2. 中药成药性与制剂制备湖南省重点实验室, 湖南 长沙 410208;
3. 湖南省中药饮片标准化及功能工程技术研究中心, 湖南 长沙 410208;
4. 中国医学科学院、北京协和医学院药物研究所, 神经科学中心, 北京 100050
2. Druggability and Preparation of Chinese Medicine Key Laboratory of Hunan Province, Changsha 410208, China;
3. Center for Standardization and Functional Engineering of Traditional Chinese Medicine in Hunan Province, Changsha 410208, China;
4. Neuroscience Center, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100050, China
血管生成是个复杂的过程[1], 参与了多种生理和病理过程, 是器官生长和修复的关键, 与严重困扰人类健康的心脑血管疾病联系密切。血管生成缺陷或不足是心肌缺血和脑缺血等心脑血管疾病中重要的病理特征, 因此调控血管生成疗法可能是治疗心脑血管疾病的有效途径之一。
“百草之王”人参是一种传统的草本植物, 具有多重医疗和保健作用, 在全球尤其是东南亚国家有着广泛的应用。人参皂苷是人参的主要有效部位, 而Rg1是人参根中最丰富的人参皂苷之一, 具有多种药理活性。Rg1主要能够通过抗氧化应激、抗炎、抗细胞凋亡、抗衰老和促增殖等机制对血管生成进行调控。多种微小核糖核酸(microRNA, miRNA) 参与到Rg1的血管保护作用中, 且因miRNA具有靶基因特异性、病理特异性、间接和直接调节等特点, 使miRNA在Rg1促血管生成作用过程中可能发挥重要作用。Rg1透血脑屏障能力弱, 生物利用度低, 利用富含miRNA的外泌体细胞间信使作用或制剂技术提高其生物利用度可能是更好发挥Rg1药理作用的途径, 鉴于此, 本文尝试总结Rg1调控的促血管生成及其血管保护作用研究进展, 尤其是miRNA的介导, 并结合新型生物制剂材料的应用, 为进一步推动Rg1作为血管生成保护剂的临床应用奠定基础。
1 Rg1的血管保护作用机制机体血管正常生理功能的维持依赖于血管内皮细胞及血管平滑肌细胞与机体其他多种细胞之间相互制约、相互调节的作用, 高血压、动脉粥样硬化、糖尿病和心肌缺血等疾病的发生发展都与内皮细胞和血管平滑肌细胞功能异常关系密切[2]。Rg1通过对血管内皮细胞的作用, 维持血管正常的生理功能, 实现血管保护作用, 进而利用外泌体细胞间信使的功能影响到机体其他细胞。
1.1 抑制血管平滑肌细胞增殖血管平滑肌细胞是许多动脉疾病的细胞水平基础, 也是构成血管中膜的主要细胞, 与血管疾病的发展关系密切, 其异常增殖及衰老对动脉粥样硬化有着显著影响。Rg1可以抑制多种生长因子的表达, 从而抑制血管内膜增生和血管平滑肌细胞增殖分化。在肿瘤坏死因子α (tumor necrosis factor α, TNF-α) 处理的抗炎模型中, Rg1降低G蛋白偶联受体激酶(G protein-coupled receptor kinases, GRKs)、蛋白激酶C (protein kinase C, PKC) 和N-ras蛋白的表达, 增加与细胞周期相关蛋白p21的表达, 促进K+流出以及抑制血管平滑肌细胞的增殖而用于治疗心肌缺血和动脉粥样硬化等老年心血管疾病[3]。在β-淀粉样蛋白(Aβ) 诱导人脑血管内皮细胞增殖抑制模型中, 得到Rg1能够促进增殖蛋白增殖细胞核抗原(proliferating cell nuclear antigen, PCNA) 和细胞增殖指标Ki-67蛋白的表达, 同时抑制炎症因子环氧化酶-2 (cyclooxygenase-2, COX-2)、白细胞介素-1 (interleukin-1, IL-1)、TNF-α的表达, 进而改善其增殖抑制作用[4]。此外, 有研究利用RNA-seq技术结合生物信息学手段, 以骨髓内皮祖细胞为模型, 发现Rg1能以浓度依赖性对细胞增殖进行调控, 而其中主要有Janus激酶/信号转导与转录激活子(Janus kinase-signal transducer and activator of transcription, Jak/Stat)、丝裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK)、Wnt (wingless/integrated)、磷脂酰肌醇3激酶/蛋白激酶B (phosphatidylinositol 3 kinase-protein kinase B, PI3K/Akt)、细胞分化、雌激素相关信号通路基因参与进来[5]。
