增加[53]。四氢姜黄素是姜黄素的主要代谢物之一, 其酚羟基和β-二酮基结构与姜黄素相似, 并具有更强的抗血管生成活性[131]。四氢姜黄素可通过下调HIF-1α、VEGF和VEGFR-2的蛋白表达水平显著降低宫颈癌动物模型中的微血管密度, 抑制肿瘤血管的异常增生[111]。
白藜芦醇(resveratrol, RES) 是中药虎杖(Polygoni Cuspidati Rhizoma Et Radix) 中的主要成分之一, 具有抗肿瘤、抗氧化、抗炎和免疫调节等多种活性。RES可通过抑制HIF-1α通路的激活下调VEGF、GLUT-1、羰基还原酶1 (carbonyl reductase 1, CBR1)、Bcl-2、β-catenin、Hedgehog信号通路以及EMT标志物的异常表达, 进而发挥抗肿瘤血管生成[112]、逆转糖酵解[113]、抗肿瘤细胞增殖[114]、促进肿瘤细胞凋亡[114, 115]、治疗增敏[116, 117]、抗肿瘤侵袭和转移[118-120]的作用。RES抑制HIF-1α表达的机制主要有以下几点: ①白藜芦醇可阻断p42/44MAPK和PI3K/AKT通路的激活, 进而下调HIF-1α的表达[112]; ②白藜芦醇促进了HIF-1α的26S蛋白酶体途径降解, 进而抑制HIF-1α蛋白积聚[112, 121]; ③白藜芦醇可通过抑制胰岛素样生长因子-1 (insulin-like growth factor-1, IGF-1) 诱导的蛋白翻译调控因子p70S6K1、S6核糖体蛋白、4E-BP1、eIF4E的磷酸化抑制HIF-1α蛋白合成[121]; ④白藜芦醇通过调节抗氧化酶活性或固有的ROS清除能力可降低肿瘤细胞的ROS水平, 继而抑制ROS诱导的HIF-1α的蛋白积聚[113]; ⑤白藜芦醇可作为sirtuin1 (Sirt1) 的激动剂, 增加Sirt1与HIF-1α的结合, 使后者的Lys674位点去乙酰化失活, 进而抑制HIF-1α与共激活因子p300之间的相互作用[122]; ⑥白藜芦醇通过阻断STAT3的激活, 抑制下游HIF-1α的基因表达和HIF-1α功能活性[115]。总而言之, 白藜芦醇可通过多种机制下调HIF-1α通路的激活, 在对抗缺氧诱导的肿瘤进展方面具有广阔的应用前景。
5.6 皂苷类皂苷是一类结构复杂的螺甾烷和其相似生源的甾体化合物以及三萜类化合物的低聚糖苷, 主要存在于五加科、薯蓣科、百合科、毛茛科、桔梗科等植物中。目前已报道的抑制HIF-1α表达的皂苷类成分相对较少(表 7)[132-142], 其中研究较为深入的为白头翁皂苷D和人参皂苷Rg3。
| Table 7 Anti-tumor components of saponins of traditional Chinese medicine inhibiting the expression of HIF-1α. GEM: Gemcitabine; PTX3: Pentraxin 3 |
白头翁皂苷D (SB365) 是从毛茛科植物白头翁[Pulsatilla chinensis (Bge.) Rege] 中分离得到的一种抗肿瘤活性成分。Hong等[132]研究表明, SB365可有效抑制PI3K/AKT/mTOR通路激活, 继而降低HIF-1α和VEGF的表达, 显著抑制肝癌移植瘤模型的生长。Son等[133]同样发现SB365可显著降低HIF-1α和VEGF的表达而发挥抗血管生成作用, 并抑制了胰腺癌细胞的瘤球形成。小鼠体内移植实验表明, SB365通过抑制血管生成和诱导肿瘤细胞凋亡显著抑制肿瘤生长, 是治疗胰腺癌的良好候选药物。
人参皂苷Rg3是我国的名贵中药人参(Ginseng Radix Et Rhizoma) 中主要的活性成分, 已经用于临床抗肿瘤治疗。近几年研究表明, Rg3可通过多种机制抑制HIF-1α信号通路的表达, 这可能是其发挥抗肿瘤血管生成[134, 135]、抗侵袭和转移[136]以及治疗增敏作用[137]的重要机制。如Rg3可阻断吉西他滨(gemcitabine, GEM) 诱导的ROS介导的AKT和ERK通路激活, 进而抑制肺癌细胞中HIF-1α和NF-κB的核堆积, 降低耐药表型PTX3的表达[138]。再如, Rg3可通过抑制PI3K/AKT/miRNA-21通路的激活下调HIF-1α、LDH、HK2的表达, 继而减缓小鼠胃癌前病变(gastric precancerous lesions, GPL) 模型的糖酵解异常, 降低胃癌的发生风险[139]。此外, 人参皂苷Rg3促进了食管癌细胞中HIF-1α的蛋白降解, 显著缩短了HIF-1α蛋白的半衰期, 并抑制了缺氧诱导的COX-2、NF-κB的表达、STAT3的磷酸化以及ERK1/2和c-Jun氨基末端激酶(C-Jun N-terminal kinase, JNK) 的磷酸化, 进而下调VEGF的表达[134]。Rg3作用于卵巢癌细胞后可抑制下游HIF-1α/HK2通路的表达和Warburg效应[135], 其机制与其抑制DNA甲基转移酶3 (DNA methyltransferase 3A, DNMT3A) 介导的miR-519a-5p前体基因启动子区域中的DNA甲基化进而上调miR-519a-5p水平有关。Rg3还可通过激活泛素-蛋白酶体途径加速HIF-1α的降解, 进而抑制Snail的转录, 促进E-钙黏素的上调和波形蛋白的下调, 进而阻断卵巢癌裸鼠异种移植模型中的EMT[136]。
5.7 其他中药活性成分的种类繁多, 除了上述介绍的化合物, 绿原酸、红景天苷等化合物也发现具有一定的抑制肿瘤细胞HIF-1α通路的作用(表 8)[48, 143-155]。
| Table 8 Other antitumor components of traditional Chinese medicine inhibiting the expression of HIF-1α. LOXL2: Lysyl oxidase-like protein 2; JAK2: Janus kinase 2; Dll4: Delta-like ligand 4; Sox2: Sex determining region Y-box 2; OCT4: Octamer binding transcription factor 4 |
绿原酸是由咖啡酸的羧基和奎尼酸的羟基缩合而成的缩酚酸, 在金银花(Lonicerae Japonicae Flos) 等中药中含量很高。绿原酸可通过促进HIF-1α的蛋白酶体途径降解抑制缺氧诱导的HIF-1α蛋白积累和转录活性[143]。绿原酸还可通过抑制鞘氨醇激酶1 (sphingosine kinase-1, SPHK-1) 的表达和功能活性下调缺氧诱导的HIF-1α表达, 并通过抑制AKT/GSK-3β通路的磷酸化降低HIF-1α蛋白的稳定性, 继而下调VEGF的基因表达, 发挥抗肿瘤血管生成的作用[144]。
红景天苷主要来源于景天科植物大花红景天[Rhodiola crenulate (Hook. f. et Thoms.) H. Ohba] 的干燥根和根茎。红景天苷可促进HIF-1α的泛素化降解, 并通过抑制HIF-1α信号通路显著增加肝癌细胞对铂类药物的敏感性, 抑制缺氧诱导的肝细胞癌上皮-间充质转化, 有望成为一种有效的铂类药物敏化剂[145]。缺氧环境会促进肿瘤细胞中氨酰氧化酶样蛋白2 (lysyl oxidase-like protein 2, LOXL2) 的表达, 进而上调MMP的表达并诱导肿瘤细胞的增殖和侵袭。而红景天苷和HIF-1α抑制剂KC7F2处理人胰腺癌BxPC-3细胞后可明显下调HIF-1α的表达和转录活性, 进而逆转HIF-1α诱导的LOXL2过表达引起的致瘤性[146]。此外, 红景天苷和红景天提取物均可下调HIF-1α/HIF-2α的蛋白表达, 抑制乳腺癌细胞中缺氧诱导的mTOR通路的激活和肿瘤血管异常增生[147, 148]。
6 总结与讨论缺氧诱导的HIF-1α过度表达在促进肿瘤细胞异常增殖、肿瘤血管生成、能量代谢异常、耐药性增加、侵袭和转移的过程中发挥着关键作用, 并与多种类型的癌症患者的不良预后密切相关。因此HIF-1α被认为是治疗缺氧肿瘤的有效靶点和评估癌症患者生物学行为和预后的独立因素。到目前为止, 已经研发出几种药物, 包括PX-478、EZN-2968、echinomycin、YC-1等用于抑制肿瘤细胞中HIF-1α的激活。此外, 一些非直接靶向HIF-1α的药物也被报道具有抑制HIF-1α表达的作用。然而, 现有大多数HIF-1α抑制剂的临床效果受到毒性过高和/或有效性不足的限制[5], 同时缺乏特异性。所以, 研发下调HIF-1α表达的强效低毒的新型抗肿瘤药物越发重要。大量研究表明, 中药活性成分在对抗HIF-1α相关的促癌作用方面具有巨大的潜力, 可通过直接或间接抑制HIF-1α表达发挥抗肿瘤细胞增殖、抗肿瘤血管生成、逆转糖酵解、抗肿瘤侵袭和转移的作用。除此之外, 中药活性成分可通过改善HIF-1α诱导的肿瘤耐药和缺氧微环境恶化显著提高化疗[145]、放疗[61]、光动力治疗[156]和栓塞治疗[107]的肿瘤杀伤作用。
但这些天然活性成分在临床转化过程中仍然面临一定的挑战。第一, 中药活性成分对HIF-1α通路可能存在双向调节作用。这种现象在白藜芦醇[157]、15, 16-双氢丹参酮Ⅰ[158]、小檗碱[100, 159]、槲皮素[54, 160]、青蒿素[161]等化合物的抗肿瘤研究中已被报道, 提示同一中药活性成分可能会因生产厂商、肿瘤细胞系、氧气水平、给药剂量等不同对HIF-1α通路产生不同的调控方式, 意味着临床条件下将中药活性成分作为HIF-1α抑制剂来使用将变得复杂化, 需要更深一步的机制探讨。第二, 许多天然化合物存在水溶性差、结构稳定性弱、代谢快、生物利用度低、靶向性劣等问题, 导致临床转化受到限制。针对中药活性成分的自身理化性质合理设计载药系统可以有效克服以上问题。如雷公藤红素胶束[162]、Sanazole-小檗碱-氧化铁纳米粒复合物[163]、小檗碱喷雾干燥(SD) 黏附性微粒[164]等均已被报道可显著提高所载药物的溶解度、稳定性、血药浓度和治疗特异性, 并有效抑制HIF-1α通路的表达。对天然活性先导化合物进行结构优化以改善其药理作用则是另一种新策略[110]。如HS-1793是一种合成的新型白藜芦醇类似物, 其不含白藜芦醇所具有的不稳定双键结构, 同时被证明具有更强的抗癌作用, 可通过阻断PI3K和AKT的磷酸化激活抑制HIF-1α/VEGF通路的表达[165, 166]。第三, 许多基于中药活性成分的HIF-1α抑制剂的临床前研究仅停留于体外研究, 而缺乏体内实验的进一步有效验证。这是由于在小鼠中建立体内缺氧微环境存在一定的困难性和局限性, 急需开发能够简单有效研究低氧诱导的体内肿瘤病理过程的动物模型。如斑马鱼[2]具有适应低氧环境、免疫豁免特性、透明性和成本低等优势, 可能更适于作为HIF-1α抑制剂体内研究的模式动物。第四, 许多HIF-1α抑制剂未能通过临床试验的原因是对接受治疗的患者的选择是随机的, 并未准确获取血清和肿瘤组织中的HIF-1α表达水平。HIF-1α在不同肿瘤类型、进展分期以及不同区域和年龄的人群中的表达水平不同[22, 167], 若能通过个体化诊疗手段选择在肿瘤组织特异性高表达HIF-1α的患者作为研究对象, 则可以更有效地评估中药活性成分对实体肿瘤的临床疗效[168]。
