中药两面针是芸香科植物两面针Zanthoxylum nitidum (Roxb.) DC.的干燥根, 最早收载于《神农本草经》, 别名蔓椒、两背针、入地金牛等[1]。两面针味苦辛, 性平, 微毒, 具有顺气止痛、活血去瘀、祛风活络等作用, 在古代中医理论中应用颇多。近年来医药学界对两面针开展了较为深入的研究, 并得到了实际应用, 如两面针牙膏[2]。氯化两面针碱(nitidine chloride, NC) 是其重要成分之一。相较于两面针的其他化学成分, NC的研究更为深入, 且其抗癌、抗炎、抗氧化等作用也多有报道, 但多数有关两面针的文献并未全面总结出其所有化学成分。相关文献对NC的抗癌、抗虫药理作用以及药效机制也总结不全。本文就近35年来国内外关于两面针的研究进行进一步总结, 并以氯化两面针碱为例, 详细综述其抗癌、抗菌、抗寄生虫等药理活性和作用机制, 以及其肝肾毒性, 为两面针的进一步研究与开发提供参考。
1 药理活性成分研究两面针含有多种化学成分, 主要有苯并菲啶类生物碱、喹啉类生物碱、香豆素类、木脂素、有机酸及其他等。NC是从两面针中提取出来的生物碱, 属于苯并菲啶类生物碱, 是两面针的化学成分中研究得较为深入的活性成分之一。这一部分将分别介绍两面针中的这几类化学成分。
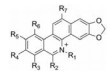
1.1 苯并菲啶类生物碱苯并菲啶类生物碱是一类自然界中广泛存在的异喹啉生物碱, 在罂粟科和芸香科植物中较为常见。苯并菲啶类生物碱大多具有抗癌、抑菌等药理活性, 如氯化两面针碱等, 能抑制肿瘤细胞生长[3]。Wang[4]最早从两面针根部得到白屈菜红碱, 鉴定结构式后进一步明确了其具有抑菌活性。迄今为止从两面针分离得到此类化合物有40多种(表 1[3-20]), 结构见表 2~4、图 1。
| Table 1 Benzophenanthridine alkaloids in Zanthoxyli Radix |
| Table 2 Structures of benzophenanthridine alkaloids in Zanthoxyli Radix (1) |
| Table 3 Structures of benzophenanthridine alkaloids in Zanthoxyli Radix (2) |
| Table 4 Structures of benzophenanthridine alkaloids in Zanthoxyli Radix (3) |
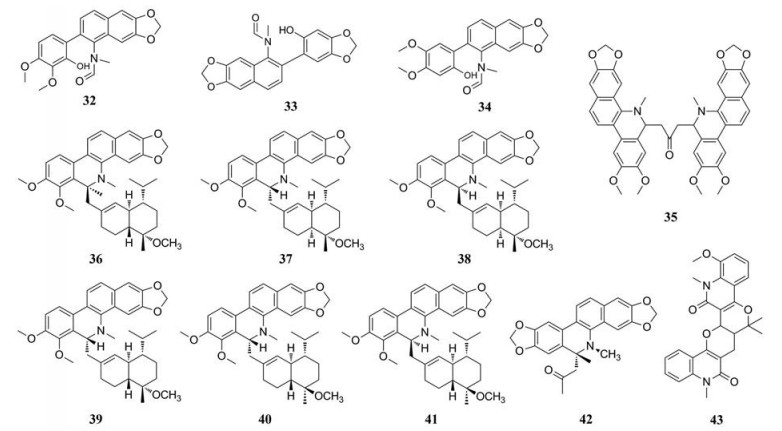
|
Figure 1 Structures of some benzophenanthridine alkaloids in Zanthoxyli Radix |
两面针中喹啉类生物碱包括有小檗碱、茵芋碱等, 都具有抗菌、镇痛[21]等多种药理活性。Hu等[22]较先从两面针中分离出小檗碱, 并确定结构式。Yang等[23]从两面针中分离得到了茵芋碱、去甲茵芋碱。此外有研究表明Yang等[23]分离出来的白鲜碱、崖椒碱, Jia等[12]分离出来的5-甲氧基白鲜碱都具有明显的抗有丝分裂和抗真菌活性。目前, 已从两面针中得到30多种喹啉类生物碱, 见表 5[3, 5, 8, 12, 16-18, 20, 22-29], 结构见图 2。
| Table 5 Quinoline alkaloids in Zanthoxyli Radix |
|
Figure 2 Quinoline alkaloids in Zanthoxyli Radix |
香豆素类化合物药理作用广泛, 主要的药理作用有抗凝血[30]、抗氧化[23]、抗感染[31]。Jia等[12]从两面针中分离出了5, 7, 8-三甲氧基香豆素等; Yang等[23]鉴定5, 6, 7-三甲氧基香豆素为两面针的活性成分之一, 且具有抗氧化功能。此外, 有研究评估了4种从两面针中提取的香豆素, 分别是5-香叶基氧基-7-甲氧基香豆素、5, 7-二甲氧基-8-异戊基香豆素、异茴芹素和异戊烯氧基呋喃香豆素的体外抗菌活性, 证实以上4种香豆素与耐甲氧西林金葡菌(MRSA) 的抗菌剂联用有协同作用[31]。另一种香豆素成分托达洛内酯可抑制纤溶酶原激活物抑制剂1 (PAI-1) 以起到活血化瘀功效[30]。两面针中含有的香豆素类化合物见表 6[12, 23], 结构见图 3。
| Table 6 Coumarin compounds in Zanthoxyli Radix |

|
Figure 3 Coumarin compounds in Zanthoxyli Radix |
木脂素类是在自然界中广泛存在的一类具有抗氧化活性的化合物。1983年, Hong等[32]首先从两面针中分离出一新的木脂素类化合物结晶-8, 并鉴定出其结构为呋喃脂素(-) 二氢芝麻素。此外, Hu等[17]从两面针中分离出d-表芝麻脂素等成分并且确定了化学结构。两面针的木脂素类化合物见表 7[17, 20, 32], 结构见图 4。
| Table 7 Lignans in Zanthoxyli Radix |
|
Figure 4 Lignans in Zanthoxyli Radix |
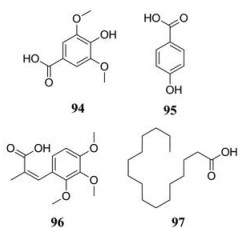
两面针中亦存在有机酸, 但能被分离出来的有机酸较少。Hu等[17]从两面针根部中分离并鉴定出紫丁香酸等几种化合物的结构。两面针中有机酸类化合物见表 8[22, 33], 结构见图 5。
| Table 8 Organic acids in Zanthoxyli Radix |
|
Figure 5 Organic acids in Zanthoxyli Radix |
两面针中还含有甾体类、黄酮类、酯类等成分。1994年, Tang[29]率先从两面针中分离出了黄酮类化合物香叶木甙, 进一步鉴定出化学结构。Yang等[18]分离得到β-谷甾醇、β-谷甾酮。其他类化合物见表 9[12, 16-18, 20, 22, 25, 33-35]。
| Table 9 Other compounds in Zanthoxyli Radix |
综上所述, 中药两面针的化学成分较多, 但对于其所有成分是否具有生物活性尚未完全研究清楚。研究进展与仪器分析方法、技术等紧密联系, 利用超高效液相色谱对口服两面针生物碱后的大鼠进行仿制药和药代动力学研究[36], 可进一步明确对有效生物活性成分的质量控制和鉴定未知化合物。有研究人员使用LC-MS的代谢组学方法[37]和生物化学统计学工具, 或者联用超滤高效液相色谱-紫外和质谱筛选[38], 从两面针中大规模分离乙酰胆碱酯酶抑制物, 并且进一步确定潜在的抗胆碱酯酶生物碱物质。由此看来, 利用新兴科技手段可有助于挖掘两面针生物活性成分在临床中的应用潜力。
2 氯化两面针碱的药理作用NC是两面针生物碱中研究得最为深入的生物碱之一, 最初被发现具有抗真菌、抗炎、抗疟疾、抗病毒和止痛等活性。进一步研究表明, NC能调控细胞内多种信号通路, 从而抑制癌细胞增殖、诱导癌细胞凋亡。
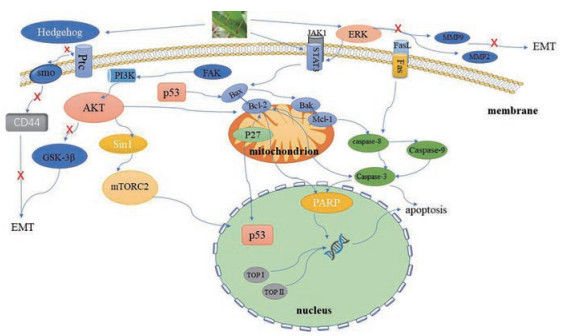
2.1 抗肿瘤作用NC可通过调控多种信号通路抑制包括卵巢癌、乳腺癌、胃癌、肝癌、胶质母细胞瘤、骨肉瘤等多种癌细胞的增殖, 还可诱导肿瘤细胞凋亡, 抑制肿瘤细胞迁移侵袭, 从而起到抗肿瘤作用[39-43] (图 6)。
|
Figure 6 The anticancer mechanism of NC |
