肝癌是全球第三大恶性肿瘤致死因素, 世界上有超过50%的肝癌病例发生在中国, 而肝细胞癌(hepatocellular carcinoma, HCC) 约占总肝癌病例的90%[1, 2]。根据中国2015年的统计数据, 每年我国大约有422 100人死于肝癌, 约占所有癌症死亡案例的15%[3]。早期诊断困难、缺乏靶向药物和疾病发展迅速是肝细胞癌存活率低的重要原因[4]。Sorafenib是临床上用于肝细胞癌患者全身治疗的一线药物。然而, 由于患者对sorafenib产生抗药性, 导致疗效降低[5, 6]。因此, 亟待开发治疗肝细胞癌的新药。
原阿片碱(C20H19O5N) 是一种来自延胡索(Corydalis yanhusuo) 的异喹啉类生物碱, 分子量为353.37 Da[7]。已有的报道表明, 原阿片碱具有抗炎、抗真菌、保护肝脏、神经保护以及抗血栓形成等广泛的生物活性[8, 9]。近来的研究表明, 原阿片碱可以通过显著降低黏附因子的表达从而抑制人乳腺癌细胞MDA-MB-231的异质性黏附[10]。原阿片碱还可以通过诱导微管蛋白聚合和有丝分裂阻滞抑制人前列腺癌细胞的增殖[11]。此外, 原阿片碱还通过上调p53的表达可以抑制对人结肠癌HCT116细胞生长[12]。但其抗肿瘤作用的分子机制仍不明确, 体内抗肿瘤活性尚未见报道, 本文研究了原阿片碱对肝癌细胞的体内外抗肿瘤活性, 证实其对肝细胞癌具有显著的抗肿瘤活性。
材料和方法药品和试剂 DMEM高糖培养基购自美国Hyclone公司, 胎牛血清来自美国Gibco公司; 单克隆抗体剪切的半胱天冬氨酸蛋白酶(cleaved-PARP)、半胱天冬氨酸蛋白酶-3 (caspase-3)、半胱天冬氨酸蛋白酶-9 (caspase-9) 和细胞色素C (cytochrome C) 购自Cell Signaling Technology公司; cleaved-caspase-3和cleaved-caspase-9购自Abcam公司; Bcl-2和Bax购自Proteintech公司; CCK-8试剂和BCA试剂盒购自上海碧云天生物技术公司; Hoechst 33342/PI双染试剂盒购自北京索莱宝科技公司; 凋亡试剂盒购自美国BD公司; JC-1试剂盒购自苏州凯基生物技术公司; SDS-PAGE凝胶试剂盒购自上海雅酶生物医药科技公司; 原阿片碱购自成都普菲德生物技术公司。其他化学试剂均为分析试剂级。
细胞株与实验动物 人肝癌细胞BEL-7402、HepG2和SMMC-7721, 人非小细胞肺癌细胞NCI-H460, 人胰腺癌细胞Panc-28, 人正常肝细胞L02和人脐静脉内皮细胞HUVEC均来自ATCC (American Type Culture Collection)。雄性Balb/c-nu无胸腺裸鼠[SCXK (京) 2019-0010] 购自重庆腾鑫生物技术有限公司。
CCK-8法检测原阿片碱对肿瘤细胞的抑制作用 分别取处于指数生长期的人肝癌细胞BEL-7402、HepG2和SMMC-7721, 人非小细胞肺癌细胞NCI-H460、人胰腺癌细胞Panc-28、人正常肝细胞L02和人脐静脉内皮细胞HUVEC, 以8 000个/孔均匀接种于96孔细胞培养板中, 每孔100 μL, 于37 ℃、5% CO2孵箱中培养, 待细胞贴壁后, 用原阿片碱干预细胞, 处理不同时间后加入CCK-8试剂染色, 使用酶标仪在450 nm处检测其OD值。
肿瘤细胞凋亡检测Hoechst 33342/PI双染法观察细胞凋亡形态 分别取处于指数生长期的BEL-7402和HepG2细胞, 均匀接种于6孔细胞培养板中, 于37 ℃、5% CO2孵箱中培养, 待细胞贴壁后, 分别用7.5、15和30 μmol·L-1原阿片碱处理细胞48 h, 加入Hoechst 33342/PI双染试剂, 置于4 ℃冰箱内避光染色20~30 min, 荧光显微镜下观察、拍照。
流式细胞术检测细胞凋亡 将细胞接种于6孔板中, 于37 ℃、5% CO2孵箱中培养, 待细胞贴壁后, 分别用7.5、15和30 μmol·L-1原阿片碱处理48 h, 胰酶消化、收集各组细胞, 加入Annexin V和PI染色液5 μL, 室温染色15 min, 加入染色缓冲液400 μL, 流式细胞仪检测凋亡率。
JC-1染色法检测线粒体膜电位变化 将细胞接种于6孔板中, 于37 ℃、5% CO2孵箱中培养, 待细胞贴壁后, 分别用7.5、15和30 μmol·L-1原阿片碱干预细胞48 h, 胰酶消化、离心收集细胞, 将细胞重悬于500 μL JC-1工作液中, 孵育30 min, 染色完成后加入适量反应缓冲液洗涤细胞1次, 将细胞重悬于500 μL培养缓冲液中, 调节每毫升细胞数至1×106个, 流式细胞仪检测其线粒体膜电位变化情况。
