2. 山西大学化学生物学与分子工程教育部重点实验室, 山西 太原 030006;
3. 地产中药功效物质研究与利用山西省重点实验室, 山西 太原 030006;
4. 中国医学科学院、北京协和医学院药物研究所, 北京 100050
2. The Key Laboratory of Chemical Biology and Molecular Engineering of Ministry of Education, Shanxi University, Taiyuan 030006, China;
3. The Key Laboratory of Effective Substances Research and Utilization in TCM of Shanxi Province, Taiyuan 030006, China;
4. Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100050, China
抑郁症是一种令人情绪低落、快感缺失、认知功能障碍并且存在高自杀风险的疾病, 因其复杂、异质、高复发等特点而难以治疗[1-3]。嘌呤类物质与抑郁症发病机制密切相关, 通过调控嘌呤类物质的代谢有望治疗抑郁症[4-6]。嘌呤能系统是嘌呤类物质在体内发挥生理作用的重要枢纽, 目前已经发现嘌呤能系统与抑郁症发病机制不可分割, 通过调控嘌呤能系统可有效改善抑郁症[7-9]。此外, 经文献报道, 抑郁症会引起抑郁模型动物海马、前额皮层、下丘脑、嗅球和外周血的嘌呤代谢物水平紊乱, 指示嘌呤代谢与抑郁症发病机制也存在关联, 但作用机制尚未研究全面, 通过调控嘌呤代谢改善抑郁症成为了当下的研究热点[10-14]。
1 嘌呤能系统假说及嘌呤代谢Drury和Szent-Györgyi[15]在1929年发现了嘌呤核苷腺苷, 并阐述了腺嘌呤类化合物在哺乳动物心脏的生理活动, 为之后的嘌呤能系统假说提供了思路。在此基础上, Burnstock[16]在20世纪60年代发现证据, 1972年首次提出嘌呤能系统假说, 即腺苷三磷酸(adenosine-5'-triphosphate, ATP)、相关的其他核苷酸和核苷作为细胞外信号分子, 从神经元、星形胶质细胞、小神经胶质细胞等各类细胞释放到细胞外, 在神经病理状态下, 如抑郁症引起的神经炎症, 会使突触前的线粒体释放大量ATP到胞外, ATP释放到胞外后, 被多种胞外酶代谢分解为足够多的腺苷一磷酸(adenosine monophosphate, AMP), 又或者在腺苷酸环化酶(adenylate cyclase, AC) 作用下形成环磷酸腺苷(cyclic adenosine monophosphate, cAMP) 后, 磷酸二酯酶(phosphodiesterases, PDEs) (PDE4、7、8) 将cAMP降解为AMP, AMP在胞外核苷酸酶CD73作用下降解为腺苷, 继而激活腺苷受体进行信号传导, 对神经元细胞和非神经元细胞(星形胶质细胞、少突胶质细胞、小胶质细胞和内皮细胞) 发挥调控作用[16-19]。该假说在20世纪90年代才被很好地接受, 现在已经确定的是, 腺苷和ATP作用于嘌呤能受体对神经传递和神经调节有广泛的生理作用[20, 21]。嘌呤能受体主要分为2个家族, 分别是由腺苷和ATP激活的P1受体和P2受体, 2个家族受体通常与G蛋白偶联发挥作用[19]。P1受体可分为A1、A2A、A2B、A3四种亚型, A1和A3亚型结合到Gi/o蛋白, 负责抑制cAMP的合成, A2A和A2B亚型结合到Gs蛋白, 负责促进cAMP的合成[6, 18]。胞外腺苷不仅可以由ATP代谢生成, 也可由胶质细胞借助平衡核苷转运体(equilibrative nucleoside transporters, ENTs) 释放到胞外, 且ENTs具有双向转运功能, 使胞内外腺苷浓度处于正常水平, 腺苷能被星形胶质细胞中的腺苷激酶转化为ATP, 也可以进入细胞内参与嘌呤代谢, 构成腺苷循环, 腺苷在神经发育、生理和病理过程中发挥重要作用[17, 18, 21]。在病理压力状态下, 腺苷降解速度比生成速度慢, 导致胞外腺苷浓度的快速增加, 腺苷与P1受体相互结合, 发挥保护作用, 然而腺苷半衰期很短, 浓度变化也是暂时的, 很难对抗病理变化[17, 22]。腺苷对脑功能的影响主要依赖于A1、A2A受体, A1受体在脑中分布最为丰富, 在海马、皮层、下丘脑中均有高表达, A2A受体在纹状体中表达较多, 其他脑区也有分布[9, 21]。突触前A1受体抑制谷氨酸(glutamic acid, Glu)、多巴胺和乙酰胆碱等神经递质的释放, 突触后A1受体会打开K+通道, 使K+外流超极化, 降低神经元信号传导, A1与A2A受体互相制衡, 共同调节神经活动[17]。P2受体主要有2种类型, 分别为通道型P2X受体(P2X1~7, 7个亚型由不同种类细胞表达) 和代谢型G蛋白偶联P2Y受体(P2Y1、2、4、6、11、12、13和14, 由ATP等嘌呤或嘧啶表达)。P2X受体是ATP门控离子通道, Na+、K+、Ca2+可通行于P2X受体, 参与不同神经递质的调节, P2Y1、2、4、6和11亚型偶联到Gq/11蛋白, 促进内质网Ca2+的释放, P2Y12、13和14亚型偶联到Gi/o蛋白, 抑制AC活性, 调控细胞内Ca2+浓度[6, 18]。嘌呤能信号通路的任何一个过程出问题都可能是精神类疾病发病的重要原因, 当前, A2A受体和P2X7受体已被确立为治疗抑郁症的潜在靶点[21]。综上所述, 细胞内外嘌呤能系统的运作机制如图 1所示。

|
Figure 1 Operating mechanism of purinergic system inside and outside cells. AMP: Adenosine monophosphate; cAMP: Cyclic adenosine monophosphate; ATP: Adenosine-5'-triphosphate; ADP: Adenosine diphosphate; AC: Adenylate cyclase; CD73: Extracellular nucleotidase; PDEs: Phosphodiesterases; P1, P2X, P2Y: Purinergic receptors; ENTs: Equilibrative nucleoside transporters; Gi/o, Gq/11, Gs: G protein coupling family; A-te: Activate; R-in: Restrain |