作者贡献: 该文章主要由史新萌收集资料和撰写; 刘玉萍提供了文章的整体思路和重要指导; 陈彦、瞿鼎、黄琳清给出了关键意见。
利益冲突: 作者声明没有任何利益冲突。
| [1] |
Sauer AG, Siegel RL, Jemal A, et al. Updated review of prevalence of major risk factors and use of screening tests for cancer in the United States[J]. Cancer Epidemiol Biomarkers Prev, 2017, 26: 1192-1208. DOI:10.1158/1055-9965.EPI-17-0219 |
| [2] |
Nalini D, Selvaraj J, Kumar GS. Herbal nutraceuticals: safe and potent therapeutics to battle tumor hypoxia[J]. J Cancer Res Clin Oncol, 2020, 146: 1-18. DOI:10.1007/s00432-019-03068-x |
| [3] |
Höckel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects[J]. J Natl Cancer Inst, 2001, 93: 266-276. DOI:10.1093/jnci/93.4.266 |
| [4] |
Masoud GN, Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy[J]. Acta Pharm Sin B, 2015, 5: 378-389. DOI:10.1016/j.apsb.2015.05.007 |
| [5] |
Burroughs SK, Kaluz S, Wang DZ, et al. Hypoxia inducible factor pathway inhibitors as anticancer therapeutics[J]. Future Med Chem, 2013, 5: 553-572. DOI:10.4155/fmc.13.17 |
| [6] |
Akanji MA, Rotimi D, Adeyemi OS. Hypoxia-inducible factors as an alternative source of treatment strategy for cancer[J]. Oxid Med Cell Longev, 2019, 2019: 8547846. |
| [7] |
Tang W, Zhao G. Small molecules targeting HIF-1α pathway for cancer therapy in recent years[J]. Bioorg Med Chem, 2020, 28: 115235. DOI:10.1016/j.bmc.2019.115235 |
| [8] |
Terzuoli E, Puppo M, Rapisarda A, et al. Aminoflavone, a ligand of the aryl hydrocarbon receptor, inhibits HIF-1α expression in an AhR-independent fashion[J]. Cancer Res, 2010, 70: 6837-6848. DOI:10.1158/0008-5472.CAN-10-1075 |
| [9] |
Shamis SAK, McMillan DC, Edwards J. The relationship between hypoxia-inducible factor 1α (HIF-1α) and patient survival in breast cancer: systematic review and meta-analysis[J]. Crit Rev Oncol Hematol, 2021, 159: 103231. DOI:10.1016/j.critrevonc.2021.103231 |
| [10] |
Wang Q, Hu DF, Rui Y, et al. Prognosis value of HIF-1α expression in patients with non-small cell lung cancer[J]. Gene, 2014, 541: 69-74. DOI:10.1016/j.gene.2014.03.025 |
| [11] |
Amankwah EK, Sellers TA, Park JY. Gene variants in the angiogenesis pathway and prostate cancer[J]. Carcinogenesis, 2012, 33: 1259-1269. DOI:10.1093/carcin/bgs150 |
| [12] |
Erpolat OP, Gocun PU, Akmansu M, et al. Hypoxia-related molecules HIF-1α, CA9, and osteopontin: predictors of survival in patients with high-grade glioma[J]. Strahlenther Onkol, 2013, 189: 147-154. DOI:10.1007/s00066-012-0262-5 |
| [13] |
Winter SC, Shah KA, Han C, et al. The relation between hypoxia-inducible factor (HIF)-1α and HIF-2α expression with anemia and outcome in surgically treated head and neck cancer[J]. Cancer, 2006, 107: 757-766. DOI:10.1002/cncr.21983 |
| [14] |
Birner P, Schindl M, Obermair A, et al. Overexpression of hypoxia-inducible factor 1α is a marker for an unfavorable prognosis in early-stage invasive cervical cancer[J]. Cancer Res, 2000, 60: 4693-4696. |
| [15] |
Ioannou M, Paraskeva E, Baxevanidou K, et al. HIF-1α in colorectal carcinoma: review of the literature[J]. J BUON, 2015, 20: 680-689. |
| [16] |
Ye LY, Zhang Q, Bai XL, et al. Hypoxia-inducible factor 1α expression and its clinical significance in pancreatic cancer: a meta-analysis[J]. Pancreatology, 2014, 14: 391-397. DOI:10.1016/j.pan.2014.06.008 |
| [17] |
Dai X, Pi G, Yang SL, et al. Association of PD-L1 and HIF-1α coexpression with poor prognosis in hepatocellular carcinoma[J]. Transl Oncol, 2018, 11: 559-566. DOI:10.1016/j.tranon.2018.02.014 |
| [18] |
Martínez-García MÁ, Riveiro-Falkenbach E, Rodríguez-Peralto JL, et al. A prospective multicenter cohort study of cutaneous melanoma: clinical staging and potential associations with HIF-1α and VEGF expressions[J]. Melanoma Res, 2017, 27: 558-564. DOI:10.1097/CMR.0000000000000393 |
| [19] |
Chen L, Shi Y, Yuan J, et al. HIF-1 alpha overexpression correlates with poor overall survival and disease-free survival in gastric cancer patients post-gastrectomy[J]. PLoS One, 2014, 9: e90678. DOI:10.1371/journal.pone.0090678 |
| [20] |
Tzao C, Lee SC, Tung HJ, et al. Expression of hypoxia-inducible factor (HIF)-1α and vascular endothelial growth factor (VEGF)-D as outcome predictors in resected esophageal squamous cell carcinoma[J]. Dis Markers, 2008, 25: 141-148. DOI:10.1155/2008/468323 |
| [21] |
Chen Y, Zhang L, Pan Y, et al. Over-expression of semaphorin 4D, hypoxia-inducible factor-1α and vascular endothelial growth factor is related to poor prognosis in ovarian epithelial cancer[J]. Int J Mol Sci, 2012, 13: 13264-13274. DOI:10.3390/ijms131013264 |
| [22] |
Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics[J]. Oncogene, 2010, 29: 625-634. DOI:10.1038/onc.2009.441 |
| [23] |
Bahrami A, Atkin SL, Majeed M, et al. Effects of curcumin on hypoxia-inducible factor as a new therapeutic target[J]. Pharmacol Res, 2018, 137: 159-169. DOI:10.1016/j.phrs.2018.10.009 |