Western blot研究原阿片碱对线粒体凋亡通路相关蛋白的影响 按照上述方法将细胞接种于6孔板中, 于37 ℃、5% CO2孵箱中培养, 待细胞贴壁后, 分别用7.5、15和30 μmol·L-1原阿片碱干预细胞48 h, 用RIPA裂解液裂解30 min后, 4 ℃离心20 min提取蛋白。使用BCA试剂盒检测各组蛋白浓度, 每组样品上样量为20 μg, 电泳分离蛋白, 湿转法将蛋白转移至PVDF膜上。使用快速封闭液封闭30 min, TBST漂洗1次, 加入一抗, 放入4 ℃冰箱中孵育过夜, TBST洗涤3次, 每次10 min, 加入二抗常温孵育1 h, TBST洗涤3次, 每次10 min。配制ECL发光试液, 检测蛋白表达。使用Image J软件分析蛋白的相对表达量。
人肝癌裸鼠移植性肿瘤模型分析原阿片碱的体内抗肿瘤活性 取指数生长期的HepG2细胞, 胰酶消化收集细胞, 调节细胞密度至每毫升3×107个。将HepG2细胞接种于适应性喂养1周后的裸鼠右侧腋下, 每只鼠接种100 μL细胞悬液, 每天记录裸鼠的体重和瘤体积, 待肿瘤长至60 mm3左右时, 将裸鼠随机分为6组: 生理盐水对照组、溶剂对照组(5% DMSO、10% PEG400和2% Tween 80)、5-氟尿嘧啶(5-fluorouracil, 5-FU) 阳性对照组(25 mg·kg-1体重)、12.5、25和50 mg·kg-1体重原阿片碱给药组。每组5只, 每次注射药物100 μL, 连续注射14天, 治疗完成后, 观察裸鼠生存状态7天。整个实验过程中, 隔日测量1次每只小鼠的体重和肿瘤体积, 并记录。待实验完成后, 取出肿瘤并拍照。动物实验严格按照西南医科大学动物伦理委员会的规定执行。
统计学方法 以上实验均重复进行3次, 统计数据结果, 用均数±标准差表示, 用GraphPad、Image J等软件分析处理数据结果, 分析组间差异时选择的统计学分析方法为t检验或one-way ANOVA, P < 0.05、P < 0.01、P < 0.001均代表组间有差异, 且差异明显具有统计学意义。
结果 1 原阿片碱能够显著抑制肝癌细胞的生长通过CCK-8法分析原阿片碱(图 1A) 对肿瘤细胞的抑制作用, 结果显示(图 1B), 原阿片碱对肿瘤细胞的抑制作用具有选择性, 其中对人肝癌细胞BEL-7402和HepG2的抑制作用最强, 半数抑制浓度(half maximal inhibitory concentration, IC50) 分别为26.32 ± 9.13和32.91 ± 8.49 μmol·L-1, 并呈现时间依赖性(图 1C、D), 对正常细胞的抑制作用相对较弱。
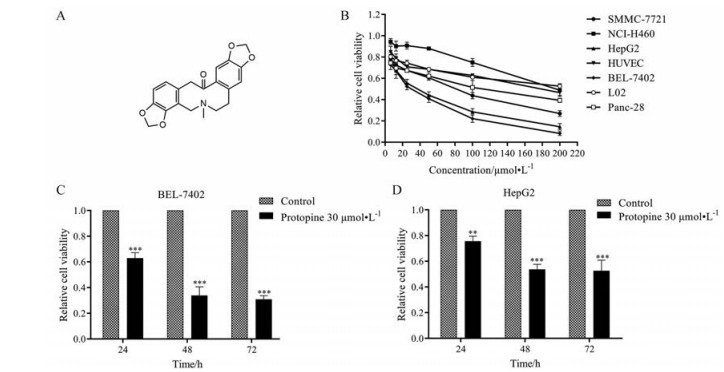
|
Figure 1 Protopine inhibited the growth of hepatocellular carcinoma. A: The chemical structure of protopine; B: Cells were treated with certain concentration of protopine for 48 h. The cell viability was measured by CCK-8 assay; C, D: Hepatocellular carcinoma cells were treated with protopine (30 μmol·L-1) for 24, 48, and 72 h respectively. The cell viability of BEL-7402 (C) and HepG2 (D) cells was measured by CCK-8 assay. n = 3, x± s. **P < 0.01, ***P < 0.001 vs control group |
采用Hoechst 33342/PI双染法在荧光显微镜下观察其凋亡形态, 结果表明经过原阿片碱干预后, 人肝癌细胞BEL-7402和HepG2出现细胞核皱缩碎裂和凋亡小体等现象(图 2A、B)。
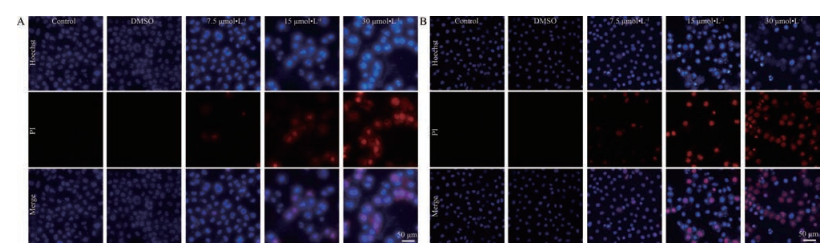
|