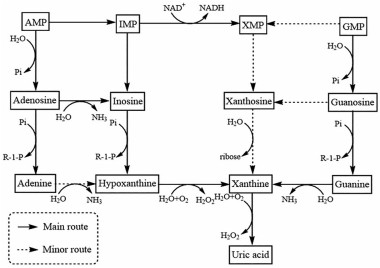
嘌呤代谢可分为合成代谢和分解代谢, 目前, 基于调控嘌呤代谢抗抑郁的作用机制研究主要集中在嘌呤分解代谢, 其走势如图 2所示。嘌呤分解代谢途径最大的特点是需要各种脱氨酶水解脱去氨基, 经过研究发现, 该途径主要有三个水平: 核苷酸水平、核苷水平和碱基水平, 都会脱氨基生成黄嘌呤, 最终在黄嘌呤氧化酶(xanthine oxidase, XO)/脱氢酶作用下生成尿酸[23, 24]。
|
Figure 2 Trends of purine catabolism. IMP: Inosine monophosphate; XMP: Xanthosine monophosphate; GMP: Guanosine monophosphate; NAD+: Nicotinamide adenine dinucleotide; NADH: Reduced nicotinamide adenine dinucleotide; Pi: Phosphate; R-1-P: Ribose-1-phosphate |
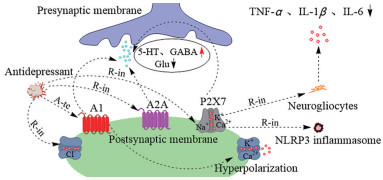
嘌呤能系统中腺苷受体在神经传递和神经保护中扮演着重要角色, 腺苷P1受体中A1、A2A亚型与神经递质释放密切相关, 与ATP结合的P2受体中P2X7亚型与抑郁症诱发神经炎症紧密相通[25-27]。经文献调研发现, 嘌呤能受体活性的改变诱使抑郁症的发生, 这为嘌呤能系统的调控可以改善抑郁症提供了证据[28-30]。研究表明, 抑郁症会抑制5-羟色胺(5-hydroxytryptamine, 5-HT) 的传递及其受体活性, 降低抑制性神经递质γ-氨基丁酸(γ-aminobutyric, GABA)、增加兴奋性神经递质Glu水平, 导致神经兴奋毒性, 不过, 有研究显示激活腺苷A1受体或抑制腺苷A2A受体, 可使抑郁症机体5-HT、GABA、Glu水平回调, 改善抑郁症状[31-33]。嘌呤能腺苷A1受体被激活后, 作用于突触后膜, 抑制Ca2+内流, 促进K+外流, 使细胞膜超极化, 从而降低兴奋毒性, 保护神经元, 除了K+、Ca2+通道可以调节神经活动, Cl-通道也能调节抑郁症病理神经递质的传递, Cl-通道主要干预神经元与神经递质甘氨酸和GABA的反应, 这两种抑制性神经递质对中枢神经系统有重要的调节作用[34-36]。另有研究报道, 部分茶叶嘌呤生物碱对抑郁模型小鼠脑区Cl-通道有明显的抑制效果, 并产生了抗抑郁作用, 作者指出该抑制作用与嘌呤结构密切相关, 具有黄嘌呤类结构的类似物效果更为显著, 而黄嘌呤是嘌呤分解代谢的下游产物, 提示嘌呤代谢产物对Cl-通道可能有调节作用, 但具体机制尚待细究[37]。随着抗抑郁药物作用机制的不断阐明, 研究重点已从神经传递转向神经保护, 抑郁症机体中嘌呤能P2X7受体是一种成熟的炎症小体激活剂, 不仅可以激活Nod样受体蛋白3 (nod-like receptor protein-3, NLRP3) 炎症小体, 还会促使神经胶质细胞释放肿瘤坏死因子-α (tumor necrosis factor-α, TNF-α)、白细胞介素(interleukin, IL)-1β、IL-6等炎症因子, P2X7受体已被公认为炎症反应的调节因子[38-42]。已有研究发现, 通过抑制或敲除抑郁模型小鼠P2X7受体, 能阻滞神经胶质细胞释放炎症因子, 保护神经, 改善抑郁症, 提示控制P2X7受体活性对抑郁症机体神经保护有重要意义[43-45]。通过在突触部位调控嘌呤能系统发挥抗抑郁作用的机制可见图 3。
|
Figure 3 Antidepressant mechanisms at synaptic sites by regulating the purinergic system. A1, A2A, P2X7: Purinergic receptors; 5-HT: 5-Hydroxytryptamine; GABA: γ-Aminobutyric; Glu: Glutamic acid; NLRP3: Nod-like receptor protein-3; TNF-α: Tumor necrosis factor-α; IL: Interleukin |
大量研究表明, 罹患抑郁症的动物和患者嘌呤代谢紊乱, 在动物实验中, 嘌呤代谢异常程度与小鼠是否易感有直接关系, 易感小鼠嘌呤代谢受外界应激影响更大, 抑郁症模型动物嘌呤分解代谢中的腺苷、肌苷、鸟苷、次黄嘌呤、黄嘌呤、尿酸含量均会发生显著变化, 在临床上, 抑郁症患者的这些嘌呤代谢物也被发现变化明显, 提示回调这些嘌呤代谢物水平对缓解抑郁症有重要意义[46-48]。除了嘌呤代谢物, 嘌呤代谢中的关键酶活性在抑郁症发病过程中也发生了显著变化。临床研究表明, 重度抑郁症患者的外周血中XO、腺苷脱氨酶(adenosine deaminase, ADA) 水平明显升高, 嘌呤分解代谢有提速趋势, 目前已有课题组提出要设计XO的特异性抑制剂, 另外还有课题组对抑制XO活性的天然产物进行了研究, 为改善抑郁症的药物开发提供了新思路[49-51]。鉴于嘌呤代谢与抑郁症发病机制脉脉相通, 通过体外调控嘌呤代谢物对抑郁症的发病机制和针对性治疗深入研究具有现实意义。近些年来, 已经陆续发现一些嘌呤代谢物具有显著的神经营养和保护作用。有证据显示: 腺苷能够调控神经递质的释放和突触反应, 具有镇静、抗惊厥、抗损伤活性及抗焦虑作用[52-54]; 肌苷对缺氧和氧化损伤的神经元、星形胶质细胞具有神经保护作用, 还能促进体外神经突的生长[55-57]; 鸟苷除了对神经元有营养作用外, 还能减少神经炎症、氧化应激和兴奋性毒性[58-60]。这些研究结果都表明腺苷、肌苷和鸟苷具有很大的抗抑郁潜力, 为这3种嘌呤代谢物的抗抑郁研究打下了基础。
3 体外增补嘌呤代谢物对抑郁症的改善作用机制大量研究表明, 体外增补适宜剂量的腺苷、肌苷和鸟苷均有较为显著的抗抑郁作用, 并且都与嘌呤能系统有关。其中腺苷和肌苷与嘌呤能系统关系密切, 都能介导腺苷A1、A2A受体发挥抗抑郁作用, 鸟苷虽然不能直接和嘌呤能受体结合, 但鸟苷对神经的积极作用与腺苷A1、A2A受体存在关联。随着腺苷、肌苷和鸟苷发挥抗抑郁作用的关键信号分子和通路不断被发现, 外源增补腺苷、肌苷和鸟苷的抗抑郁机制也逐渐清晰。