NC能抑制细胞周期相关蛋白[39]、拓扑异构酶(topoisomerase, TOP)[44], 以及磷脂酰肌醇3-激酶/丝氨酸特异性蛋白激酶/哺乳动物雷帕霉素靶蛋白(phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin, PI3K/Akt/mTOR) 通路的活性[41, 42], 从而抑制卵巢癌、肝癌、胶质母细胞瘤、骨肉瘤等恶性肿瘤细胞增殖。
S期激酶相关蛋白2 (S-phase kinase associated protein 2, Skp2) 参与调控细胞增殖、凋亡、信号转导等生理活动[45, 46]。研究表明, Skp2的表达与上皮性卵巢癌[47]和晚期卵巢癌的恶性程度显著相关[48]。Mou等[39]实验表明NC能显著抑制卵巢癌细胞Skp2的表达, 阻滞卵巢癌细胞细胞周期的G2/M期, 从而抑制卵巢癌细胞增殖。TOP能催化DNA双链断裂进而有利于DNA进行复制, 因此TOP现已被认为是多种抗肿瘤药物的有效靶点[49]。Liu等[40]的研究显示, 在肝癌细胞中, NC与拓扑异构酶1 (topoisomerase Ⅰ, TOP 1) 或拓扑异构酶2A (topoisomerase, TOP ⅡA) 直接结合后可抑制DNA转录表达, 进而诱导肝癌细胞生长周期阻滞。Prado等[44]在实验中观察到小剂量NC可完全抑制TOP1的催化活性, 而大剂量的NC能在一定程度上抑制拓扑异构酶2 (topoisomerase Ⅱ, TOP 2) 的催化活性。此外, 现已有合成的NC类似物, 能有效地阻止肝癌细胞增殖[50]。
之前的研究表明激活PI3K/Akt/mTOR通路后能够刺激生长因子, 以促进细胞增殖、转录[51], 此外有研究证明该信号通路可维持胶质母细胞瘤干细胞的自我更新[52], 因此抑制PI3K/Akt/mTOR信号通路, 对肿瘤细胞的增殖、迁移活动等都有阻断作用[52-54]。在研究NC对骨肉瘤细胞的作用时发现, NC可抑制Akt对Sin1的磷酸化, 降低Sin1的表达, 从而降低(mammalian target of rapamycin complex 2, mTORC2) 的活性, 最终遏制骨肉瘤细胞增殖[41]。此外, Liu等[42]表明NC通过抑制PI3K/Akt/mTOR信号通路的蛋白表达, 也可降低胶质母细胞瘤细胞的活力, 从而使该种细胞的增殖停滞。随着对PI3K/Akt/mTOR信号通路的不断深入研究, 现已开发mTOR抑制剂作为抗癌药物, 且已被应用于治疗肾癌和套细胞淋巴瘤[54]。
此外, Deshpande等[55]发现NC可促进DNA修复酶(poly ADP-ribose polymerase, PARP) 的裂解并增加裂解的半胱氨酸蛋白酶3 (caspase-3) 蛋白的表达, 抑制神经胶质瘤细胞中的蛋白激酶2 (protein kinases 2, PK2) 的活性, 使细胞周期阻滞在G2/M期从而中断细胞增殖。
2.1.2 诱导肿瘤细胞凋亡NC诱导肿瘤细胞凋亡主要包括死亡受体途径如: 在人凋亡相关因子/人凋亡相关因子配体(factor associated suicide, Fas/factor associated suicide ligand, FasL) 通路中, 提高Fas的表达, 诱导肿瘤细胞凋亡[56]。还有线粒体凋亡途径如: 抑制B淋巴细胞瘤-2基因(B-cell lymphoma-2, Bcl-2) 家族蛋白表达或促进Bcl相关蛋白X (Bcl-2-associated X, Bax) 以及一系列促凋亡蛋白基因、抑癌基因的表达, 从而诱导肿瘤细胞凋亡[57, 58]。此外, NC还能通过抑制Akt信号通路[59]和酪氨酸激酶/信号传导与活化转录因子3 (Janus kinase/signal transducer and activator of transcription 3, JAK/STAT3) 信号通路途径[60], 来调节线粒体凋亡途径中下游蛋白的活性, 从而诱导细胞凋亡。
NC通过死亡受体途径诱导癌细胞凋亡。Fas/FasL系统在维持淋巴细胞稳态和自身免疫起关键作用[61], 它是死亡受体凋亡通路之一[62], Fas相关因子1 (Fas-associated factor 1, FAF1) 可以增强凋亡效应[63]。有研究指出, 与正常卵巢细胞比较, 卵巢癌细胞中Fas表达减少而FasL表达增多[64]。此外还有卵巢癌细胞中FAF1的表达低于正常卵巢细胞[65]。深入研究后表明, 卵巢癌细胞经NC处理后, 细胞中Fas的表达被提高, 可促进Fas与FasL结合并激活半胱氨酸蛋白酶8 (caspase-8) 和caspase-3发挥凋亡作用, 以此使癌变细胞凋亡, 起到有效治疗卵巢癌的作用[56]。
NC通过线粒体途径诱导癌细胞凋亡。Bcl-2蛋白可通过多种途径抑制靶细胞凋亡进而表现出抗凋亡效果[66], 而Bax蛋白则能与Bcl-2蛋白结合, 以对抗Bcl-2蛋白的抗凋亡作用, 从而促进细胞凋亡[67]。p53是一种抑癌基因, 被证实具有潜在抑癌作用[68]。Min等[69]表明NC可激活p53基因介导的凋亡信号通路, 抑制鼻咽癌细胞的增殖并诱导其凋亡。此外, Li等[57]实验发现NC可以抑制急性髓细胞性白血病细胞增殖, 具体机制是通过下调细胞周期蛋白B1、CDK1、Bcl-2, 裂解PARP, 上调p27、Bax和激活caspase-3从而使细胞凋亡。除此之外, Sun等[70]研究表明, NC还可通过提高表达凋亡蛋白、增加裂解PARP来抑制细胞增殖并诱导乳腺癌细胞周期停滞进而促进凋亡; 实验进一步还发现NC可与阿霉素协同诱导乳腺癌周期停滞和表现出细胞毒性, 进而促进细胞凋亡[70]。基因myeloid cell leukemia-1 (Mcl-1) 属于Bcl-2基因家族, 对细胞的生长发展起重要作用[71]。通过蛋白印迹和免疫组化等实验表明, NC可通过溶酶体依赖性降解并下调Mcl-1蛋白水平[58], 从而导致癌变的口腔鳞状细胞凋亡。
NC通过Akt或JAK/STAT3通路途径诱导癌细胞凋亡。NC除了能显著下调Bcl-2家族蛋白表达水平, 上调Bax等凋亡蛋白的表达外, 还能抑制Akt磷酸化, 并通过其他的Akt信号通路诱导细胞凋亡[59]。Fang等[72]发现在肾癌细胞中, NC可抑制肾癌细胞中Akt的磷酸化, 下调Bcl-2蛋白以触发肾癌细胞的凋亡。
NC还可通过作用于JAK1/STAT3信号通路中的细胞因子或下游蛋白等使细胞凋亡。Chen等[43]实验表明NC能显著降低胃癌细胞中STAT3、CD31和血管内皮生长因子的表达, 阻断体内肿瘤血管生成, 导致胃肿瘤细胞凋亡。Liao等[73]实验表明在肝癌中, NC通过抑制肝细胞癌中JAK1/STAT3信号通路, 提高Bax等凋亡蛋白的表达来影响细胞增殖, 促进细胞的凋亡[74]。此外, Kim等[75]发现在带有口腔鳞状细胞癌中, NC通过抑制STAT3信号传导从而抑制了肿瘤生长并诱导了癌细胞凋亡, 并且无肝或肾毒性。
除此之外, NC还可以通过其他途径诱导癌细胞凋亡。Xu等[76]实验证实NC阻止了骨肉瘤细胞的生长, 并观察到caspase-3、caspase-9被激活和Bcl-2的表达减少, 具体机制可能是caspase依赖性途径诱导细胞凋亡, 但具体的凋亡通路还有待探索。NC还能通过损伤肿瘤细胞的DNA导致遗传信息损害, 中断增殖最后使细胞凋亡, 组蛋白Ser139磷酸化是DNA损伤的典型标志[77]。Kwon等[78]用NC处理人类宫颈癌细胞后, 发现组蛋白Ser139的磷酸化增加, 即说明NC能直接损伤肿瘤细胞DNA进而导致人类宫颈癌细胞凋亡。