Figure 2 Protopine induced apoptosis as analyzed by Hoechst 33342/PI double staining. BEL-7402 cells (A) and HepG2 cells (B) were treated with protopine (7.5, 15, and 30 μmol·L-1) for 48 h and the cells were stained with Hoechst 33342 (blue, staining apoptotic cells) and PI (red, staining dying cells). Cancer cells apoptosis was observed by fluorescence microscopy. The blue color indicated the apoptotic cells while the red color represented the dying cells. PI: Propidium iodide |
进一步采用Annexin V-FITC/PI双染流式细胞术检测人肝癌细胞BEL-7402 (图 3A) 和HepG2 (图 3B) 的凋亡率, 定量分析结果表明, 原阿片碱能够以浓度依赖的方式显著诱导肝癌细胞凋亡, BEL-7402细胞经过原阿片碱处理后, 凋亡率由5.26%升高至55.16% (图 3C), 原阿片碱处理HepG2细胞也获得类似结果, 凋亡率由5.13%升高至40.51% (图 3D)。

|
Figure 3 Protopine induced apoptosis on BEL-7402 cells and HepG2 cells. Cancer cells were treated with certain concentration of protopine, and the cells were stained with Annexin V/PI. Apoptotic effect of BEL-7402 cells (A) and HepG2 cells (B) were detected by flow cytometry approach. (C) and (D) were quantitative analysis results. n = 3, x± s. *P < 0.05, **P < 0.01, ***P < 0.001 vs control group. FITC: Fluorescein isothiocyanate; DMSO: Dimethyl sulfoxide |
采用JC-1染色法检测了原阿片碱对肝癌细胞线粒体膜电位的影响, 结果表明, 原阿片碱能够显著降低人肝癌细胞BEL-7402 (图 4A) 和HepG2 (图 4B) 的线粒体膜电位, 定量结果分析表明, 经过原阿片碱的处理, 人肝癌细胞BEL-7402的绿色荧光比率由11.36%升高至57.99% (图 4C), 而人肝癌细胞HepG2的绿色荧光比率由14.92%升高至56.79% (图 4D)。以上结果显示, 原阿片碱能够以浓度依赖的方式诱导肝癌细胞线粒体膜去极化。
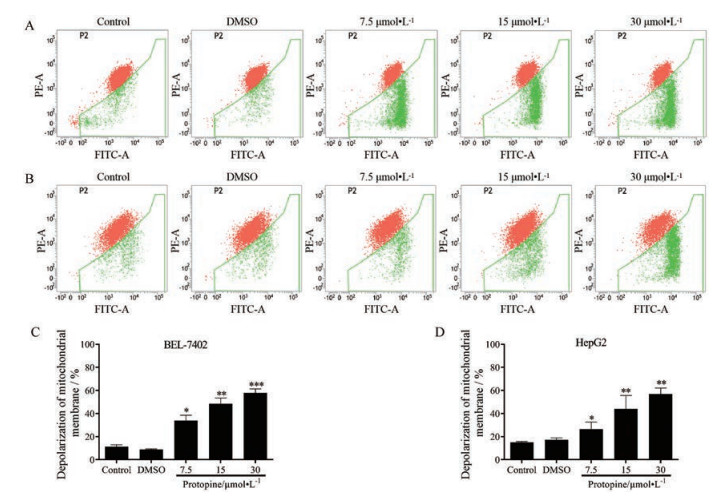
|
Figure 4 Protopine decreased the mitochondrial membrane potential on BEL-7402 and HepG2 cells. Cells were treated with certain concentration of protopine. After incubated for 48 h, cells were stained with JC-1. Mitochondrial membrane potential of BEL-7402 cells (A) and HepG2 cells (B) were detected by flow cytometry approach. (C) and (D) were the quantitative analysis results. n = 3, x± s. *P < 0.05, **P < 0.01, ***P < 0.001 vs control group |
采用Western blot分析检测了原阿片碱对线粒体凋亡途径相关蛋白表达的影响。结果显示, 原阿片碱可以活化caspase-3与caspase-9 (图 5A), 促进Bax、细胞色素C、cleaved-PARP和Apaf-1表达, 降低Bcl-2表达水平(图 5B), 并呈现剂量依赖性, 说明原阿片碱可以通过线粒体途径诱导肝癌细胞凋亡。

|