3.1 体外增补腺苷的抗抑郁作用腺苷是嘌呤能系统中的关键化合物, 本身具有神经保护特性, 尤其是激活腺苷A1受体后产生的神经保护作用, 抑郁症是涉及神经损伤的精神类疾病, 存在证据表明, 外源增补腺苷具有抗抑郁作用[61-64]。早期研究发现[61], 通过腹腔注射(5~10 mg·kg-1) 和脑室内注射(0.01~10 μg·site-1) 的腺苷均可介导腺苷A1、A2A受体的激活发挥抗抑郁作用, 显著减少抑郁症模型小鼠的强迫游泳实验(forced swimming test, FST) 静止时间。随后的研究发现[62], 外源增补腺苷的抗抑郁作用涉及5-羟色胺能系统的调节, 腺苷可能作为5-HT1A受体的部分激动剂, 但是腺苷对5-羟色胺能系统的这种调节作用与腺苷A1、A2A受体活性关系不显著。研究还发现[63], 腺苷介导A1、A2A受体发挥的抗抑郁作用与阿片系统有关。内源性阿片系统由不同的阿片肽家族组成, 作用于3种不同亚型阿片受体: μ、δ、κ受体, 这3种亚型都是G蛋白偶联受体超家族成员, 而嘌呤能系统腺苷A1、A2A受体也是G蛋白偶联受体家族的成员。结果显示, 腺苷介导A1、A2A受体发挥的抗抑郁作用可能是由中枢μ-、δ-阿片受体的激活和κ-阿片受体的抑制产生的。药理学证据还表明, 外源性腺苷抗抑郁作用与激活A1受体介导谷氨能系统N-甲基-D-天冬氨酸受体(N-methyl-D-aspartate receptor, NMDAR) 的抑制有关, 而临床前和临床已有数据表明, 抑制NMDAR活性是抗抑郁化合物的特征, 并且抑郁症患者也被观察到NMDAR受体活性异常, 再次证明外源增补腺苷具有抗抑郁作用[64]。
3.2 体外增补肌苷的抗抑郁作用肌苷是在腺苷分解过程中产生的, 由ADA分解腺苷而成, 腺苷到肌苷的脱氨作用主要发生在细胞内有高浓度腺苷的情况下, 当胞内肌苷达到高浓度时, 也能通过双向平衡核苷转运体进入胞外, 并且像腺苷一样, 结合腺苷P1受体发挥作用, 腺苷已被发现介导嘌呤能系统A1、A2A受体的激活发挥抗抑郁作用, 肌苷作为腺苷的主要下游产物, 被假设有类似腺苷的抗抑郁功能[65]。近年来, 已有大量研究发现体外增补肌苷确实存在抗抑郁作用[65-69]。初期研究发现, 外源增补肌苷处理的抑郁模型小鼠在FST (5和50 mg·kg-1) 和悬尾实验(tail suspension test, TST, 1和10 mg·kg-1) 中表现出比对照组更好的抗静止作用, 腺苷A1、A2A受体拮抗剂均可抑制肌苷在FST中的抗抑郁功能, 指示肌苷有可能通过A1、A2A受体的激活发挥抗抑郁作用[65]。有课题组证实了肌苷的抗抑郁潜力, 在细胞水平, 发现肌苷有较好的促神经元生长作用, 100 μmol·L-1肌苷培养Wistar大鼠胚胎中分离的新皮层神经元比载体培养的神经元突起数量更多, 并且30~300 μmol·L-1肌苷能够显著浓度依赖性地提升细胞存活率, 10~300 μmol·L-1肌苷能够显著浓度依赖性地增加神经元数量; 在动物水平, 肌苷对慢性不可预测应激和慢性社交挫败应激抑郁模型小鼠均有抗抑郁作用, 并且肌苷给药后, 脑肌苷水平短暂升高, 给药1 h后, 大脑中肌苷含量显著增加, 2 h后又恢复到基础水平, 给药前和给药后1 h脑肌苷含量差异为130 μmol·kg-1, 体外增补肌苷还能促进抑郁模型小鼠海马体内丝裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK) 的激活和脑源性神经营养因子(brain derived neurotrophic factor, BDNF) 的增殖, 而BDNF的增殖受A1受体活性调控, 提供了腺苷A1受体的激活参与肌苷抗抑郁作用的依据, 不过在这次检测中, A2A受体的积极作用不显著[66]。为了进一步了解腺苷A1、A2A受体参与肌苷抗抑郁作用的机制, 有课题组对这种效应背后的信号通路进行了研究, 因为腺苷A1、A2A受体的激活能够调节丝裂原活化蛋白激酶激酶(mitogen-activated protein kinase kinase, MAPKK, MEK)/细胞外调节蛋白激酶(extracellular regulated protein kinase, ERK)1/2、钙离子依赖性蛋白激酶(Ca2+-dependent protein kinase, CaMKⅡ)、cAMP/蛋白激酶A (protein kinase A, PKA)、磷脂酰肌醇3-激酶(phosphatidylinositol 3-kinase, PI3K)/丝氨酸/苏氨酸激酶(serine/threonine kinase, Akt) 和糖原合成酶激酶3β (glycogen synthase kinase 3β, GSK-3β) 等与神经可塑性和细胞存活相关的信号通路, 于是该小组对这些通路进行了检测, 结果表明肌苷抗抑郁机制与激活MEK/ERK 1/2、CaMKⅡ、cAMP/PKA、PI3K/Akt和抑制GSK-3β通路有关, 不仅如此, 在这次研究中还发现CaMKⅡ拮抗剂KN-62会阻断嘌呤能P2受体, 如P2X7受体, 虽然肌苷并不能直接激活P2受体, 但不能排除肌苷有间接激活P2受体的可能性[67]。另外, 有研究表明肌苷也能像腺苷一样通过NMDAR的抑制发挥抗抑郁作用, 鉴于腺苷A1、A2A受体参与雷帕霉素靶蛋白(mammalian target of rapamycin, mTOR) 上游信号通路的调节, 研究者通过验证又发现肌苷抗抑郁的作用机制与mTOR的激活密切相关[68]。除此之外, 研究发现外源增补肌苷发挥的抗抑郁作用还与cAMP反应元件结合蛋白活性的增强存在联系, 但是否通过腺苷A1、A2A受体的激活而增强还有待研究[69]。
3.3 体外增补鸟苷的抗抑郁作用鸟苷的神经保护作用已经在体内和体外研究中得到证实, 并有研究表明鸟苷的上游产物鸟苷一磷酸急性治疗小鼠有抗抑郁效果, 指示鸟苷可能也有类似的抗抑郁作用[70-72]。不断有文献报道增补外源鸟苷具有抗抑郁作用[73-77]。前期研究发现[73], 体外增补鸟苷在抑郁模型小鼠FST (0.5~5 mg·kg-1) 和TST (0.05~0.5 mg·kg-1) 中均表现出抗抑郁作用, 并且相比于腺苷和肌苷, 鸟苷发挥抗抑郁作用的剂量要低得多, 脑室内注射鸟苷只需1 nmol·site-1就有抗抑郁作用, 鸟苷发挥抗抑郁作用的机制与NMDAR、L-精氨酸-一氧化氮-环鸟苷酸通路的抑制和PI3K-mTOR通路的激活有关。随着机制研究的深入, 有研究小组考察了鸟苷对急性束缚应激小鼠的抗抑郁作用与氧化应激之间的关系[74], 结果表明体外增补鸟苷对急性束缚应激诱导的抑郁模型小鼠海马脂质过氧化有保护作用, 不仅增强了海马体超氧化物歧化酶活性, 还对谷胱甘肽过氧化物酶、谷胱甘肽还原酶和过氧化氢酶活性产生了调节作用, 指示鸟苷的抗抑郁作用与抗氧化应激有关。研究还发现[75], 鸟苷的抗抑郁机制与MAPK/ERK、核转录因子NF-E2相关因子2—血红素加氧酶-1通路的激活和GSK-3β的抑制有关, 不过以上鸟苷的抗抑郁机制研究均还未报道有嘌呤能系统的参与。腺苷和肌苷都能通过嘌呤能A1、A2A受体发挥抗抑郁作用, 也有文献报道鸟苷的营养和神经保护作用可能与嘌呤能系统有关, 虽然鸟苷不是这些受体的直接配体, 但阻断腺苷A1、A2A腺苷受体会部分抑制鸟苷的神经营养和保护作用, 指示体外增补鸟苷发挥的抗抑郁作用可能与A1、A2A腺苷受体的间接激活有关[76]。