2.1.3 抑制肿瘤转移在适宜条件下, 肿瘤细胞迁移扩散至身体其他重要器官可导致患者死亡。NC可通过抑制细胞外调节蛋白激酶(extracellular regulated protein kinases, ERK) 信号通路下游蛋白的活性, 如基质金属蛋白酶-2 (matrixmetallo proteinase-2, MMP-2) 和基质金属蛋白酶-9 (matrixmetallo proteinase-9, MMP-9) 的活性, 从而影响细胞增殖与分化与细胞迁移、凋亡等活动[79]; 还可以抑制非受体酪氨酸激酶/类固醇受体共激活因子(steroid receptor coactivator/focal adhesionkinase, c-Src/FAK) 信号通路, 阻止癌细胞大范围扩散, 从而尽可能地减少肿瘤细胞扩散后的不良后果。
ERK参与的众多信号通路能够调控细胞增殖与分化与细胞迁移、凋亡等活动[79], 因此在肿瘤细胞内, 可通过抑制ERK活性来削弱细胞内的信号转导继而改变肿瘤细胞发展状态。Sun等[80]实验表明在卵巢癌细胞中, NC通过抑制ERK信号传导途径, 抑制MMP-2和MMP-9酶活性, 可减少蛋白质水解, 来阻断卵巢癌细胞的迁移并降低侵袭活性。在肾癌细胞、乳腺癌细胞中, NC可分别抑制Akt信号通路[81]、c-Src/FAK相关的信号通路[82], 并且伴随上调p53、Bax, 裂解caspase-3和裂解PARP, 下调Bcl-2、caspase-3和PARP从而抑制细胞的侵袭和转移。此外, 在结肠直肠癌细胞中NC也能抑制ERK信号传导途径, 增加Bax等蛋白表达以诱导其凋亡[83]。
2.1.4 抑制上皮-间质转化(epithelial-mesenchymal transition, EMT) 和血管生成EMT是一种至关重要的生物调控方式[84]。肿瘤转移过程中常伴随EMT[85], 在此过程中, 肿瘤细胞迁移能力会大大增强并获得间质表型特征[86]。Vijay等[87]发现在乳腺癌细胞中加入EMT抑制剂后, 肿瘤干细胞(cancer stem cell, CSC) 特性减弱, 说明EMT与CSC表型特性激活有关。CSC激活的具体表现为肿瘤转移, 耐药性和肿瘤复发[88]。Hedgehog (Hh) 信号通路参与控制细胞增殖, 该信号通路被异常活化时, 会引起肿瘤细胞增殖[89]。Sun等[90]发现NC通过抑制乳腺癌细胞Hh途径中的Nanog基因、巢蛋白(Nestin)、转录因子Oct-4和CD44蛋白的表达而抑制EMT和CSC样表型, 从而使肿瘤细胞迁移和侵袭能力下降。此外有研究指出, 糖原合成酶激酶-3β (glycogen synthase kinase 3β, GSK-3β), 能在多条信号通路中调节细胞的增殖分化进程[91]。Cheng等[92]通过实验发现NC可降低骨肉瘤细胞的侵袭能力, 并且伴随GSK-3β活化能力降低、snail蛋白表达下降使EMT调控减弱, 说明NC通过抑制Akt/GSK-3β/snail信号通路抑制了EMT, 阻止肿瘤细胞迁移进程。
血管生成包括整合不稳定的血管, 内皮细胞增殖、迁移和肾小管生成[93]。有研究报道STAT3基因在胃癌中过度表达, 并且促使肿瘤的血管生成[94], 因此降低STAT3活性可使其诱导的血管生成减少以阻滞细胞迁移。Cook等[95, 96]发现在肝癌细胞中, NC可抑制STAT3、ERK和Sonic hedgehog (SHH) 途径的激活, 破坏肿瘤的血管生成, 从而极大抑制其增殖和迁移能力, 可较好地阻滞肿瘤细胞迁移[97]。
2.2 抗炎作用NC在抗炎方面的活性已有诸多报道[98, 99]。银屑病属于一种常见的由炎症引起的皮肤病, 该病会导致持续的炎症以及角质形成细胞增殖和功能障碍[100]。有研究指出, NC能够通过减低细胞周期蛋白A (cyclin A) 和细胞周期蛋白D1 (cyclin D1) 水平以及增加p53蛋白表达, 抑制人类永生化表皮细胞增殖并诱导S期细胞周期停滞[101], 从而缓解银屑病患者的皮肤炎症。IL-10家族细胞因子对人体免疫功能起重要作用, 能够激活固有免疫, 同时促进组织修复, 使机体抵御炎症[102]。Yang等[103]实验证实NC和TOP1抑制剂能够通过增强Akt介导的信号通路, 促进产生IL-10而发挥抗炎作用, 有助于炎症的治疗。
2.3 抗菌作用此前已有研究表明NC具有一定的抗菌活性[104, 105]。幽门螺杆菌导致人体感染有赖于脲酶存在[106], 表现为慢性活动性胃炎及一系列胃肠道炎症反应[107]。Schmalstig等[108]实验表明NC可抑制脲酶活性, 使得胃大部分仍保持强酸性并且增强胃的抗氧化作用, 使幽门螺旋杆菌生长受限而抑制其生长[109], 从而有效治疗由幽门螺杆菌引起的消化性溃疡。此外, 最近Cesari等[110]报道NC还具有孢子原芽孢杆菌和化脓性链球菌的抑制活性。
2.4 抗寄生虫作用据报道, NC对疟原虫等寄生虫有抑制甚至灭活作用[111, 112], 其具体机制是NC以与氯喹相同的效价强度[113]抑制结晶状疟色素的形成[114], 血红素游离出来, 对细胞产生巨大的毒性作用从而杀灭疟原虫, 达到抗疟疾的效果。Lempereur等[115]研究表明巴贝虫属和泰勒虫属寄生虫能够感染人类, 此后有进一步研究认为NC能够有效地抑制巴贝虫属和泰勒虫属寄生虫的生长[116], 从而可能治疗此类寄生虫病。
2.5 其他药理作用小胶质细胞是中枢神经系统中具有免疫作用的一类细胞。当脑部神经元受到创伤并感染炎症时, 为维持中枢神经系统稳态, 小胶质细胞会加剧神经元自噬[117], 但这也会导致一系列不良反应, 因此常把抑制小胶质细胞活性作为治疗创伤性枢神经系统的方法。Yuan等[118]实验表明NC通过调节ERK和蛋白质复合物NF-κB信号通路促使中枢神经系统恢复并且抑制抑制小胶质细胞增生, 从而保护创伤性中枢神经。此外, Yang等[119]报道NC显示出对乙型肝炎病毒的体外抗病毒作用, 具体是对逆转录酶抑制从而产生抗病毒的效果[120]。此外, NC还能通过抑制RANKL诱导的NF-κB和NFATc1信号通路抑制破骨细胞生成并预防卵巢切除诱导的骨丢失[121]。Addae-Mensah等[122]在研究抗高血压的化学成分时, 发现NC能够降低新西兰白兔的血压, 因而NC可能具备一定的对哺乳动物的降压能力, 但具体降血压机制还有待研究。
3 氯化两面针碱的肝肾毒性肝脏和肾脏是人体非常重要的器官, 肝脏和肾脏受到了损害之后可能会诱导一些疾病和并发症的发生, 甚至可能会威胁生命以及引发死亡。据Wei等[123, 124]的实验, NC在体外对肿瘤细胞具有较强的增殖抑制作用, 但同时对人正常肝细胞和肾细胞有一定的增殖抑制作用。Li等[125]通过实验进一步表明NC引起肾毒性是因为: 有机阳离子转运蛋白2 (organic cation transporter 2, OCT2) 在肾脏对NC的摄取作用, 以及多药物和有毒化合物排出家族蛋白1 (multidrug and toxic compound extrusion family, MATE1) 对NC的排出减弱。此外, 实验证实通过有机阳离子转运蛋白1 (organic cation transporter 1, OCT1) 和有机阳离子转运蛋白3 (organic cation transporter 3, OCT3) 介导途径, 还会导致NC聚集于心脏从而产生蓄积的心肌细胞毒作用。由于存在以上所述的细胞毒性, NC的应用可能会受到限制, 因此考虑NC药理活性的同时, 它对正常细胞的毒性作用也应该受到重视。
4 小结与展望两面针是一种重要的中药资源, 其分布较广, 且含有多种药理活性成分。目前仍在研究两面针的活性成分, 就已分离鉴定出的100多种化学成分而言, 只有少部分生物成分被证实具有药理活性。因此两面针的真正药用潜力并未完全开发。虽然现在已明确NC发挥抗癌、抗炎、抗虫等作用的部分机制, 但还有其他活性成分的药理机制尚未充分阐明, 还应进一步深入研究。此外, 关于NC发挥药理活性的具体结构仍只有少许研究涉及。