Figure 5 Protopine affected the expression of proteins related to mitochondrial apoptosis pathway in hepatocellular carcinoma cells. Cells were treated with certain concentration of protopine. After incubated for 48 h, Western blot was performed to detect the expression level of apoptotic related proteins, including caspase-9, cleaved-caspase-9, caspase-3, and cleaved-caspase-3 (A), and Bcl-2, Bax, Cytochrome C (Cyto-C), Apaf-1, and cleaved-PARP (B) |
采用人肝癌细胞HepG2裸鼠皮下移植瘤测定了原阿片碱的体内抗肿瘤活性。结果表明, 原阿片碱能够显著抑制肿瘤生长(图 6A、B), 50 mg·kg-1原阿片碱i.p.给药抑瘤率达到72.46% (图 6C)。原阿片碱给药组的裸鼠体重没有明显改变(图 6D), 说明原阿片碱的毒副作用较小。

|
Figure 6 Protopine inhibited tumor growth in xenograft nude mice model. The xenograft nude mice model bearing HepG2 cells was established and the model mice were injected ip with protopine (12.5, 25, and 50 mg·kg-1) and 5-FU (25 mg·kg-1). Tumor volume (A) and mouse body weight (D) were measured every other day. After being treated with protopine for 14 days, the tumor tissues were removed and photographed (B). C: The quantitative analysis of tumor weight after treating the mice with protopine for 14 days. n = 5, x± s. *P < 0.05, **P < 0.01, ***P < 0.001 vs control group |
天然产物是新型抗癌药物开发的重要发展方向, 并已成为癌症研究的新兴领域[13]。目前, 临床天然来源的生物碱包括喜树碱和紫杉醇在临床上广泛应用, 但这类药物多存在骨髓抑制等严重的不良反应, 另外由于耐药性的产生影响了这类药物的疗效[14-16]。本研究初步表明, 原阿片碱对肿瘤细胞有较好的选择性, 对裸鼠生长没有显著的抑制作用, 提示原阿片碱可以作为抗肿瘤药物的研发前体。此外, 本课题组发现原阿片碱水溶性差, 这可能是其体内抗肿瘤活性不强的原因之一, 因此, 课题组正在应用化学修饰或纳米给药系统等方法提高原阿片碱的生物利用度, 以提高其体内抗肿瘤效果。
细胞凋亡又称程序性死亡, 主要包括线粒体凋亡与死亡受体凋亡两种途径。死亡受体途径的凋亡主要由肿瘤坏死因子受体及其配体(Fas/Fas L)、肿瘤坏死因子相关的凋亡诱导配体(TNF-related apoptosis-inducing ligand, TRAIL) 信号转导, 细胞表面的死亡受体与死亡配体的特异性结合导致caspase-8的激活, 引起细胞凋亡[17]。线粒体凋亡途径主要包括细胞色素C和非caspase依赖的细胞凋亡诱导因子(apoptosis inducing factor, AIF) 介导的两种途径, 当细胞接收到凋亡信号时, 线粒体膜发生去极化、通透性增加导致线粒体膜内的促凋亡因子如细胞色素C、Apaf-1释放到胞质中, 激活caspase级联反应, 引起细胞凋亡; 另一种途径作用方式为线粒体膜内的AIF释放到胞质中, 并转移至细胞核内破坏DNA, 导致细胞死亡[18]。细胞凋亡是抗肿瘤药物抑制肿瘤细胞生长的主要方式, 临床上常用的抗肿瘤药物包括紫杉醇[19]、5-FU[20]和喜树碱[21]都可以通过凋亡途径抑制肿瘤生长。已有的报道表明, 原阿片碱可以通过诱导微管蛋白聚合和有丝分裂阻滞抑制人前列腺癌细胞的增殖[11]。本研究证实, 原阿片碱对人肝癌细胞具有良好的抑制作用。原阿片碱能够诱导肝癌细胞线粒体膜去极化, 上调Bax、细胞色素C、cleaved-caspase-9、cleaved-caspase-3、cleaved-PARP和Apaf-1的表达, 下调癌基因Bcl-2的表达, 启动caspase级联反应, 导致细胞凋亡。原阿片碱是否可以抑制肝癌细胞微管聚合, 其与微管蛋白相互作用的位点还需要进一步研究证实。本研究的体内实验表明, 原阿片碱对人肝癌细胞裸鼠移植性肿瘤呈现良好的抗肿瘤作用, 对裸鼠体重没有明显影响, 提示原阿片碱可以作为抗肿瘤药物的先导药物。