4 总结与展望综上所述, 体外增补嘌呤代谢物腺苷、肌苷和鸟苷可以介导多条信号通路发挥抗抑郁作用, 并且抗抑郁机制均与嘌呤能系统有关, 但互作机制关系尚未完全清晰, 这些嘌呤代谢物作用于抑郁模型小鼠的主要生理部位和发挥作用的神经细胞及其生化过程还需进一步研究阐明。
目前, 以嘌呤能系统为靶点的抗抑郁药物开发主要集中于P2X7受体拮抗剂。P2X7受体拮抗剂的研发针对性已从外周炎症转向神经炎症, 需要通过血脑屏障。已有制药公司确定了2种脑渗透P2X7受体拮抗剂作为抗抑郁的临床候选药物: 11号和12号。11号经过Ⅰ期临床试验(NCT02475148) 被发现是安全、无不良反应、有良好的耐受性且药效明显, 之后, 英国又招募抑郁症患者进行11号的抗抑郁试验(ISRCTN44411633); 12号对不同抑郁症动物模型均表现出了较好的抗抑郁作用, 12号已进入Ⅰ期临床试验(NCT03151486)[78]。此外, 嘌呤能系统中A1受体的激动剂已被开发用于治疗与抑郁症类似的焦虑症, 但是因其存在复杂的不良反应, 随即转向了A1受体变构调节剂的研发, 目前A1受体的正向变构调节剂已被开发为强效抗焦虑药物[79]。有趣的是, 嘌呤能A2A受体与抑郁症的关系悬而未决, 有文献报道抑制嘌呤能A2A受体有抗抑郁作用, 这样的结果可能与所用的A2A拮抗剂剂量有关, 已有研究表明, 低剂量的选择性A2A拮抗剂才有神经保护作用, 随着剂量增加, 保护作用消失, 具体机制还需深入研究[17]。
嘌呤能系统与嘌呤代谢虽密切相关, 但嘌呤代谢中可以调控嘌呤能系统的嘌呤类物质尚未完全发现, 需进一步寻找。在临床试验中, 样本的获取较为受限, 一般只能获取外周血、分泌物和排泄物, 嘌呤代谢的测定部位也因此被局限; 嘌呤能受体主要存在于脑区, 所以临床上嘌呤能受体的生理变化还难以表征, 有待技术突破。在动物实验中, 抑郁症模型动物的外周和脑区均有嘌呤代谢紊乱的现象, 但引起嘌呤代谢紊乱的原因、嘌呤代谢与嘌呤能系统之间的联系尚不清楚, 需进一步研究。代谢组学作为系统生物学的重要组成部分, 可对低分子代谢物进行定性、定量分析, 该技术便于寻找嘌呤代谢物之间的关系, 在研究抑郁症与嘌呤能系统及嘌呤代谢的机制关系中具有广泛的应用前景。
作者贡献: 陈佳俊是综述的主要撰写者, 搜集资料并分析, 撰写草稿; 周玉枝、秦雪梅、杜冠华负责综述思路的提出; 周玉枝指导并修改论文。所有作者阅读并认可终稿。
利益冲突: 所有作者均声明不存在利益冲突。
| [1] |
Aricioglu F, Yalcinkaya C, Ozkartal CS, et al. NLRP1-mediated antidepressant effect of ketamine in chronic unpredictable mild stress model in rats[J]. Psychiat Invest, 2020, 17: 283-291. DOI:10.30773/pi.2019.0189 |
| [2] |
Li M, Fu X, Xie W, et al. Effect of early life stress on the epigenetic profiles in depression[J]. Front Cell Dev Biol, 2020, 8: 867. DOI:10.3389/fcell.2020.00867 |
| [3] |
Zhou X, Liu L, Lan X, et al. Polyunsaturated fatty acids metabolism, purine metabolism and inosine as potential independent diagnostic biomarkers for major depressive disorder in children and adolescents[J]. Mol Psychiatry, 2019, 24: 1478-1488. DOI:10.1038/s41380-018-0047-z |
| [4] |
He Y, Wang Y, Wu Z, et al. Metabolomic abnormalities of purine and lipids implicated olfactory bulb dysfunction of CUMS depressive rats[J]. Metab Brain Dis, 2020, 35: 649-659. DOI:10.1007/s11011-020-00557-8 |
| [5] |
Ali-sisto T, Tolmunen T, Toffol E, et al. Purine metabolism is dysregulated in patients with major depressive disorder[J]. Psychoneuroendocrinology, 2016, 70: 25-32. DOI:10.1016/j.psyneuen.2016.04.017 |
| [6] |
Cheffer A, Castillo A, Correa-Velloso J, et al. Purinergic system in psychiatric diseases[J]. Mol Psychiatry, 2018, 23: 94-106. DOI:10.1038/mp.2017.188 |
| [7] |
Ribeiro DE, Roncalho AL, Glaser T, et al. P2X7 receptor signaling in stress and depression[J]. Int J Mol Sci, 2019, 20: 2778. DOI:10.3390/ijms20112778 |
| [8] |