| [24] |
Vaupel P, Multhoff G. Fatal alliance of hypoxia-/HIF-1α-driven microenvironmental traits promoting cancer progression[J]. Adv Exp Med Biol, 2020, 1232: 169-176. |
| [25] |
Wang Z, Li Q, Xia L, et al. Borneol promotes apoptosis of human glioma cells through regulating HIF-1α expression via mTORC1/eIF4E pathway[J]. J Cancer, 2020, 11: 4810-4822. DOI:10.7150/jca.45304 |
| [26] |
Balamurugan K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer[J]. Int J Cancer, 2016, 138: 1058-1066. DOI:10.1002/ijc.29519 |
| [27] |
Palazon A, Goldrath A, Nizet V, et al. HIF transcription factors, inflammation, and immunity[J]. Immunity, 2014, 41: 518-528. DOI:10.1016/j.immuni.2014.09.008 |
| [28] |
Ma Z, Xiang X, Li S, et al. Targeting hypoxia-inducible factor-1, for cancer treatment: recent advances in developing small-molecule inhibitors from natural compounds[J]. Semin Cancer Biol, 2020. DOI:10.1016/j.semcancer.2020.09.011 |
| [29] |
Wang GL, Jiang BH, Rue EA, et al. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension[J]. Proc Natl Acad Sci U S A, 1995, 92: 5510-5514. DOI:10.1073/pnas.92.12.5510 |
| [30] |
Min JH, Yang H, Ivan M, et al. Structure of an HIF-1α-pVHL complex: hydroxyproline recognition in signaling[J]. Science, 2002, 296: 1886-1889. DOI:10.1126/science.1073440 |
| [31] |
Jeong JW, Bae MK, Ahn MY, et al. Regulation and destabilization of HIF-1α by ARD1-mediated acetylation[J]. Cell, 2002, 111: 709-720. DOI:10.1016/S0092-8674(02)01085-1 |
| [32] |
Tam SY, Wu VWC, Law HKW. Hypoxia-induced epithelial-mesenchymal transition in cancers: HIF-1α and beyond[J]. Front Oncol, 2020, 10: 486. DOI:10.3389/fonc.2020.00486 |
| [33] |
Liu ZJ, Semenza GL, Zhang HF. Hypoxia-inducible factor 1 and breast cancer metastasis[J]. J Zhejiang Univ Sci B, 2015, 16: 32-43. DOI:10.1631/jzus.B1400221 |
| [34] |
Luo W, Zhong J, Chang R, et al. Hsp70 and CHIP selectively mediate ubiquitination and degradation of hypoxia-inducible factor (HIF)-1α but not HIF-2α[J]. J Biol Chem, 2010, 285: 3651-3663. DOI:10.1074/jbc.M109.068577 |
| [35] |
Liu Fi, Huang X, Luo Z, et al. Hypoxia-activated PI3K/AKT inhibits oxidative stress via the regulation of reactive oxygen species in human dental pulp cells[J]. Oxid Med Cell Longev, 2019, 2019: 6595189. |
| [36] |
Zhang J, Guo H, Zhu JS, et al. Inhibition of phosphoinositide 3-kinase/AKT pathway decreases hypoxia inducible factor-1α expression and increases therapeutic efficacy of paclitaxel in human hypoxic gastric cancer cells[J]. Oncol Lett, 2014, 7: 1401-1408. DOI:10.3892/ol.2014.1963 |
| [37] |
Zundel W, Schindler C, Haas-Kogan D, et al. Loss of PTEN facilitates HIF-1-mediated gene expression[J]. Genes Dev, 2000, 14: 391-396. |
| [38] |
Guo YJ, Pan WW, Liu SB, et al. ERK/MAPK signalling pathway and tumorigenesis[J]. Exp Ther Med, 2020, 19: 1997-2007. |
| [39] |
Cam H, Easton JB, High A, et al. mTORC1 signaling under hypoxic conditions is controlled by ATM-dependent phosphorylation of HIF-1α[J]. Mol Cell, 2010, 40: 509-520. DOI:10.1016/j.molcel.2010.10.030 |
| [40] |
Ravi R, Mookerjee B, Bhujwalla ZM, et al. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1α[J]. Genes Dev, 2000, 14: 34-44. |
| [41] |
van de Sluis B, Mao X, Zhai Y, et al. COMMD1 disrupts HIF-1α/β dimerization and inhibits human tumor cell invasion[J]. J Clin Invest, 2010, 120: 2119-2130. DOI:10.1172/JCI40583 |
| [42] |
Sapra P, Kraft P, Pastorino F, et al. Potent and sustained inhibition of HIF-1α and downstream genes by a polyethyleneglycol-SN38 conjugate, EZN-2208, results in anti-angiogenic effects[J]. Angiogenesis, 2011, 14: 245-253. DOI:10.1007/s10456-011-9209-1 |
| [43] |
Kim YH, Coon A, Baker AF, et al. Antitumor agent PX-12 inhibits HIF-1α protein levels through an Nrf2/PMF-1-mediated increase in spermidine/spermine acetyl transferase[J]. Cancer Chemother Pharmacol, 2011, 68: 405-413. DOI:10.1007/s00280-010-1500-0 |
| [44] |
Goey AKL, Chau CH, Sissung TM, et al. Screening and biological effects of marine pyrroloiminoquinone alkaloids: potential inhibitors of the HIF-1α/p300 interaction[J]. J Nat Prod, 2016, 79: 1267-1275. DOI:10.1021/acs.jnatprod.5b00846 |
| [45] |
Choi HJ, Eun JS, Kim DK, et al. Icariside Ⅱ from epimedium koreanum inhibits hypoxia-inducible factor-1α in human osteosarcoma cells[J]. Eur J Pharmacol, 2008, 579: 58-65. DOI:10.1016/j.ejphar.2007.10.010 |
| [46] |
Shi L, Zhang G, Zheng Z, et al. Andrographolide reduced VEGFA expression in hepatoma cancer cells by inactivating HIF-1α: the involvement of JNK and MTA1/HDCA[J]. Chem Biol Interact, 2017, 273: 228-236. DOI:10.1016/j.cbi.2017.06.024 |
| [47] |
Tong EJ. The Correlation of Radiosensitizing Effect of Elemene to Anoxia Lung Cancer Cells with MTOR and HIF-1α/Survivin Signal Pathway (榄香烯对乏氧肺癌细胞的放射增敏作用与mTOR及HIF-1α/Survivin通路的相关性研究)[D]. Dalian: Dalian Medical University, 2013.