NC的毒性研究也尚未深入, 由信号传导异常而导致的肿瘤细胞生长抑制, 也会影响机体正常细胞的生命活动[126], 产生毒性反应。明确药物的治疗剂量与中毒剂量才能为中药应用提出合理用药指导, 因此毒性反应应引起重视。有研究提出将NC制成超分子制剂可有效降低肝毒性[127], 这将有望解决NC的毒性问题, 其他更有效降低毒性的方法还有待探索。
两面针作为一种实用性强的药材, 此前关于它的应用主要是两面针牙膏, 因此应继续挖掘出两面针在其他方面, 如医药、生物、化学等领域的应用潜力, 更好地造福于人类健康事业。
作者贡献: 扶佳俐负责文章初稿撰写; 杨璐铭负责化合物结构式的绘制; 范欣悦负责文献的调研; 郭乔如和周雯敏负责文章初稿的修改; 张建业负责综述的框架设计和修改。
利益冲突: 本文无任何利益冲突。
| [1] |
National Pharmacopoeia Committee. Pharmacopoeia of the People's Republic of China (中华人民共和国药典)[M]. Beijing: China Medical Science Press, 2015: 159.
|
| [2] |
Guo Q. Speech at industry market working conference[J]. Toothpaste Ind (口腔护理用品工业), 2020, 30: 50-51. |
| [3] |
Liu HG, Feng J, Feng K, et al. Optimization of the extraction conditions and quantification by RP-LC analysis of three alkaloids in Zanthoxylum nitidum roots[J]. Pharm Biol, 2014, 52: 255-261. DOI:10.3109/13880209.2013.826244 |
| [4] |
Wang MX. Studies on the chemical constituents of Zanthoxylum nitidum. Isolation of anticancer alkaloids and structure of alkaloid C[J]. Acta Acad Med Zhong Shan (中山医学院学报), 1980, 1: 341-349, 402. |
| [5] |
Shen XH, Mu SZ, Wang QY, et al. Isolation and identification of chemical constituents from the roots of Zanthoxylum nitidum (Roxb.) DC. in Yunnan province[J]. J Shenyang Pharm Univ (沈阳药科大学学报), 2016, 33: 275-279, 292. |
| [6] |
Wang XL, Ma YY, Ke Y, et al. Two new alkaloids from Zanthoxylum nitidum[J]. Chin Tradit Herb Drugs (中草药), 2010, 41: 340-342. |
| [7] |
Geng D, Li DX, Shi Y, et al. A new benzophenanthridine alkaloid from Zanthoxylum nitidum[J]. Chin J Nat Med (中国天然药物), 2009, 7: 274-277. DOI:10.3724/SP.J.1009.2009.00274 |
| [8] |
Wang XL, Ma MF, Ding LS. Chemical constituents of Zanthoxylum nitidum[J]. Chin Pharm J (中国药学杂志), 2008, 43: 253-256. |
| [9] |
Li DX, Min ZD. Isolation of alkaloids from Zanthoxylum nitidum[J]. Chin J Nat Med (中国天然药物), 2004, 2: 32-35. |
| [10] |
Chen YZ, Yang LL, Xu BJ. Crystal structure of 7-demethyl-6-methoxy-5, 6-dihydrochelerythrine from Zanthoxylum nitidum[J]. Acta Chem Sin (化学学报), 1989, 47: 1048-1051. |
| [11] |
Chen YZ, Xu BJ, Huang ZX. The crystal strcture of oxynitidine[J]. Chin Sci Bull, 1990, 35: 558-561. |
| [12] |
Jia W, Cen YH. Research progress on chemical constituents and clinical application of Zanthoxylum nitidum[J]. Chin J Ethnic Med (中国民族医药杂志), 2016, 22: 1-3. |
| [13] |
Ishii H, Chen IS, Ueki S, et al. Studies on the chemical constituents of Rutaceous plants. LXIV. Structural establishment of oxyterihanine, a phenolic benzo[c] phenanthridone alkaloid. Syntheses of phenolic benzo[c] phenanthridine alkaloids, terihanine and isoterihanine, and related compounds[J]. Chem Pharm Bull, 1987, 35: 2717-2725. DOI:10.1248/cpb.35.2717 |
| [14] |
Cui XG, Zhao QJ, Chen QL. Two new benzophenanthridine alkaloids from Zanthoxylum nitidum[J]. Helv Chim Acta, 2008, 91: 155-158. DOI:10.1002/hlca.200890006 |
| [15] |
Fang SD, Wang LK, Hecht SM. Inhibitors of DNA topoisomerase I isolated from the roots of Zanthoxylum nitidum[J]. J Org Chem, 1993, 58: 5025-5027. DOI:10.1021/jo00071a001 |
| [16] |
Ye YS, Liu JW. Antibacterial constituents from roots of Zanthoxylum nitidum[J]. Chin Tradit Herb Drugs (中草药), 2013, 44: 1546-1551. |
| [17] |
Hu J. Studies on the Active Components in Zanthoxylum nitidum (两面针中活性成分的研究) [D]. Shanghai: the Second Military Medical University, 2006.