作者贡献: 林秀坤、叶汉林负责实验设计; 叶汉林、乔淦和王琳琳负责具体实验的实施; 程丽参与了实验数据分析; 叶汉林完成文章初稿; 林秀坤对文章进行修订。
利益冲突: 文章内容不涉及相关利益冲突, 该研究未涉及任何厂家及相关雇主或其他经济组织直接或间接的经济或利益的资助, 所有作者均声明不存在利益冲突。
| [1] |
Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012[J]. CA Cancer J Clin, 2015, 65: 87-108. DOI:10.3322/caac.21262 |
| [2] |
Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods[J]. Int J Cancer, 2019, 144: 1941-1953. DOI:10.1002/ijc.31937 |
| [3] |
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016, 66: 115-132. DOI:10.3322/caac.21338 |
| [4] |
Fu J, Wang H. Precision diagnosis and treatment of liver cancer in China[J]. Cancer Lett, 2017, 412: 283-288. |
| [5] |
Faivre S, Rimassa L, Finn RS. Molecular therapies for HCC: looking outside the box[J]. J Hepatol, 2020, 72: 342-352. DOI:10.1016/j.jhep.2019.09.010 |
| [6] |
Villanueva A, Llovet JM. Second-line therapies in hepatocellular carcinoma: emergence of resistance to sorafenib[J]. Clin Cancer Res, 2012, 18: 1824-1826. DOI:10.1158/1078-0432.CCR-12-0151 |
| [7] |
Sung DK, Kim YH, Pan CH, et al. Protopine reduces the inflammatory activity of lipopolysaccharide-stimulated murine macrophages[J]. BMB Rep, 2012, 45: 108-113. DOI:10.5483/BMBRep.2012.45.2.108 |
| [8] |
Orhan I, Ozçelik B, Karaoğlu T, et al. Antiviral and antimicrobial profiles of selected isoquinoline alkaloids from Fumaria and Corydalis species[J]. Z Naturforsch C J Biosci, 2007, 62: 19-26. DOI:10.1515/znc-2007-1-204 |
| [9] |
Rathi A, Srivastava AK, Shirwaikar A, et al. Hepatoprotective potential of Fumaria indica Pugsley whole plant extracts, fractions and an isolated alkaloid protopine[J]. Phytomedicine, 2008, 15: 470-477. DOI:10.1016/j.phymed.2007.11.010 |
| [10] |
He K, Gao J. Protopine inhibits heterotypic cell adhesion in MDA-MB-231 cells through down-regulation of multi-adhesive factors[J]. Afr J Tradit Complement Altern Med, 2014, 11: 415-424. DOI:10.4314/ajtcam.v11i2.28 |
| [11] |