|
| [48] |
Yang MH, Zang YS, Huang H, et al. Arsenic trioxide exerts anti-lung cancer activity by inhibiting angiogenesis[J]. Curr Cancer Drug Targets, 2014, 14: 557-566. DOI:10.2174/1568009614666140725090000 |
| [49] |
Deng M, Xue YJ, Xu LR, et al. Chrysophanol suppresses hypoxia-induced epithelial-mesenchymal transition in colorectal cancer cells[J]. Anat Rec (Hoboken), 2019, 302: 1561-1570. DOI:10.1002/ar.24081 |
| [50] |
Kim HS, Wannatung T, Lee S, et al. Quercetin enhances hypoxia-mediated apoptosis via direct inhibition of AMPK activity in HCT116 colon cancer[J]. Apoptosis, 2012, 17: 938-949. DOI:10.1007/s10495-012-0719-0 |
| [51] |
Du G, Lin H, Wang M, et al. Quercetin greatly improved therapeutic index of doxorubicin against 4T1 breast cancer by its opposing effects on HIF-1α in tumor and normal cells[J]. Cancer Chemother Pharmacol, 2010, 65: 277-287. DOI:10.1007/s00280-009-1032-7 |
| [52] |
Ye MX, Zhao YL, Li Y, et al. Curcumin reverses cis-platin resistance and promotes human lung adenocarcinoma A549/DDP cell apoptosis through HIF-1α and caspase-3 mechanisms[J]. Phytomedicine, 2012, 19: 779-787. DOI:10.1016/j.phymed.2012.03.005 |
| [53] |
Du Y, Long Q, Zhang L, et al. Curcumin inhibits cancer-associated fibroblast-driven prostate cancer invasion through MAOA/mTOR/HIF-1α signaling[J]. Int J Oncol, 2015, 47: 2064-2072. DOI:10.3892/ijo.2015.3202 |
| [54] |
Lee DH, Lee YJ. Quercetin suppresses hypoxia-induced accumulation of hypoxia-inducible factor-1 (HIF-1) through inhibiting protein synthesis[J]. J Cell Biochem, 2008, 105: 546-553. DOI:10.1002/jcb.21851 |
| [55] |
Oh SJ, Kim O, Lee JS, et al. Inhibition of angiogenesis by quercetin in tamoxifen-resistant breast cancer cells[J]. Food Chem Toxicol, 2010, 48: 3227-3234. DOI:10.1016/j.fct.2010.08.028 |
| [56] |
Lin TH, Hsu WH, Tsai PH, et al. Dietary flavonoids, luteolin and quercetin, inhibit invasion of cervical cancer by reduction of UBE2S through epithelial-mesenchymal transition signaling[J]. Food Funct, 2017, 8: 1558-1568. DOI:10.1039/C6FO00551A |
| [57] |
Shiau AL, Shen YT, Hsieh JL, et al. Scutellaria barbata inhibits angiogenesis through downregulation of HIF-1α in lung tumor[J]. Environ Toxicol, 2014, 29: 363-370. DOI:10.1002/tox.21763 |
| [58] |
Ansó E, Zuazo A, Irigoyen M, et al. Flavonoids inhibit hypoxia-induced vascular endothelial growth factor expression by a HIF-1 independent mechanism[J]. Biochem Pharmacol, 2010, 79: 1600-1609. DOI:10.1016/j.bcp.2010.02.004 |
| [59] |
Mukund V, Saddala MS, Farran B, et al. Molecular docking studies of angiogenesis target protein HIF-1α and genistein in breast cancer[J]. Gene, 2019, 701: 169-172. DOI:10.1016/j.gene.2019.03.062 |
| [60] |
Li S, Li J, Dai W, et al. Genistein suppresses aerobic glycolysis and induces hepatocellular carcinoma cell death[J]. Br J Cancer, 2017, 117: 1518-1528. DOI:10.1038/bjc.2017.323 |
| [61] |
Singh-Gupta V, Zhang H, Yunker CK, et al. Daidzein effect on hormone refractory prostate cancer in vitro and in vivo compared to genistein and soy extract: potentiation of radiotherapy[J]. Pharm Res, 2010, 27: 1115-1127. DOI:10.1007/s11095-010-0107-9 |
| [62] |
Chen F, Zhuang M, Zhong C, et al. Baicalein reverses hypoxia-induced 5-FU resistance in gastric cancer AGS cells through suppression of glycolysis and the PTEN/AKT/HIF-1α signaling pathway[J]. Oncol Rep, 2015, 33: 457-463. DOI:10.3892/or.2014.3550 |
| [63] |
Chen J, Li Z, Chen AY, et al. Inhibitory effect of baicalin and baicalein on ovarian cancer cells[J]. Int J Mol Sci, 2013, 14: 6012-6025. DOI:10.3390/ijms14036012 |
| [64] |
Song X, Yao J, Wang F, et al. Wogonin inhibits tumor angiogenesis via degradation of HIF-1α protein[J]. Toxicol Appl Pharmacol, 2013, 271: 144-155. DOI:10.1016/j.taap.2013.04.031 |
| [65] |
Seo S, Seo K, Ki SH, et al. Isorhamnetin inhibits reactive oxygen species-dependent hypoxia inducible factor (HIF)-1α accumulation[J]. Biol Pharm Bull, 2016, 39: 1830-1838. DOI:10.1248/bpb.b16-00414 |
| [66] |
Kim KM, Heo DR, Lee J, et al. 5, 3'-Dihydroxy-6, 7, 4'-trimethoxyflavanone exerts its anticancer and antiangiogenesis effects through regulation of the AKT/mTOR signaling pathway[J]. Chem Biol Interact, 2015, 225: 32-39. DOI:10.1016/j.cbi.2014.10.033 |
| [67] |
Fang J, Xia C, Cao Z, et al. Apigenin inhibits VEGF and HIF-1 expression via PI3K/AKT/p70S6K1 and HDM2/p53 pathways[J]. FASEB J, 2005, 19: 342-353. DOI:10.1096/fj.04-2175com |
| [68] |
Huang H, Chen AY, Rojanasakul Y, et al. Dietary compounds galangin and myricetin suppress ovarian cancer cell angiogenesis[J]. J Funct Foods, 2015, 15: 464-475. DOI:10.1016/j.jff.2015.03.051 |
| [69] |
Gao H, Xie J, Peng J, et al. Hispidulin inhibits proliferation and enhances chemosensitivity of gallbladder cancer cells by targeting HIF-1α[J]. Exp Cell Res, 2015, 332: 236-246. DOI:10.1016/j.yexcr.2014.11.021 |