|
| [18] |
Yang CH, Cheng MJ, Lee S, et al. Secondary metabolites and cytotoxic activities from the stem bark of Zanthoxylum nitidum[J]. Chem Biodiv, 2009, 6: 846-857. DOI:10.1002/cbdv.200800107 |
| [19] |
Yang CH, Cheng MJ, Chiang MY, et al. Dihydrobenzo[c] phenan-thridine alkaloids from stem bark of Zanthoxylum nitidum[J]. J Nat Prod, 2008, 71: 669-673. DOI:10.1021/np700745f |
| [20] |
Zhao LN, Wang J, Wang Z, et al. Chemical and cytotoxic constituents of Zanthoxylum nitidum[J]. China J Chin Mater Med (中国中药杂志), 2018, 43: 4659-4664. |
| [21] |
Yang G, Chen D. Alkaloids from the roots of Zanthoxylum nitidum and their antiviral and antifungal effects[J]. Chem Biodiv, 2008, 5: 1718-1722. DOI:10.1002/cbdv.200890160 |
| [22] |
Hu J, Zhang WD. Chemical constituents of Zanthoxylum nitidum[J]. China J Chin Mater Med (中国中药杂志), 2006, 31: 1689-1691. |
| [23] |
Yang ZD, Zhong DB, Ren J, et al. Skimmianine, a furoquinoline alkaloid from Zanthoxylum nitidum as a potential acetylcholinesterase inhibitor[J]. Med Chem Res, 2011, 21: 722-725. |
| [24] |
Zhu LJ, Ren M, Yang TC, et al. Four new alkylamides from the roots of Zanthoxylum nitidum[J]. J Asian Nat Prod Res, 2015, 17: 711-716. DOI:10.1080/10286020.2015.1049161 |
| [25] |
Fan J, Li HX. Isolation, identification and activity determination of chemical constituents from Zanthoxylum nitidum[J]. J Shenyang Pharm Univ (沈阳医科大学学报), 2013, 30: 100-105, 131. |
| [26] |
Hu J, Shi X, Mao X, et al. Cytotoxic mannopyranosides of indole alkaloids from Zanthoxylum nitidum[J]. Chem Biodiv, 2014, 11: 970-974. DOI:10.1002/cbdv.201300381 |
| [27] |
Hu J, Zhang W, Shen Y. Two novel alkaloids from Zanthoxylum nitidum[J]. Helv Chim Acta, 2007, 90: 720-722. DOI:10.1002/hlca.200790071 |
| [28] |
Deng Y, Shen XH, Deng LL, et al. Study on the alkaloids and their anticancer activity from Zanthoxylum nitidum[J]. Nat Prod Res Dev(天然产物研究与开发), 2020, 32: 1370-1378. |
| [29] |
Tang YM. Studies on the chemical constituents of Zanthoxylum nitidum[J]. Chin Tradit Herb Drugs (中草药), 1994, 29: 550. |
| [30] |
Yu B, Zhang G, Jin L, et al. Inhibition of PAI-1 activity by todda-lolactone as a mechanism for promoting blood circulation and removing stasis by Chinese herb Zanthoxylum nitidum var. tomentosum[J]. Front Pharmacol, 2017, 8: 489. DOI:10.3389/fphar.2017.00489 |
| [31] |
Zuo GY, Wang CJ, Han J, et al. Synergism of coumarins from the Chinese drug Zanthoxylum nitidum with antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA)[J]. Phytomedicine, 2016, 23: 1814-1820. DOI:10.1016/j.phymed.2016.11.001 |
| [32] |
Hong GX, Zeng XY. Study on analgesic mechanism of Zanthoxylum nitidum crystal-8[J]. Acta Pharm Sin (药学学报), 1983, 18: 227-230. |
| [33] |
He ZN, Liu JW. Comparative analysis of supercritical CO2 extraction from roots and stems of Zanthoxylum nitidum by GC-MS and in vitro cytotoxicity evaluation[J]. China J Chin Mater Med (中国中药杂志), 2014, 39: 710-714. |
| [34] |
Wang XL, Ma YY, Ke Y, et al. New benzenoids and anti-inflam-matory constituents from Zanthoxylum nitidum[J]. Food Chem, 2010, 41: 340-342. |
| [35] |
Guthrie N, Kurowska EM, Carroll KK. Compositions and methods of treatment of neoplastic diseases and hypercholesterolemia with citrus limonoids and flavonoids and tocotrienols: US, 6251400 B1[P]. 2001-06-26.