Wang P, Jia J, Zhang D. Purinergic signalling in liver diseases: Pathological functions and therapeutic opportunities[J]. JHEP Rep, 2020, 2: 100165. DOI:10.1016/j.jhepr.2020.100165 |
| [9] |
Ballesteros-Yanez I, Castillo CA, Merighi S, et al. The role of adenosine receptors in psychostimulant addiction[J]. Front Pharmacol, 2017, 8: 985. |
| [10] |
Park DI, Dournes C, Sillaber I, et al. Purine and pyrimidine metabolism: convergent evidence on chronic antidepressant treatment response in mice and humans[J]. Sci Rep, 2016, 6: 35317. DOI:10.1038/srep35317 |
| [11] |
Michel TM, Camara S, Tatschner T, et al. Increased xanthine oxidase in the thalamus and putamen in depression[J]. World J Biol Psychiatry, 2010, 11: 314-320. DOI:10.3109/15622970802123695 |
| [12] |
Wu Y, Li Y, Jia Y, et al. Imbalance in amino acid and purine metabolisms at the hypothalamus in inflammation-associated depression by GC-MS[J]. Mol Biosyst, 2017, 13: 2715-2728. DOI:10.1039/C7MB00494J |
| [13] |
Meng X, Huang X, Deng W, et al. Serum uric acid a depression biomarker[J]. PLoS One, 2020, 15: e0229626. DOI:10.1371/journal.pone.0229626 |
| [14] |
Zhu YL, Li SL, Zhu CY, et al. Metabolomics analysis of the antidepressant prescription Danzhi xiaoyao powder in a rat model of chronic unpredictable mild stress (CUMS)[J]. J Ethnopharmacol, 2020, 260: 112832. DOI:10.1016/j.jep.2020.112832 |
| [15] |
Drury AN, Szent-Györgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart[J]. J Physiol, 1929, 68: 213-237. DOI:10.1113/jphysiol.1929.sp002608 |
| [16] |
Burnstock G. Purinergic nerves[J]. Pharmacol Rev, 1972, 24: 509-581. |
| [17] |
Liu YJ, Chen J, Li X, et al. Research progress on adenosine in central nervous system diseases[J]. CNS Neurosci Ther, 2019, 25: 899-910. DOI:10.1111/cns.13190 |
| [18] |
Krugel U. Purinergic receptors in psychiatric disorders[J]. Neuropharmacology, 2016, 104: 212-225. DOI:10.1016/j.neuropharm.2015.10.032 |
| [19] |
Burnstock G. An introduction to the roles of purinergic signalling in neurodegeneration, neuroprotection and neuroregeneration[J]. Neuropharmacology, 2016, 104: 4-17. DOI:10.1016/j.neuropharm.2015.05.031 |
| [20] |
Burnstock G. Purinergic signalling: therapeutic developments[J]. Front Pharmacol, 2017, 8: 661. |
| [21] |
Bartoli F, Burnstock G, Crocamo C, et al. Purinergic signaling and related biomarkers in depression[J]. Brain Sci, 2020, 10: 160. DOI:10.3390/brainsci10030160 |
| [22] |
Lofgren L, Pehrsson S, Hagglund G, et al. Accurate measurement of endogenous adenosine in human blood[J]. PLoS One, 2018, 13: e0205707. DOI:10.1371/journal.pone.0205707 |
| [23] |
Sun ML. Characterization of mRNA Splicing Related SNU 66 and Purine Nucleotide Metabolism Pathway Genes in Fusarium graminearum (禾谷镰刀菌mRNA剪接基因SNU66和嘌呤核苷酸代谢途径基因的功能研究)[D]. Yangling: University of Northwest A&F, 2019.
|
| [24] |