| [70] |
Xu B, Jiang C, Han H, et al. Icaritin inhibits the invasion and epithelial-to-mesenchymal transition of glioblastoma cells by targeting EMMPRIN via PTEN/AKT/HIF-1α signalling[J]. Clin Exp Pharmacol Physiol, 2015, 42: 1296-1307. DOI:10.1111/1440-1681.12488 |
| [71] |
Hou HX, Li DR, Cheng DH, et al. Cellular redox status regulates emodin-induced radiosensitization of nasopharyngeal carcinoma cells in vitro and in vivo[J]. J Pharm (Cairo), 2013, 2013: 218297. |
| [72] |
Lu HF, Lai KC, Hsu SC, et al. Involvement of matrix metalloproteinases on the inhibition of cells invasion and migration by emodin in human neuroblastoma SH-SY5Y cells[J]. Neurochem Res, 2009, 34: 1575-1583. DOI:10.1007/s11064-009-9946-3 |
| [73] |
Shi GH, Zhou L. Emodin suppresses angiogenesis and metastasis in anaplastic thyroid cancer by affecting TRAF6-mediated pathways in vivo and in vitro[J]. Mol Med Rep, 2018, 18: 5191-5197. |
| [74] |
Hu L, Cui R, Liu H, et al. Emodin and rhein decrease levels of hypoxia-inducible factor-1α in human pancreatic cancer cells and attenuate cancer cachexia in athymic mice carrying these cells[J]. Oncotarget, 2017, 8: 88008-88020. DOI:10.18632/oncotarget.21330 |
| [75] |
Yuan X, Tian W, Hua Y, et al. Rhein enhances the cytotoxicity of effector lymphocytes in colon cancer under hypoxic conditions[J]. Exp Ther Med, 2018, 16: 5350-5358. |
| [76] |
Fernand VE, Losso JN, Truax RE, et al. Rhein inhibits angiogenesis and the viability of hormone-dependent and -independent cancer cells under normoxic or hypoxic conditions in vitro[J]. Chem Biol Interact, 2011, 192: 220-232. DOI:10.1016/j.cbi.2011.03.013 |
| [77] |
Ding Z, Xu F, Tang J, et al. Physcion 8-O-β-glucopyranoside prevents hypoxia-induced epithelial-mesenchymal transition in colorectal cancer HCT116 cells by modulating EMMPRIN[J]. Neoplasma, 2016, 63: 351-361. DOI:10.4149/303_150723N405 |
| [78] |
Chen X, Gao H, Han Y, et al. RETRACTED: physcion induces mitochondria-driven apoptosis in colorectal cancer cells via downregulating EMMPRIN[J]. Eur J Pharmacol, 2015, 764: 124-133. DOI:10.1016/j.ejphar.2015.07.008 |
| [79] |
Fu P, Du F, Chen W, et al. Tanshinone ⅡA blocks epithelial-mesenchymal transition through HIF-1α downregulation, reversing hypoxia-induced chemotherapy resistance in breast cancer cell lines[J]. Oncol Rep, 2014, 31: 2561-2568. DOI:10.3892/or.2014.3140 |
| [80] |
Dat NT, Jin X, Lee JH, et al. Abietane diterpenes from Salvia miltiorrhiza inhibit the activation of hypoxia-inducible factor-1[J]. J Nat Prod, 2007, 70: 1093-1097. DOI:10.1021/np060482d |
| [81] |
Yang YF, Cao Y, Chen LH, et al. Cryptotanshinone suppresses cell proliferation and glucose metabolism via STAT3/SIRT3 signaling pathway in ovarian cancer cells[J]. Cancer Med, 2018, 7: 4610-4618. DOI:10.1002/cam4.1691 |
| [82] |
Guo Y, Han B, Luo K, et al. NOX2-ROS-HIF-1α signaling is critical for the inhibitory effect of oleanolic acid on rectal cancer cell proliferation[J]. Biomed Pharmacother, 2017, 85: 733-739. DOI:10.1016/j.biopha.2016.11.091 |
| [83] |
Li Y, Xu Q, Yang W, et al. Oleanolic acid reduces aerobic glycolysis-associated proliferation by inhibiting yes-associated protein in gastric cancer cells[J]. Gene, 2019, 712: 143956. DOI:10.1016/j.gene.2019.143956 |
| [84] |
Ma J, Han L Z, Liang H, et al. Celastrol inhibits the HIF-1α pathway by inhibition of mTOR/p70S6K/eIF4E and ERK1/2 phosphorylation in human hepatoma cells[J]. Oncol Rep, 2014, 32: 235-242. DOI:10.3892/or.2014.3211 |
| [85] |
Huang L, Zhang Z, Zhang S, et al. Inhibitory action of celastrol on hypoxia-mediated angiogenesis and metastasis via the HIF-1α pathway[J]. Int J Mol Med, 2011, 27: 407-415. |
| [86] |
Zhu Y, Liu X, Zhao P, et al. Celastrol suppresses glioma vasculogenic mimicry formation and angiogenesis by blocking the PI3K/AKT/mTOR signaling pathway[J]. Front Pharmacol, 2020, 11: 25. DOI:10.3389/fphar.2020.00025 |
| [87] |
Li W, Yang L, Wang D, et al. Effects of triptolide on epithelial-mesenchymal transition and invasion of melanoma A375 cells[J]. Shanghai J Tradit Chin Med (上海中医药杂志), 2020, 54: 153-155. |
| [88] |
Li T, Jin MM, Song SL, et al. Triptolide inhibits human hepatocarcinoma SMMC-7721 cells by regulating glycolysis[J]. World J Integr Tradit West Med (世界中西医结合杂志), 2020, 15: 981-985, 990. |
| [89] |
Dawood M, Ooko E, Efferth T. Collateral sensitivity of parthenolide via NF-κB and HIF-α inhibition and epigenetic changes in drug-resistant cancer cell lines[J]. Front Pharmacol, 2019, 10: 542. DOI:10.3389/fphar.2019.00542 |
| [90] |
Lv Y. The Effect of Excisanin A on the HIF-1α and Its Target Genes in Hepatocellular Carcinoma Cells (尾叶香茶菜素A对肝癌细胞中HIF-1α及其靶基因的影响)[D]. Yanji: Yanbian University, 2017.