|
| [36] |
Huang A, Chi Y, Liu J, et al. Profiling and pharmacokinetic studies of alkaloids in rats after oral administration of Zanthoxylum nitidum decoction by UPLC-Q-TOF-MS/MS and HPLC-MS/MS[J]. Molecules, 2019, 24: 585. DOI:10.3390/molecules24030585 |
| [37] |
Plazas E, Casoti R, Avila Murillo M, et al. Metabolomic profiling of Zanthoxylum species: identification of anti-cholinesterase alkaloids candidates[J]. Phytochemistry, 2019, 168: 112128. DOI:10.1016/j.phytochem.2019.112128 |
| [38] |
Liu M, Liu Q, Chen M, et al. Large-scale separation of acetyl-cholinesterase inhibitors from Zanthoxylum nitidum by pH-zone-refining counter-current chromatography target-guided by ultra-filtration high-performance liquid chromatography with ultra-violet and mass spectrometry screening[J]. J Sep Sci, 2019, 42: 1194-1201. DOI:10.1002/jssc.201801238 |
| [39] |
Mou HP, Guo P, Li XM, et al. Nitidine chloride inhibited the expression of S phase kinase-associated protein 2 in ovarian cancer cells[J]. Cell Cycle, 2017, 16: 1366-1375. DOI:10.1080/15384101.2017.1327490 |
| [40] |
Liu LM, Xiong DD, Lin P, et al. DNA topoisomerase 1 and 2A function as oncogenes in liver cancer and may be direct targets of nitidine chloride[J]. Int J Oncol, 2018, 53: 1897-1912. |
| [41] |
Xu H, Cao T, Zhang X, et al. Nitidine chloride inhibits SIN1 expression in osteosarcoma cells[J]. Mol Ther Oncol, 2019, 12: 224-234. DOI:10.1016/j.omto.2019.01.005 |
| [42] |
Liu M, Wang J, Qi Q, et al. Nitidine chloride inhibits the malignant behavior of human glioblastoma cells by targeting the PI3K/AKT/mTOR signaling pathway[J]. Oncol Rep, 2016, 36: 2160-2168. DOI:10.3892/or.2016.4998 |
| [43] |
Chen J, Wang J, Lin L, et al. Inhibition of STAT3 signaling pathway by nitidine chloride suppressed the angiogenesis and growth of human gastric cancer[J]. Mol Cancer Ther, 2011, 11: 277-287. |
| [44] |
Prado S, Michel S, Tillequin F, et al. Synthesis and cytotoxic activity of benzo[c] [1, 7] and[1, 8] phenanthrolines analogues of nitidine and fagaronine[J]. Bioorg Med Chem, 2004, 12: 3943-3953. DOI:10.1016/j.bmc.2004.04.038 |
| [45] |
Lough L, Sherman D, Ni E, et al. Chemical probes of Skp2-mediated p27 ubiquitylation and degradation[J]. Medchemcomm, 2018, 9: 1093-1104. DOI:10.1039/C8MD00140E |
| [46] |
Chan CH, Morrow JK, Zhang S, et al. Skp2:a dream target in the coming age of cancer therapy[J]. Cell Cycle, 2014, 13: 679-680. DOI:10.4161/cc.27853 |
| [47] |
Sui L, Dong Y, Watanabe Y, et al. Clinical significance of Skp2 expression, alone and combined with Jab1 and p27 in epithelial ovarian tumors[J]. Oncol Rep, 2006, 15: 765-771. |
| [48] |
Hafez MM, Alhoshani AR, Al-Hosaini KA, et al. SKP2/P27Kip1 pathway is associated with advanced ovarian cancer in saudi patients[J]. Asian Pac J Cancer P, 2015, 16: 5807-5815. DOI:10.7314/APJCP.2015.16.14.5807 |
| [49] |
Delgado JL, Hsieh CM, Chan NL, et al. Topoisomerases as anti-cancer targets[J]. Biochem J, 2018, 475: 373-398. DOI:10.1042/BCJ20160583 |
| [50] |
Qin SQ, Li LC, Song JR, et al. Structurally simple phenanthridine analogues based on nitidine and their antitumor activities[J]. Molecules, 2019, 24: 437. DOI:10.3390/molecules24030437 |
| [51] |
Humphrey SJ, Yang G, Yang P, et al. Dynamic adipocyte phosphoproteome reveals that Akt directly regulates mTORC2[J]. Cell Metab, 2013, 17: 1009-1020. DOI:10.1016/j.cmet.2013.04.010 |
| [52] |
Sunayama J, Matsuda K, Sato A, et al. Crosstalk between the PI3K/mTOR and MEK/ERK pathways involved in the maintenance of self-renewal and tumorigenicity of glioblastoma stemlike cells[J]. Stem Cells, 2010, 28: 1930-1939. DOI:10.1002/stem.521 |
| [53] |
Hervieu A, Kermorgant S. The role of PI3K in met driven cancer: a recap[J]. Front Mol Biosci, 2018, 5: 86. DOI:10.3389/fmolb.2018.00086 |
| [54] |
Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR signaling in cancer[J]. Front Oncol, 2014, 4: 64. |
| [55] |
Deshpande RP, Babu PP. pDok2, caspase 3 dependent glioma cell growth arrest by nitidine chloride[J]. Pharmacoll Rep, 2018, 70: 48-54. DOI:10.1016/j.pharep.2017.07.013 |
| [56] |
Chen S, Yang L, Feng J. Nitidine chloride inhibits proliferation and induces apoptosis in ovarian cancer cells by activating the Fas signalling pathway[J]. J Pharm Pharmacol, 2018, 70: 778-786. DOI:10.1111/jphp.12901 |
| [57] |
Li P, Yan SX, Dong X, et al. Cell cycle arrest and apoptosis induction activity of nitidine chloride on acute myeloid leukemia cells[J]. Med Chem, 2018, 14: 60-66. |
| [58] |
Yang IH, Jung W, Kim LH, et al. Nitidine chloride represses Mcl-1 protein via lysosomal degradation in oral squamous cell carcinoma[J]. J Oral Pathol Med, 2018, 47: 823-829. DOI:10.1111/jop.12755 |
| [59] |
Feng D, Liu TF, Yu N, et al. Nitidine chloride inhibits proliferation, induces apoptosis via the Akt pathway and exhibits a synergistic effect with doxorubicin in ovarian cancer cells[J]. Mol Med Rep, 2016, 14: 2853-2859. DOI:10.3892/mmr.2016.5577 |
| [60] |
Quick L, Young R, Henrich IC, et al. Jak1-STAT3 signals are essential effectors of the USP6/TRE17 oncogene in tumorigenesis[J]. Cancer Res, 2016, 76: 5337-5347. DOI:10.1158/0008-5472.CAN-15-2391 |
| [61] |
Abrahams V, Kamsteeg M, Mor G. The Fas/Fas ligand system and cancer: immune privilege and apoptosis[J]. Mol Biotechnol, 2003, 25: 19-30. DOI:10.1385/MB:25:1:19 |
| [62] |
Rossin A, Miloro G, Hueber AO. TRAIL and fasl functions in cancer and autoimmune diseases: towards an increasing complexity[J]. Cancers (Basel), 2019, 11: 639. DOI:10.3390/cancers11050639 |
| [63] |
Chu K, Niu X, Williams LT. A Fas-associated protein factor, FAF1, potentiates Fas-mediated apoptosis[J]. Proc Natl Acad Sci U S A, 1995, 92: 11894-11898. DOI:10.1073/pnas.92.25.11894 |
| [64] |
Ma XY, He FX, Wu S, et al. Expression of survivin in ovarian epithelial carcinoma and its correlation with expression of Fas and FasL[J]. Chin J Cancer (癌症), 2004, 23: 173-176. |
| [65] |
Kang HJ, Moon HS, Chung HW. The expression of FAS-associated factor 1 and heat shock protein 70 in ovarian cancer[J]. Obstet Gynecol, 2014, 57: 281-290. |
| [66] |
Pena-Blanco A, Garcia-Saez AJ. Bax, Bak and beyond-mitochondrial performance in apoptosis[J]. FEBS J, 2018, 285: 416-431. DOI:10.1111/febs.14186 |