Wang YC, Chen T, Shi T, et al. Progress in biosynthesis of purine nucleosides and their derivatives by metabolic engineering[J]. Chin Biotechnol J (中国生物工程杂志), 2015, 35: 87-95. |
| [25] |
Leem YH, Jang JH, Park JS, et al. Exercise exerts an anxiolytic effect against repeated restraint stress through 5-HT2A-mediated suppression of the adenosine A2A receptor in the basolateral amygdala[J]. Psychoneuroendocrinology, 2019, 108: 182-189. DOI:10.1016/j.psyneuen.2019.06.005 |
| [26] |
Rombo DM, Dias RB, Duarte ST, et al. Adenosine A1 receptor suppresses tonic GABAA receptor currents in hippocampal pyramidal cells and in a defined subpopulation of interneurons[J]. Cereb Cortex, 2016, 26: 1081-1095. DOI:10.1093/cercor/bhu288 |
| [27] |
Manzoni OJ, Manabe T, Nicoll RA. Release of adenosine by activation of NMDA receptors in the hippocampus[J]. Science, 1994, 265: 2098-2101. DOI:10.1126/science.7916485 |
| [28] |
Fan KQ, Li YY, Wang HL, et al. Stress-induced metabolic disorder in peripheral CD4+ T cells leads to anxiety-like behavior[J]. Cell, 2019, 179: 864-879. DOI:10.1016/j.cell.2019.10.001 |
| [29] |
Iwata M, Ota KT, Li XY, et al. Psychological stress activates the inflammasome via release of adenosine triphosphate and stimulation of the purinergic type 2X7 receptor[J]. Biol Psychiatry, 2016, 80: 12-22. DOI:10.1016/j.biopsych.2015.11.026 |
| [30] |
van Calker D, Biber K, Domschke K, et al. The role of adenosine receptors in mood and anxiety disorders[J]. J Neurochem, 2019, 151: 11-27. DOI:10.1111/jnc.14841 |
| [31] |
Duman RS, Sanacora G, Krystal JH. Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments[J]. Neuron, 2019, 102: 75-90. DOI:10.1016/j.neuron.2019.03.013 |
| [32] |
Ulrich D, Huguenard JR. Purinergic inhibition of GABA and glutamate release in the thalamus: implications for thalamic network activity[J]. Neuron, 1995, 15: 909-918. DOI:10.1016/0896-6273(95)90181-7 |
| [33] |
Shen KZ, Johnson SW. Presynaptic GABAB and adenosine A1 receptors regulate synaptic transmission to rat substantia nigra reticulata neurones[J]. J Physiol, 1997, 505: 153-163. DOI:10.1111/j.1469-7793.1997.153bc.x |
| [34] |
Menzikov SA, Zaichenko DM, Moskovtsev AA, et al. Ectopic GABAA receptor beta 3 subunit determines Cl-/HCO3--ATPase and chloride transport in HEK 293FT cells[J]. FEBS J, 2021, 288: 699-712. DOI:10.1111/febs.15359 |
| [35] |
Bukanova JV, Solntseva EI, Kudova E. Neurosteroids as selective inhibitors of glycine receptor activity: structure-activity relationship study on endogenous androstanes and androstenes[J]. Front Mol Neurosci, 2020, 13: 44. DOI:10.3389/fnmol.2020.00044 |
| [36] |
Wang HQ, Wang ZZ, Chen NH. Advance in relationship between receptor gene abnormality and depression[J]. Acta Pharm Sin (药学学报), 2020, 55: 384-391. |
| [37] |
Xie G. Study on the Antidepressant Effects and Mechanisms of Tea Purine Alkaloids (茶叶嘌呤生物碱抗抑郁作用及其机制的研究)[D]. Guangzhou: University of Jinan, 2010.