|
| [91] |
Dong J, Chen Y, Yang W, et al. Antitumor and anti-angiogenic effects of artemisinin on breast tumor xenografts in nude mice[J]. Res Vet Sci, 2020, 129: 66-69. DOI:10.1016/j.rvsc.2020.01.005 |
| [92] |
Huynh N, Beutler JA, Shulkes A, et al. Glaucarubinone inhibits colorectal cancer growth by suppression of hypoxia-inducible factor 1α and β-catenin via a p-21 activated kinase 1-dependent pathway[J]. Biochim Biophys Acta, 2015, 1853: 157-165. DOI:10.1016/j.bbamcr.2014.10.013 |
| [93] |
Lingyi F, Wangbing C, Wei G, et al. Berberine targets AP-2/hTERT, NF-κB/COX-2, HIF-1α/VEGF and cytochrome-c/caspase signaling to suppress human cancer cell growth[J]. PLoS One, 2013, 8: e69240. DOI:10.1371/journal.pone.0069240 |
| [94] |
Zeng X, Wan L, Wang Y, et al. Effect of low dose of berberine on the radioresistance of cervical cancer cells via a PI3K/HIF-1 pathway under nutrient-deprived conditions[J]. Int J Radiat Biol, 2020, 96: 1060-1067. DOI:10.1080/09553002.2020.1770358 |
| [95] |
Lin SK, Tsai SC, Lee CC, et al. Berberine inhibits HIF-1α expression via enhanced proteolysis[J]. Mol Pharmacol, 2004, 66: 612-619. |
| [96] |
Tsang CM, Cheung KCP, Cheung YC, et al. Berberine suppresses Id-1 expression and inhibits the growth and development of lung metastases in hepatocellular carcinoma[J]. Biochim Biophys Acta, 2015, 1852: 541-551. DOI:10.1016/j.bbadis.2014.12.004 |
| [97] |
Wu YY, Li TM, Zang LQ, et al. Effects of berberine on tumor growth and intestinal permeability in HCT116 tumor-bearing mice using polyamines as targets[J]. Biomed Pharmacother, 2018, 107: 1447-1453. DOI:10.1016/j.biopha.2018.08.130 |
| [98] |
Zhang Q, Zhang C, Yang X, et al. Berberine inhibits the expression of hypoxia induction factor-1α and increases the radiosensitivity of prostate cancer[J]. Diagn Pathol, 2014, 9: 98. DOI:10.1186/1746-1596-9-98 |
| [99] |
Zhang C, Yang X, Zhang Q, et al. Berberine radiosensitizes human nasopharyngeal carcinoma by suppressing hypoxia-inducible factor-1α expression[J]. Acta Otolaryngol, 2014, 134: 185-192. DOI:10.3109/00016489.2013.850176 |
| [100] |
Pan Y, Zhang F, Zhao YW, et al. Berberine enhances chemosensitivity and induces apoptosis through dose-orchestrated AMPK signaling in breast cancer[J]. J Cancer, 2017, 8: 1679-1689. DOI:10.7150/jca.19106 |
| [101] |
Su Q, Wang J, Fan M, et al. Sanguinarine disrupts the colocalization and interaction of HIF-1α with tyrosine and serine phosphorylated-STAT3 in breast cancer[J]. J Cell Mol Med, 2020, 24: 3756-3761. DOI:10.1111/jcmm.15056 |
| [102] |
Su Q, Fan M, Wang J, et al. Sanguinarine inhibits epithelial-mesenchymal transition via targeting HIF-1α/TGF-β feed-forward loop in hepatocellular carcinoma[J]. Cell Death Dis, 2019, 10: 939. DOI:10.1038/s41419-019-2173-1 |
| [103] |
Hong X, Zhong L, Xie Y, et al. Matrine reverses the warburg effect and suppresses colon cancer cell growth negatively regulating HIF-1α[J]. Front Pharmacol, 2019, 10: 1437. DOI:10.3389/fphar.2019.01437 |
| [104] |
Huang J, Chen ZH, Ren CM, et al. Antiproliferation effect of evodiamine in human colon cancer cells is associated with IGF-1/HIF-1α downregulation[J]. Oncol Rep, 2015, 34: 3203-3211. DOI:10.3892/or.2015.4309 |
| [105] |
Ramu A, Kathiresan S, Ali AB. Gramine inhibits angiogenesis and induces apoptosis via modulation of TGF-β signalling in 7, 12 dimethylbenz[J]. Phytomedicine, 2017, 33: 69-76. DOI:10.1016/j.phymed.2017.05.008 |
| [106] |
Wang JY, Wang Z, Li MY, et al. Dictamnine promotes apoptosis and inhibits epithelial-mesenchymal transition, migration, invasion and proliferation by downregulating the HIF-1α and Slug signaling pathways[J]. Chem Biol Interact, 2018, 296: 134-144. DOI:10.1016/j.cbi.2018.09.014 |
| [107] |
Liang B, Zheng CS, Feng GS, et al. Experimental evaluation of inhibitory effect of 10-hydroxycamptothecin on hypoxia-inducible factor-1α expression and angiogenesis in liver tumors after transcatheter arterial embolization[J]. J Vasc Interv Radiol, 2010, 21: 1565-1572. DOI:10.1016/j.jvir.2010.05.028 |
| [108] |
Liu RM, Xu P, Chen Q, et al. A multiple-targets alkaloid nuciferine overcomes paclitaxel-induced drug resistance in vitro and in vivo[J]. Phytomedicine, 2020, 79: 153342. DOI:10.1016/j.phymed.2020.153342 |
| [109] |
Lou S, Wang Y, Yu Z, et al. Curcumin induces apoptosis and inhibits proliferation in infantile hemangioma endothelial cells via downregulation of MCL-1 and HIF-1α[J]. Medicine (Baltimore), 2018, 97: e9562. DOI:10.1097/MD.0000000000009562 |
| [110] |
Thomas SL, Zhong D, Zhou W, et al. EF24, a novel curcumin analog, disrupts the microtubule cytoskeleton and inhibits HIF-1[J]. Cell Cycle, 2008, 7: 2409-2417. DOI:10.4161/cc.6410 |
| [111] |
Yoysungnoen B, Bhattarakosol P, Patumraj S, et al. Effects of tetrahydrocurcumin on hypoxia-inducible factor-1α and vascular endothelial growth factor expression in cervical cancer cell-induced angiogenesis in nude mice[J]. Biomed Res Int, 2015, 2015: 391748. |
| [112] |
Zhang Q, Tang X, Lu QY, et al. Resveratrol inhibits hypoxia-induced accumulation of hypoxia-inducible factor-1α and VEGF expression in human tongue squamous cell carcinoma and hepatoma cells[J]. Mol Cancer Ther, 2005, 4: 1465-1474. DOI:10.1158/1535-7163.MCT-05-0198 |
| [113] |
Jung KH, Lee JH, Thien Quach CH, et al. Resveratrol suppresses cancer cell glucose uptake by targeting reactive oxygen species-mediated hypoxia-inducible factor-1α activation[J]. J Nucl Med, 2013, 54: 2161-2167. DOI:10.2967/jnumed.112.115436 |
| [114] |
Zhang M, Zhou X, Zhou K. Resveratrol inhibits human nasopharyngeal carcinoma cell growth via blocking pAKT/p70S6K signaling pathways[J]. Int J Mol Med, 2013, 31: 621-627. DOI:10.3892/ijmm.2013.1237 |
| [115] |
Wang H, Jia R, Lv T, et al. Resveratrol suppresses tumor progression via inhibiting STAT3/HIF-1α/VEGF pathway in an orthotopic rat model of non-small-cell lung cancer (NSCLC)[J]. Onco Targets Ther, 2020, 13: 7057-7063. DOI:10.2147/OTT.S259016 |
| [116] |
Firouzi F, Khoei S, Mirzaei HR. Role of resveratrol on the cytotoxic effects and DNA damages of iododeoxyuridine and megavoltage radiation in spheroid culture of U87MG glioblastoma cell line[J]. Gen Physiol Biophys, 2015, 34: 43-50. DOI:10.4149/gpb_2014023 |