| [67] |
Adams JM. The Bcl-2 protein family: arbiters of cell survival[J]. Science, 1998, 281: 1322-1326. DOI:10.1126/science.281.5381.1322 |
| [68] |
Levine A. p53, the cellular gatekeeper for growth and division[J]. Cell, 1997, 88: 323-331. DOI:10.1016/S0092-8674(00)81871-1 |
| [69] |
Min K, Ou HS. The effect of nitidine chloride on the proliferation and apoptosis of nasopharyngeal carcinoma cells[J]. J BUON, 2014, 19: 130-136. |
| [70] |
Sun M, Zhang N, Wang X, et al. Nitidine chloride induces apoptosis, cell cycle arrest, and synergistic cytotoxicity with doxorubicin in breast cancer cells[J]. Tumor Biol, 2014, 35: 10201-10212. DOI:10.1007/s13277-014-2327-9 |
| [71] |
Xiang W, Yang CY, Bai L. MCL-1 inhibition in cancer treatment[J]. Onco Targets Ther, 2018, 11: 7301-7314. DOI:10.2147/OTT.S146228 |
| [72] |
Fang Z, Tang Y, Jiao W, et al. Nitidine chloride inhibits renal cancer cell metastasis via suppressing AKT signaling pathway[J]. Food Chem Toxicol, 2013, 60: 246-251. DOI:10.1016/j.fct.2013.07.062 |
| [73] |
Liao J, Xu T, Zheng JX, et al. Nitidine chloride inhibits hepato-cellular carcinoma cell growth in vivo through the suppression of the JAK1/STAT3 signaling pathway[J]. Int J Mol Med, 2013, 32: 79-84. DOI:10.3892/ijmm.2013.1358 |
| [74] |
Ou XH, Lu Y, Liao LF, et al. Nitidine chloride induces apoptosis in human hepatocellular carcinoma cells through a pathway involving p53, p21, Bax and Bcl-2[J]. Oncol Rep, 2015, 33: 1264-1274. DOI:10.3892/or.2014.3688 |
| [75] |
Kim L, Khadka S, Shin J, et al. Nitidine chloride acts as an apoptosis inducer in human oral cancer cells and a nude mouse xenograft model via inhibition of STAT3[J]. Oncotarget, 2017, 8: 91306-91315. DOI:10.18632/oncotarget.20444 |
| [76] |
Xu Q, Li ZX, Ye ZM. Nitidine chloride-induced apoptosis of human osteosarcoma cells and its mechanism[J]. J South Med Univ (南方医科大学学报), 2011, 31: 361-364. |
| [77] |
Baritaud M, Cabon L, Delavallee L, et al. AIF-mediated caspase-independent necroptosis requires ATM and DNA-PK-induced histone H2AX Ser139 phosphorylation[J]. Cell Death Dis, 2012, 3: e390. DOI:10.1038/cddis.2012.120 |
| [78] |
Kwon HJ, Kim LH, Ahn CH, et al. A new insight into the apoptotic effect of nitidine chloride targeting Checkpoint kinase 2 in human cervical cancer in vitro[J]. J Clin Biochem Nutr, 2019, 65: 193-202. DOI:10.3164/jcbn.19-28 |
| [79] |
Muta Y, Matsuda M, Imajo M. Dynamic ERK signaling regulation in intestinal tumorigenesis[J]. Mol Cell Oncol, 2018, 5: e1506684. DOI:10.1080/23723556.2018.1506684 |
| [80] |
Sun X, Lin L, Chen Y, et al. Nitidine chloride inhibits ovarian cancer cell migration and invasion by suppressing MMP-2/9 production via the ERK signaling pathway[J]. Mol Med Rep, 2016, 13: 3161-3168. DOI:10.3892/mmr.2016.4929 |
| [81] |
Fang Z, Tang Y, Jiao W, et al. Nitidine chloride induces apoptosis and inhibits tumor cell proliferation via suppressing ERK signaling pathway in renal cancer[J]. Food Chem Toxicol, 2014, 66: 210-216. DOI:10.1016/j.fct.2014.01.049 |
| [82] |
Pan XH, Han HH, Wang L, et al. Nitidine chloride inhibits breast cancer cells migration and invasion by suppressing c-Src/FAK associated signaling pathway[J]. Cancer Lett, 2011, 313: 181-191. DOI:10.1016/j.canlet.2011.09.001 |
| [83] |
Zhai H, Hu S, Liu T, et al. Nitidine chloride inhibits proliferation and induces apoptosis in colorectal cancer cells by suppressing the ERK signaling pathway[J]. Mol Med Rep, 2016, 13: 2536-2542. DOI:10.3892/mmr.2016.4827 |
| [84] |
Ye X, Tam WL, Shibue T, et al. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells[J]. Nature, 2015, 525: 256-260. DOI:10.1038/nature14897 |
| [85] |
Stemmler MP, Eccles RL, Brabletz S, et al. Non-redundant functions of EMT transcription factors[J]. Nat Cell Biol, 2019, 21: 102-112. DOI:10.1038/s41556-018-0196-y |
| [86] |
Alix Panabieres C, Mader S, Pantel K. Epithelial-mesenchymal plasticity in circulating tumor cells[J]. J Molr Med (Berl), 2017, 95: 133-142. DOI:10.1007/s00109-016-1500-6 |
| [87] |
Vijay GV, Zhao N, Den Hollander P, et al. GSK3beta regulates epithelial-mesenchymal transition and cancer stem cell properties in triple-negative breast cancer[J]. Breast Cancer Res, 2019, 21: 37. DOI:10.1186/s13058-019-1125-0 |
| [88] |
Lytle NK, Barber AG, Reya T. Stem cell fate in cancer growth, progression and therapy resistance[J]. Nat Rev Cancer, 2018, 18: 669-680. DOI:10.1038/s41568-018-0056-x |
| [89] |
Girardi D, Barrichello A, Fernandes G, et al. Targeting the hedgehog pathway in cancer: current evidence and future perspectives[J]. Cells, 2019, 8: 153. DOI:10.3390/cells8020153 |
| [90] |
Sun M, Zhang N, Wang X, et al. Hedgehog pathway is involved in nitidine chloride induced inhibition of epithelial-mesenchymal transition and cancer stem cells-like properties in breast cancer cells[J]. Cell Biosci, 2016, 6: 44. DOI:10.1186/s13578-016-0104-8 |
| [91] |
McCubrey J, Steelman L, Bertrand F, et al. GSK-3 as potential target for therapeutic intervention in cancer[J]. Oncotarget, 2014, 5: 2881-2911. DOI:10.18632/oncotarget.2037 |
| [92] |
Cheng Z, Guo Y, Yang Y, et al. Nitidine chloride suppresses epithelial-to-mesenchymal transition in osteosarcoma cell migration and invasion through Akt/GSK-3beta/Snail signaling pathway[J]. Oncol Rep, 2016, 36: 1023-1029. DOI:10.3892/or.2016.4846 |
| [93] |
Page DJ, Thuret R, Venkatraman L, et al. Positive feedback defines the timing, magnitude, and robustness of angiogenesis[J]. Cell Rep, 2019, 27: 3139-3151. DOI:10.1016/j.celrep.2019.05.052 |
| [94] |
Yang S, Ngo VC, Lew GB, et al. AZD6244(ARRY-142886) enhances the therapeutic efficacy of sorafenib in mouse models of gastric cancer[J]. Mol Cancer Ther, 2009, 8: 2537-2545. DOI:10.1158/1535-7163.MCT-09-0213 |
| [95] |
Cook KM, Figg WD. Angiogenesis inhibitors: current strategies and future prospects[J]. CA Cancer J Clin, 2010, 60: 222-243. DOI:10.3322/caac.20075 |
| [96] |
Angelidis A, Racekova E, Arnoul P, et al. Disrupted migration and proliferation of neuroblasts after postnatal administration of angiogenesis inhibitor[J]. Brain Res, 2018, 1698: 121-129. DOI:10.1016/j.brainres.2018.08.010 |