|
| [38] |
Cao X, Li LP, Wang Q, et al. Astrocyte-derived ATP modulates depressive-like behaviors[J]. Nat Med, 2013, 19: 773-777. DOI:10.1038/nm.3162 |
| [39] |
Burnstock G, Boeynaems JM. Purinergic signalling and immune cells[J]. Purinergic Signal, 2014, 10: 529-564. |
| [40] |
Su W, Zhang T, Jiang C, et al. Clemastine alleviates depressive-like behavior through reversing the imbalance of microglia-related pro-inflammatory state in mouse hippocampus[J]. Front Cell Neurosci, 2018, 12: 412. |
| [41] |
Li K, Yan L, Zhang Y, et al. Seahorse treatment improves depression-like behavior in mice exposed to CUMS through reducing inflammation/oxidants and restoring neurotransmitter and neurotrophin function[J]. J Ethnopharmacol, 2020, 250: 112487. DOI:10.1016/j.jep.2019.112487 |
| [42] |
Wang MY, Tao JH, Li XP. Association of purine receptor P2X ligand-gated ion channel 7 and associated inflammatory factors with depressive disorder in systemic lupus erythematosus[J]. Chin Rheumatol J (中华风湿病学杂志), 2011, 15: 52-55. |
| [43] |
Boucher AA, Arnold JC, Hunt GE, et al. Resilience and reduced C-FOS expression in P2X7 receptor knockout mice exposed to repeated forced swim test[J]. Neuroscience, 2011, 189: 170-177. DOI:10.1016/j.neuroscience.2011.05.049 |
| [44] |
Csoelle C, Baranyi M, Zsilla G, et al. Neurochemical changes in the mouse hippocampus underlying the antidepressant effect of genetic deletion of P2X7 receptors[J]. PLoS One, 2013, 8: e66547. DOI:10.1371/journal.pone.0066547 |
| [45] |
Aricioglu F, Ozkartal CS, Bastaskin T, et al. Antidepressant-like effects induced by chronic blockade of the purinergic 2X7 receptor through inhibition of non-like receptor protein 1 inflammasome in chronic unpredictable mild stress model of depression in rats[J]. Clin Psychopharmacol Neurosci, 2019, 17: 261-272. DOI:10.9758/cpn.2019.17.2.261 |
| [46] |
Hamilton PJ, Chen EY, Tolstikov V, et al. Chronic stress and antidepressant treatment alter purine metabolism and beta oxidation within mouse brain and serum[J]. Sci Rep, 2020, 10: 18134. DOI:10.1038/s41598-020-75114-5 |
| [47] |
Liu L, Zhou X, Zhang Y, et al. Hippocampal metabolic differences implicate distinctions between physical and psychological stress in four rat models of depression[J]. Transl Psychiatry, 2018, 8: 4. DOI:10.1038/s41398-017-0018-1 |
| [48] |
Umehara H, Numata S, Watanabe SY, et al. Altered KYN/TRP, Gln/Glu, and Met/methionine sulfoxide ratios in the blood plasma of medication-free patients with major depressive disorder[J]. Sci Rep, 2017, 7: 4855. DOI:10.1038/s41598-017-05121-6 |
| [49] |
Herken H, Gurel A, Selek S, et al. Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: impact of antidepressant treatment[J]. Arch Med Res, 2007, 38: 247-252. DOI:10.1016/j.arcmed.2006.10.005 |
| [50] |
Martorell M, Lucas X, Alarcon-Zapata P, et al. Targeting xanthine oxidase by natural products as a therapeutic approach for mental disorders[J]. Curr Pharm Des, 2021, 27: 367-382. DOI:10.2174/1381612826666200621165839 |
| [51] |
Jiang N, Zhang XL, Tian JY, et al. Recent studies on the natural products with xanthine oxidase inhibitory effect[J]. Acta Pharm Sin (药学学报), 2021. DOI:10.16438/j.0513-4870.2020-1952 |
| [52] |
Florio C, Prezioso A, Papaioannou A, et al. Adenosine A1 receptors modulate anxiety in CD1 mice[J]. Psychopharmacology (Berl), 1998, 136: 311-319. DOI:10.1007/s002130050572 |
| [53] |
Mendonca A, Sebastiao AM, Ribeiro JA. Adenosine: does it have a neuroprotective role after all?[J]. Brain Res Brain Res Rev, 2000, 33: 258-274. |
| [54] |
Ribeiro JA, Sebastiao AM, Mendonca A. Adenosine receptors in the nervous system: pathophysiological implications[J]. Prog Neurobiol, 2002, 68: 377-392. |
| [55] |
Haun SE, Segeleon JE, Trapp VL, et al. Inosine mediates the protective effect of adenosine in rat astrocyte cultures subjected to combined glucose-oxygen deprivation[J]. J Neurochem, 1996, 67: 2051-2059. |
| [56] |
Cipriani S, Bakshi R, Schwarzschild MA. Protection by inosine in a cellular model of Parkinson's disease[J]. Neuroscience, 2014, 274: 242-249. |
| [57] |
Chen P, Goldberg DE, Kolb B, et al. Inosine induces axonal rewiring and improves behavioral outcome after stroke[J]. Proc Natl Acad Sci U S A, 2002, 99: 9031-9036. |
| [58] |
Bettio LE, Gil-Mohapel J, Rodrigues AL. Guanosine and its role in neuropathologies[J]. Purinergic Signal, 2016, 12: 411-426. |