| [117] |
Mitani T, Ito Y, Harada N, et al. Resveratrol reduces the hypoxia-induced resistance to doxorubicin in breast cancer cells[J]. J Nutr Sci Vitaminol (Tokyo), 2014, 60: 122-128. DOI:10.3177/jnsv.60.122 |
| [118] |
Li W, Cao L, Chen X, et al. Resveratrol inhibits hypoxia-driven ROS-induced invasive and migratory ability of pancreatic cancer cells via suppression of the hedgehog signaling pathway[J]. Oncol Rep, 2016, 35: 1718-1726. DOI:10.3892/or.2015.4504 |
| [119] |
Sun Y, Wang H, Liu M, et al. Resveratrol abrogates the effects of hypoxia on cell proliferation, invasion and EMT in osteosarcoma cells through downregulation of the HIF-1α protein[J]. Mol Med Rep, 2015, 11: 1975-1981. DOI:10.3892/mmr.2014.2913 |
| [120] |
Xu QH, Xiao Y, Li XQ, et al. Resveratrol counteracts hypoxia-induced gastric cancer invasion and EMT through hedgehog pathway suppression[J]. Anticancer Agents Med Chem, 2020, 20: 1105-1114. DOI:10.2174/1871520620666200402080034 |
| [121] |
Cao Z, Fang J, Xia C, et al. Trans-3, 4, 5'-trihydroxystibene inhibits hypoxia-inducible factor 1α and vascular endothelial growth factor expression in human ovarian cancer cells[J]. Clin Cancer Res, 2004, 10: 5253-5263. DOI:10.1158/1078-0432.CCR-03-0588 |
| [122] |
Subbaramaiah K, Iyengar NM, Morrow M, et al. Prostaglandin E down-regulates sirtuin 1 (SIRT1), leading to elevated levels of aromatase, providing insights into the obesity-breast cancer connection[J]. J Biol Chem, 2019, 294: 361-371. DOI:10.1074/jbc.RA118.005866 |
| [123] |
Mitani T, Harada N, Tanimori S, et al. Resveratrol inhibits hypoxia-inducible factor-1α-mediated androgen receptor signaling and represses tumor progression in castration-resistant prostate cancer[J]. J Nutr Sci Vitaminol (Tokyo), 2014, 60: 276-282. DOI:10.3177/jnsv.60.276 |
| [124] |
Jung DB, Lee HJ, Jeong SJ, et al. Rhapontigenin inhibited hypoxia inducible factor 1 alpha accumulation and angiogenesis in hypoxic PC-3 prostate cancer cells[J]. Biol Pharm Bull, 2011, 34: 850-855. DOI:10.1248/bpb.34.850 |
| [125] |
Butt NA, Kumar A, Dhar S, et al. Targeting MTA1/HIF-1α signaling by pterostilbene in combination with histone deacetylase inhibitor attenuates prostate cancer progression[J]. Cancer Med, 2017, 6: 2673-2685. DOI:10.1002/cam4.1209 |
| [126] |
Li X, Feng Y, Liu J, et al. Epigallocatechin-3-gallate inhibits IGF-I-stimulated lung cancer angiogenesis through downregulation of HIF-1α and VEGF expression[J]. J Nutrigenet Nutrigenomics, 2013, 6: 169-178. DOI:10.1159/000354402 |
| [127] |
Liu CC, Lin WW, Wu CC, et al. In vitro lauryl gallate induces apoptotic cell death through caspase-dependent pathway in U87 human glioblastoma cells[J]. In Vivo, 2018, 32: 1119-1127. DOI:10.21873/invivo.11354 |
| [128] |
Luo LX, Li Y, Liu ZQ, et al. Honokiol induces apoptosis, G1 arrest, and autophagy in KRAS mutant lung cancer cells[J]. Front Pharmacol, 2017, 8: 199. |
| [129] |
Lan KL, Lan KH, Sheu ML, et al. Honokiol inhibits hypoxia-inducible factor-1 pathway[J]. Int J Radiat Biol, 2011, 87: 579-590. DOI:10.3109/09553002.2011.568572 |
| [130] |
Kim A, Ma JY. Piceatannol-3-O-β-D-glucopyranoside (PG) exhibits in vitro anti-metastatic and anti-angiogenic activities in HT1080 malignant fibrosarcoma cells[J]. Phytomedicine, 2019, 57: 95-104. DOI:10.1016/j.phymed.2018.12.017 |
| [131] |
Yoysungnoen P, Wirachwong P, Changtam C, et al. Anti-cancer and anti-angiogenic effects of curcumin and tetrahydrocurcumin on implanted hepatocellular carcinoma in nude mice[J]. World J Gastroenterol, 2008, 14: 2003-2009. DOI:10.3748/wjg.14.2003 |
| [132] |
Hong SW, Jung KH, Lee HS, et al. SB365 inhibits angiogenesis and induces apoptosis of hepatocellular carcinoma through modulation of PI3K/AKT/mTOR signaling pathway[J]. Cancer Sci, 2012, 103: 1929-1937. DOI:10.1111/j.1349-7006.2012.02409.x |
| [133] |
Son MK, Jung KH, Lee HS, et al. SB365, Pulsatilla saponin D suppresses proliferation and induces apoptosis of pancreatic cancer cells[J]. Oncol Rep, 2013, 30: 801-808. DOI:10.3892/or.2013.2517 |
| [134] |
Chen QJ, Zhang MZ, Wang LX. Gensenoside Rg3 inhibits hypoxia-induced VEGF expression in human cancer cells[J]. Cell Physiol Biochem, 2010, 26: 849-858. DOI:10.1159/000323994 |
| [135] |
Lu J, Chen H, He F, et al. Ginsenoside 20(S)-Rg3 upregulates HIF-1α-targeting miR-519a-5p to inhibit the Warburg effect in ovarian cancer cells[J]. Clin Exp Pharmacol Physiol, 2020, 47: 1455-1463. DOI:10.1111/1440-1681.13321 |
| [136] |
Liu T, Zhao L, Zhang Y, et al. Ginsenoside 20(S)-Rg3 targets HIF-1α to block hypoxia-induced epithelial-mesenchymal transition in ovarian cancer cells[J]. PLoS One, 2014, 9: e103887. DOI:10.1371/journal.pone.0103887 |
| [137] |
Ge X, Zhen F, Yang B, et al. Ginsenoside Rg3 enhances radiosensitization of hypoxic oesophageal cancer cell lines through vascular endothelial growth factor and hypoxia inducible factor 1α[J]. J Int Med Res, 2014, 42: 628-640. DOI:10.1177/0300060513505491 |
| [138] |
Ahmmed B, Kampo S, Khan M, et al. Rg3 inhibits gemcitabine-induced lung cancer cell invasiveness through ROS-dependent, NF-κB- and HIF-1α-mediated downregulation of PTX3[J]. J Cell Physiol, 2019, 234: 10680-10697. DOI:10.1002/jcp.27731 |
| [139] |
Liu W, Pan HF, Yang LJ, et al. Panax ginseng C.A. Meyer (Rg3) ameliorates gastric precancerous lesions in Atp4a-/- mice via inhibition of glycolysis through PI3K/AKT/miRNA-21 pathway[J]. Evid Based Complement Alternat Med, 2020, 2020: 2672648. |
| [140] |
Qiu SP, Li HL, Shi HL, et al. Notoginsenoside Ft1 down-regulates HIF-1α, inhibits cell proliferation, decreases migration and promotes apoptosis in breast cancer cells[J]. Acta Pharm Sin (药学学报), 2016, 51: 1091-1097. |
| [141] |
Qiu P, Man S, Yang H, et al. Utilization of metabonomics to identify serum biomarkers in murine H22 hepatocarcinoma and deduce antitumor mechanism of Rhizoma Paridis saponins[J]. Chem Biol Interact, 2016, 256: 55-63. DOI:10.1016/j.cbi.2016.06.026 |
| [142] |
Law PC, Auyeung KK, Chan LY, et al. Astragalus saponins downregulate vascular endothelial growth factor under cobalt chloride-stimulated hypoxia in colon cancer cells[J]. BMC Complement Altern Med, 2012, 12: 160. DOI:10.1186/1472-6882-12-160 |