| [97] |
Lin JM, Shen A, Chen H. Nitidine chloride inhibits hepatic cancer growth via modulation of multiple signaling pathway[J]. BMC Cancer, 2014, 14: 729. DOI:10.1186/1471-2407-14-729 |
| [98] |
Hu J, Zhang WD, Liu RH, et al. Benzophenanthridine alkaloids from Zanthoxylum nitidum (Roxb.) DC, and their analgesic and anti-inflammatory activities[J]. Chem Biodiv, 2006, 3: 990-995. DOI:10.1002/cbdv.200690108 |
| [99] |
Zhang HL, Gan XQ, Fan QF, et al. Chemical constituents and antiinflammatory activities of Maqian (Zanthoxylum myriacanthum var. pubescens) bark extracts[J]. Sci Rep, 2017, 7: 45805. DOI:10.1038/srep45805 |
| [100] |
Rendon A, Schakel K. Psoriasis pathogenesis and treatment[J]. Int J Mol Sci, 2019, 20: 1475. DOI:10.3390/ijms20061475 |
| [101] |
Yang X, Jiang BW, Jing QQ, et al. Nitidine chloride induces S phase cell cycle arrest and mitochondria-dependent apoptosis in HaCaT cells and ameliorates skin lesions in psoriasis-like mouse models[J]. Eur J Clin Pharmacol, 2019, 863: 172680. DOI:10.1016/j.ejphar.2019.172680 |
| [102] |
Ouyang WJ, O'Garra A. IL-10 family cytokines IL-10 and IL-22:from basic science to clinical translation[J]. Immunity, 2019, 50: 871-891. DOI:10.1016/j.immuni.2019.03.020 |
| [103] |
Yang N, Yue RC, Ma JZ, et al. Nitidine chloride exerts anti-inflammatory action by targeting topoisomerase I and enhancing IL-10 production[J]. Pharmacol Res, 2019, 148: 104368. DOI:10.1016/j.phrs.2019.104368 |
| [104] |
Zhang Y, Luo Z, Wang D, et al. Phytochemical profiles and anti-oxidant and antimicrobial activities of the leaves of Zanthoxylum bungeanum[J]. Sci World J, 2014, 2014: 181072. |
| [105] |
Tankeo SB, Damen F, Awouafack MD, et al. Antibacterial activities of the methanol extracts, fractions and compounds from Fagara tessmannii[J]. J Ethnopharmacol, 2015, 169: 275-279. DOI:10.1016/j.jep.2015.04.041 |
| [106] |
Debowski AW, Walton SM, Chua EG, et al. Helicobacter pylori gene silencing in vivo demonstrates urease is essential for chronic infection[J]. PLoS Pathog, 2017, 13: e1006464. DOI:10.1371/journal.ppat.1006464 |
| [107] |
Abadi ATB. Strategies used by Helicobacter pylori to establish persistent infection[J]. World J Gastroenterol, 2017, 23: 2870-2882. DOI:10.3748/wjg.v23.i16.2870 |
| [108] |
Schmalstig A, Benoit S, Misra S, et al. Noncatalytic antioxidant role for Helicobacter pylori urease[J]. J Bacteriol, 2018, 200: e00124-18. |
| [109] |
Lu Q, Li C, Wu G. Insight into the inhibitory effects of Zanthoxylum nitidum against Helicobacter pylori urease and jack bean urease: kineticsandmechanism[J]. JEthno-pharmacol, 2020, 249: 112419. |
| [110] |
Cesari I, Grisoli P, Paolillo M, et al. Isolation and characterization of the alkaloid nitidine responsible for the traditional use of Phyllanthus muellerianus (Kuntze) excell stem bark against bacterial infections[J]. J Pharmaceut Biomed Anal, 2015, 105: 115-120. DOI:10.1016/j.jpba.2014.11.051 |
| [111] |
Nyangulu JM, Hargreaves SL, Sharples SL, et al. Antimalarial benzo[c] phenanthridines[J]. Bioorg Med Chem Lett, 2005, 15: 2007-2010. DOI:10.1016/j.bmcl.2005.02.074 |
| [112] |
Jullian V, Bourdy G, Georges S, et al. Validation of use of a traditional antimalarial remedy from French Guiana, Zanthoxylum rhoifolium Lam[J]. J Ethnopharmacol, 2006, 106: 348-352. DOI:10.1016/j.jep.2006.01.011 |
| [113] |
Gilles HM. Malaria--an overview[J]. J Infect, 1989, 18: 11-23. DOI:10.1016/S0163-4453(89)93517-2 |
| [114] |
Bouquet J, Rivaud M, Chevalley S, et al. Biological activities of nitidine, a potential anti-malarial lead compound[J]. Malaria J, 2012, 11: 67. DOI:10.1186/1475-2875-11-67 |
| [115] |
Lempereur L, Beck R, Fonseca I, et al. Guidelines for the detection of Babesia and Theileria parasites[J]. Vector Borne Zoonot, 2017, 17: 51-65. DOI:10.1089/vbz.2016.1955 |
| [116] |
Tayebwa DS, Tuvshintulga B, Guswanto A, et al. The effects of nitidine chloride and camptothecin on the growth of Babesia and Theileria parasites[J]. Ticks Tick-borne Dis, 2018, 9: 1192-1201. DOI:10.1016/j.ttbdis.2018.04.019 |
| [117] |
Chen ZZ, Zhong D, Li GZ. The role of microglia in viral encephalitis: a review[J]. J Neuroinflamm, 2019, 16: 76. DOI:10.1186/s12974-019-1443-2 |
| [118] |
Yuan Y, Zhu F, Pu Y, et al. Neuroprotective effects of nitidine against traumatic CNS injury via inhibiting microglia activation[J]. Brain Behav Immun, 2015, 48: 287-300. DOI:10.1016/j.bbi.2015.04.008 |
| [119] |
Yang G, Chen D. Alkaloids from the roots of Zanthoxylum nitidum and their antiviral and antifungal effects[J]. Chem Biodiv, 2008, 5: 1718-1722. DOI:10.1002/cbdv.200890160 |
| [120] |
Kakiuchi N, Hattori M, Namba T, et al. Inhibitory effect of tannins on reverse transcriptase from RNA tumor virus[J]. J Nat Prod, 1985, 48: 614-621. DOI:10.1021/np50040a016 |
| [121] |
Liu Q, Tao Wang, Zhou L, et al. Nitidine chloride prevents OVX-induced bone loss via suppressing NFATc1-mediated osteoclast differentiation[J]. Sci Rep, 2016, 6: 36662. DOI:10.1038/srep36662 |
| [122] |
Addae-Mensah I, Muriuki G, Sofowora A. Structure and anti-hypertensive properties of nitidine chloride from Fagara species[J]. Planta Med, 1986, 52: 538-539. |
| [123] |
Li LP, Song FF, Wang YY, et al. Role of OCT2 and MATE1 in renal disposition and toxicity of nitidine chloride[J]. Br J Pharmacol, 2016, 173: 2543-2554. DOI:10.1111/bph.13537 |
| [124] |
Wei M, Liu HG, Liu LM, et al. In vitro nephrotoxicity of nitidine chloride in human liver L-O2 and kidney 293 cells[J]. Chin New Drug J (中国新药杂志), 2010, 19: 56-59, 63. |
| [125] |
Li C, Chen YC, Zeng QQ, et al. Accumulation of nitidine chloride in rat heart and the underlying mechanism[J]. Acta Pharm Sin (药学学报), 2019, 54: 913-918. |
| [126] |
Lovejoy KS, Lippard SJ. Non-traditional platinum compounds for improved accumulation, oral bioavailability, and tumor targeting[J]. Dalton Trans, 2009, 48: 10813-10823. |
| [127] |
Li W, Yin H, Bardelang D, et al. Supramolecular formulation of nitidine chloride can alleviate its hepatotoxicity and improve its anticancer activity[J]. Food Chem Toxicol, 2017, 109: 923-929. DOI:10.1016/j.fct.2017.02.022 |
 2021, Vol. 56
2021, Vol. 56