Chen CH, Liao CH, Chang YL, et al. Protopine, a novel microtubule-stabilizing agent, causes mitotic arrest and apoptotic cell death in human hormone-refractory prostate cancer cell lines[J]. Cancer Lett, 2012, 315: 1-11. DOI:10.1016/j.canlet.2011.09.042 |
| [12] |
Son YL, An YJ, Jung J, et al. Protopine isolated from Nandina domestica induces apoptosis and autophagy in colon cancer cells by stabilizing p53[J]. Phytother Res, 2019, 33: 1689-1696. DOI:10.1002/ptr.6357 |
| [13] |
Zhang X, Meng LH. Progress in molecularly targeted anti-tumor drugs derived from natural products or their derivatives[J]. Acta Pharm Sin (药学学报), 2020, 55: 2491-2500. |
| [14] |
Yang CP, Horwitz S. Taxol: the first microtubule stabilizing agent[J]. Int J Mol Sci, 2017, 18: 1733. DOI:10.3390/ijms18081733 |
| [15] |
Martino E, Della VS, Terribile E, et al. The long story of camptothecin: from traditional medicine to drugs[J]. Bioorg Med Chem Lett, 2016, 27: 701-707. |
| [16] |
Shah NN, Merchant MS, Cole DE, et al. Vincristine sulfate liposomes injection (VSLI, Marqibo): results from a phase Ⅰ study in children, adolescents, and young adults with refractory solid tumors or leukemias[J]. Pediatr Blood Cancer, 2016, 63: 997-1005. DOI:10.1002/pbc.25937 |
| [17] |
Hermeking H. The miR-34 family in cancer and apoptosis[J]. Cell Death Differ, 2010, 17: 193-199. DOI:10.1038/cdd.2009.56 |
| [18] |
Wiraswati HL, Hangen E, Sanz AB, et al. Apoptosis inducing factor (AIF) mediates lethal redox stress induced by menadione[J]. Oncotarget, 2016, 7: 76496-76507. DOI:10.18632/oncotarget.12562 |
| [19] |
Weaver BA. How taxol/paclitaxel kills cancer cells[J]. Mol Biol Cell, 2014, 25: 2677-2681. DOI:10.1091/mbc.e14-04-0916 |
| [20] |
Wyatt MD, Wilson DM. Participation of DNA repair in the response to 5-fluorouracil[J]. Cell Mol Life Sci, 2009, 66: 788-799. DOI:10.1007/s00018-008-8557-5 |
| [21] |
Liew ST, Yang LX. Design, synthesis and development of novel camptothecin drugs[J]. Curr Pharm Des, 2008, 14: 1078-1097. DOI:10.2174/138161208784246180 |
 2021, Vol. 56
2021, Vol. 56