| [143] |
Park JJ, Hwang SJ, Park JH, et al. Chlorogenic acid inhibits hypoxia-induced angiogenesis via down-regulation of the HIF-1α/AKT pathway[J]. Cell Oncol (Dordr), 2015, 38: 111-118. |
| [144] |
Lee MS, Lee SO, Kim KR, et al. Sphingosine kinase-1 involves the inhibitory action of HIF-1α by chlorogenic acid in hypoxic DU145 cells[J]. Int J Mol Sci, 2017, 18: 325. DOI:10.3390/ijms18020325 |
| [145] |
Qin Y, Liu HJ, Li M, et al. Salidroside improves the hypoxic tumor microenvironment and reverses the drug resistance of platinum drugs via HIF-1α signaling pathway[J]. EBioMedicine, 2018, 38: 25-36. DOI:10.1016/j.ebiom.2018.10.069 |
| [146] |
Chen X, Kou Y, Lu Y, et al. Salidroside ameliorated hypoxia-induced tumorigenesis of BxPC-3 cells via downregulating hypoxia-inducible factor (HIF)-1α and LOXL2[J]. J Cell Biochem, 2020, 121: 165-173. DOI:10.1002/jcb.29000 |
| [147] |
Li Y, Pham V, Bui M, et al. Rhodiola rosea L.: an herb with anti-stress, anti-aging, and immunostimulating properties for cancer chemoprevention[J]. Curr Pharmacol Rep, 2017, 3: 384-395. DOI:10.1007/s40495-017-0106-1 |
| [148] |
Qi YJ, Cui S, Lu DX, et al. Effects of the aqueous extract of a Tibetan herb, Rhodiola algida var. tangutica on proliferation and HIF-1α, HIF-2α expression in MCF-7 cells under hypoxic condition in vitro[J]. Cancer Cell Int, 2015, 15: 81. DOI:10.1186/s12935-015-0225-x |
| [149] |
Su C, Zhang P, Liu J, et al. Erianin inhibits indoleamine 2, 3-dioxygenase -induced tumor angiogenesis[J]. Biomed Pharmacother, 2017, 88: 521-528. DOI:10.1016/j.biopha.2017.01.090 |
| [150] |
Xing Y, Mi C, Wang Z, et al. Fraxinellone has anticancer activity in vivo by inhibiting programmed cell death-ligand 1 expression by reducing hypoxia-inducible factor-1α and STAT3[J]. Pharmacol Res, 2018, 135: 166-180. DOI:10.1016/j.phrs.2018.08.004 |
| [151] |
Kim DH, Sung B, Kang YJ, et al. Sulforaphane inhibits hypoxia-induced HIF-1α and VEGF expression and migration of human colon cancer cells[J]. Int J Oncol, 2015, 47: 2226-2232. DOI:10.3892/ijo.2015.3200 |
| [152] |
Li Y, Zhang Y, Liu X, et al. Lutein inhibits proliferation, invasion and migration of hypoxic breast cancer cells via downregulation of HES1[J]. Int J Oncol, 2018, 52: 2119-2129. |
| [153] |
Le Y, Zhang X, Li K. Esculetin regulates triple negative breast cancer cell stemness in hypoxia microenvironment through HIF-1α[J]. Chin J New Drugs Clin Rem (中国新药与临床杂志), 2020, 39: 558-563. |
| [154] |
Sui W, Zhang W, Wu L, et al. Inhibitory mechanism of polypeptide from scorpion venom combined with 5-fluorouacil on angiogenesis of H22 hepatoma[J]. Chin Tradit Herb Drugs (中草药), 2014, 45: 392-397. |
| [155] |
Ren F, Wu K, Yang Y, et al. Dandelion polysaccharide exerts anti-angiogenesis effect on hepatocellular carcinoma by regulating VEGF/HIF-1α expression[J]. Front Pharmacol, 2020, 11: 460. DOI:10.3389/fphar.2020.00460 |
| [156] |
Zhang Z, Wang R, Huang X, et al. Self-delivered and self-monitored chemo-photodynamic nanoparticles with light-triggered synergistic antitumor therapies by downregulation of HIF-1α and depletion of GSH[J]. ACS Appl Mater Interfaces, 2020, 12: 5680-5694. DOI:10.1021/acsami.9b23325 |
| [157] |
Wang D, Gao Z, Zhang X. Resveratrol induces apoptosis in murine prostate cancer cells via hypoxia-inducible factor 1-alpha (HIF-1α)/reactive oxygen species (ROS)/P53 signaling[J]. Med Sci Monit, 2018, 24: 8970-8976. DOI:10.12659/MSM.913290 |
| [158] |
Chuang MT, Ho FM, Wu CC, et al. 15, 16-Dihydrotanshinone I, a compound of Salvia miltiorrhiza Bunge, induces apoptosis through inducing endoplasmic reticular stress in human prostate carcinoma cells[J]. Evid Based Complement Alternat Med, 2011, 2011: 865435. |
| [159] |
Pan Y, Shao D, Zhao Y, et al. Berberine reverses hypoxia-induced chemoresistance in breast cancer through the inhibition of AMPK- HIF-1α[J]. Int J Biol Sci, 2017, 13: 794-803. DOI:10.7150/ijbs.18969 |
| [160] |
Wang K, Liu R, Li J, et al. Quercetin induces protective autophagy in gastric cancer cells: involvement of AKT-mTOR-and hypoxia-induced factor 1α-mediated signaling[J]. Autophagy, 2011, 7: 966-978. DOI:10.4161/auto.7.9.15863 |
| [161] |
Riganti C, Doublier S, Viarisio D, et al. Artemisinin induces doxorubicin resistance in human colon cancer cells via calcium-dependent activation of HIF-1α and P-glycoprotein overexpression[J]. Br J Pharmacol, 2009, 156: 1054-1066. DOI:10.1111/j.1476-5381.2009.00117.x |
| [162] |
Li Z, Guo Z, Chu D, et al. Effectively suppressed angiogenesis-mediated retinoblastoma growth using celastrol nanomicelles[J]. Drug Deliv, 2020, 27: 358-366. DOI:10.1080/10717544.2020.1730522 |
| [163] |
Sreeja S, Krishnan NCK. Tumor control by hypoxia-specific chemotargeting of iron-oxide nanoparticle-berberine complexes in a mouse model[J]. Life Sci, 2018, 195: 71-80. DOI:10.1016/j.lfs.2017.12.036 |
| [164] |
Godugu C, Patel AR, Doddapaneni R, et al. Approaches to improve the oral bioavailability and effects of novel anticancer drugs berberine and betulinic acid[J]. PLoS One, 2014, 9: e89919. DOI:10.1371/journal.pone.0089919 |
| [165] |
Choi YJ, Heo K, Park HS, et al. The resveratrol analog HS-1793 enhances radiosensitivity of mouse-derived breast cancer cells under hypoxic conditions[J]. Int J Oncol, 2016, 49: 1479-1488. DOI:10.3892/ijo.2016.3647 |
| [166] |
Kim DH, Sung B, Kim JA, et al. HS-1793, a resveratrol analogue, downregulates the expression of hypoxia-induced HIF-1 and VEGF and inhibits tumor growth of human breast cancer cells in a nude mouse xenograft model[J]. Int J Oncol, 2017, 51: 715-723. DOI:10.3892/ijo.2017.4058 |
| [167] |
Talks KL, Turley H, Gatter KC, et al. The expression and distribution of the hypoxia-inducible factors HIF-1α and HIF-2α in normal human tissues, cancers, and tumor-associated macrophages[J]. Am J Pathol, 2000, 157: 411-421. DOI:10.1016/S0002-9440(10)64554-3 |
| [168] |
He J, Hu Y, Hu M, et al. The relationship between the preoperative plasma level of HIF-1α and clinic pathological features, prognosis in non-small cell lung cancer[J]. Sci Rep, 2016, 6: 20586. DOI:10.1038/srep20586 |
 2021, Vol. 56
2021, Vol. 56