| [59] |
Oleskovicz SP, Martins WC, Leal RB, et al. Mechanism of guanosine-induced neuroprotection in rat hippocampal slices submitted to oxygen-glucose deprivation[J]. Neurochem Int, 2008, 52: 411-418. |
| [60] |
Courtes AA, Carvalho NR, Goncalves DF, et al. Guanosine protects against Ca2+-induced mitochondrial dysfunction in rats[J]. Bioed Pharmacother, 2019, 111: 1438-1446. |
| [61] |
Kaster MP, Rosa AO, Rosso MM, et al. Adenosine administration produces an antidepressant-like effect in mice: evidence for the involvement of A1 and A2A receptors[J]. Neurosci Lett, 2004, 355: 21-24. |
| [62] |
Kaster MP, Santos AR, Rodrigues AL. Involvement of 5-HT1A receptors in the antidepressant-like effect of adenosine in the mouse forced swimming test[J]. Brain Res Bull, 2005, 67: 53-61. |
| [63] |
Kaster MP, Budni J, Santos AR, et al. Pharmacological evidence for the involvement of the opioid system in the antidepressant-like effect of adenosine in the mouse forced swimming test[J]. Eur J Pharmacol, 2007, 576: 91-98. |
| [64] |
Kaster MP, Machado DG, Santos AR, et al. Involvement of NMDA receptors in the antidepressant-like action of adenosine[J]. Pharmacol Rep, 2012, 64: 706-713. |
| [65] |
Kaster MP, Budni J, Gazal M, et al. The antidepressant-like effect of inosine in the FST is associated with both adenosine A1 and A 2A receptors[J]. Purinergic Signal, 2013, 9: 481-486. |
| [66] |
Muto J, Lee H, Uwaya A, et al. Oral administration of inosine produces antidepressant-like effects in mice[J]. Sci Rep, 2014, 4: 4199. |
| [67] |
Goncalves FM, Neis VB, Rieger DK, et al. Signaling pathways underlying the antidepressant-like effect of inosine in mice[J]. Purinergic Signal, 2017, 13: 203-214. |
| [68] |
Goncalves FM, Neis VB, Rieger DK, et al. Glutamatergic system and mTOR-signaling pathway participate in the antidepressant-like effect of inosine in the tail suspension test[J]. J Neural Transm (Vienna), 2017, 124: 1227-1237. DOI:10.1007/s00702-017-1753-4 |
| [69] |
Yuan S, Jiang X, Zhou X, et al. Inosine alleviates depression-like behavior and increases the activity of the ERK-CREB signaling in adolescent male rats[J]. Neuroreport, 2018, 29: 1223-1229. |
| [70] |
Dal-Cim T, Martins WC, Santos AR, et al. Guanosine is neuroprotective against oxygen/glucose deprivation in hippocampal slices via large conductance Ca2+-activated K+ channels, phosphatidilinositol-3 kinase/protein kinase B pathway activation and glutamate uptake[J]. Neuroscience, 2011, 183: 212-220. |
| [71] |
Tarozzi A, Merlicco A, Morroni F, et al. Guanosine protects human neuroblastoma cells from oxidative stress and toxicity induced by amyloid-beta peptide oligomers[J]. J Biol Regul Homeost Agents, 2010, 24: 297-306. |
| [72] |
Eckeli AL, Dach F, Rodrigues AL. Acute treatments with GMP produce antidepressant-like effects in mice[J]. Neuroreport, 2000, 11: 1839-1843. |
| [73] |
Bettio LE, Cunha MP, Budni J, et al. Guanosine produces an antidepressant-like effect through the modulation of NMDA receptors, nitric oxide-cGMP and PI3K/mTOR pathways[J]. Behav Brain Res, 2012, 234: 137-148. |
| [74] |
Bettio LE, Freitas AE, Neis VB, et al. Guanosine prevents behavioral alterations in the forced swimming test and hippocampal oxidative damage induced by acute restraint stress[J]. Pharmacol Biochem Behav, 2014, 127: 7-14. |
| [75] |
Bettio LE, Gil-Mohapel J, Rodrigues AL. Current perspectives on the antidepressant-like effects of guanosine[J]. Neural Regen Res, 2016, 11: 1411-1413. |
| [76] |
Rosa PB, Bettio L, Neis VB, et al. The antidepressant-like effect of guanosine is dependent on GSK-3beta inhibition and activation of MAPK/ERK and Nrf2/heme oxygenase-1 signaling pathways[J]. Purinergic Signal, 2019, 15: 491-504. DOI:10.1007/s11302-019-09681-2 |
| [77] |
Almeida RF, Pocharski CB, Rodrigues A, et al. Guanosine fast onset antidepressant-like effects in the olfactory bulbectomy mice model[J]. Sci Rep, 2020, 10: 8429. |
| [78] |
Gelin CF, Bhattacharya A, Letavic MA. P2X7 receptor antagonists for the treatment of systemic inflammatory disorders[J]. Prog Med Chem, 2020, 59: 63-99. |
| [79] |
Vincenzi F, Ravani A, Pasquini S, et al. Positive allosteric modulation of A1 adenosine receptors as a novel and promising therapeutic strategy for anxiety[J]. Neuropharmacology, 2016, 111: 283-292. |
 2021, Vol. 56
2021, Vol. 56