1.2 促进一氧化氮(NO) 产生和血管内皮生长因子(vascular endothelial growth factor, VEGF) 表达VEGF和NO是关键的血管生成刺激剂, 两者在调节血管生成方面彼此紧密合作, 而NO既是VEGF依赖性血管生成的上游和下游介质或效应子。结合到其受体后, VEGF启动复杂的信号传导级联反应, 生成NO, 随后激活血管内皮细胞和血管平滑肌细胞[6]。在TNF-α刺激的人脐静脉内皮细胞(human umbilical vein endothelial cells, HUVECs) 中, 有丝分裂原活化蛋白激酶激酶激酶3 (mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase 3, MEKK3) 和磷酸甘油酸突变酶等蛋白的表达水平升高, 而一氧化氮合酶(NOS) 和盐皮质激素受体的表达降低, Rg1可以阻止这种改变, 促进TNF-α刺激的HUVECs的NO生成和内皮型一氧化氮合酶(endothelial nitric oxide synthase, eNOS) mRNA的表达, 保护内皮细胞免受TNF-α激活[7, 8]。同时Rg1是糖皮质激素受体的功能性配体, 能和纤维细胞生长因子受体1共同激活非基因组信号传导级联反应[9], 通过PI3K/Akt/糖原合成酶激酶-3β (glycogen synthase kinase-3β, GSK3β) 途径诱导eNOS迅速产生NO[10], 导致β-catenin的水平增加影响T细胞因子/淋巴增强因子(T-cell factor-lymphoid enhancer factor, TCF/LEF) 转录活性, 最终导致其核积累, 并随后激活VEGF表达, 而促进血管生成活性[11]。由于NO能够抑制血细胞黏附于内皮, 增加微血管的通透性, 降低自由基造成的血管内皮功能障碍, 因此具有舒张血管作用, 而Rg1能通过调节PI3K/Akt/eNOS途径、L-精氨酸[L(+)-arginine]、环状鸟嘌呤核苷-磷酸(GMP) 形成在内皮细胞中的转运, 以此激活NO的生成, 从而增加内皮依赖性血管扩张[12-14]。
同样, 进一步研究VEGF和NO信号通路, 发现许多上下游蛋白基因被Rg1以各种方式进行调控, 如VEGF 5个结构相关因子VEGFA、VEGFB、VEGFC、VEGFD和胎盘生长因子(placental growth factor, PLGF), 以及血管内皮细胞生长因子受体(vascular endothelial growth factor receptor, VEGFR)。而NO则主要涉及几种一氧化氮合酶[诱导型一氧化氮合酶(inducible nitric oxide synthase, iNOS)、eNOS和神经元型一氧化氮合酶(neuronsal nitric oxide synthase, nNOS)]。在此基础上, 有关Rg1促进血管生成作用的更多研究重点便集中在RNA的水平上, 而其中最为显著的便是miRNA。miRNA是一类内源性非编码RNA, 承载外泌体细胞间信号转导的部分作用, 具有独特的表达谱, 并在各种生理和病理过程中发挥重要作用[15], 许多miRNA参与血管生成, 是调节血管生物学稳态的关键因子, 在调节内皮细胞功能和血管生成中起到重要作用[16]。Rg1作用于内皮细胞, 调节不同的miRNA及miRNA网络, 进而刺激内皮细胞外泌体携带miRNA作用于基质细胞, 转录翻译出各种具有特异性功能的细胞因子、蛋白质, 推动血管完整性的建立和维持。因此推测, Rg1可能通过miRNA参与VEGF和NO信号通路的调节, 实现了血管生成相关疾病的治疗, 相关的miRNA主要有miR-15b、miR-23a、miR-214等。
1.2.1 miR-15bmiR-15b是miR-15/16家族的成员, miR-15/16家族在人类的血管内皮中广泛分布, 也是天然的反义B细胞淋巴瘤2 (B-cell lymphoma 2, Bcl2) 相互作用因子, miR-15b特异性表达于血管内皮细胞中, 可有效调节脂肪细胞和乳腺上皮细胞中的脂质代谢[17, 18]。Rg1能够快速稳定地降低miR-15b的水平, 进而诱导血管内皮生长因子受体2 (vascular endothelial cell growth factor receptor 2, VEGFR-2) 表达, 导致细胞迁移和肾小管生成[19], VEGFR-2可能是miR-15b在介导Rg1血管生成作用中的靶点之一。
1.2.2 miR-23amiR-23a在人肝癌、胶质母细胞瘤、膀胱癌、胰腺癌和鼻咽癌等发生过程中均呈现负调控作用, 其可调控细胞凋亡诱导上皮间质转化和促进肿瘤发生[20], 并对血管生成具有调节作用[21]。来自鼻咽癌细胞的外泌体可通过转移miR-23a直接靶向睾丸特异性基因抗原10 (testis-specific gene antigen 10, TSGA10) 影响血管生成[22]。研究表明, miR-23a可通过靶向矮小相关转录因子2 (runt-related transcription factor 2, RUNX2) 调节VEGFA, 抑制内皮细胞推动的血管生成作用, RUNX2是与成骨细胞分化相关的重要生物标志物, 被确定为潜在抗血管生成的治疗靶标[23]。有关Rg1上调RUNX2表达的结果早在人牙周膜干细胞中得到证实[24], 因此, Rg1可能通过作用于内皮细胞降低miR-23a的表达, 从而上调RUNX2表达, 促进血管生成。研究发现, 在糖尿病足溃疡大鼠模型中, Rg1通过miR-23a抑制胰岛素样生长因子-1 (insulin like growth factor 1, IGF-1) 蛋白表达, 进而作用于iNOS发挥促血管作用[25]。miR-23a通过靶向MET (肝细胞生长因子受体, 具有酪氨酸激酶活性), 激活肝细胞生长因子(hepatocyte growth factor, HGF)-MET途径诱导血管生成。HGF-MET途径是癌症发展的关键驱动因素, 也是上皮细胞迁移和心血管重塑中的基础过程之一[26, 27]。Rg1可能是通过下调miR-23a的表达来上调MET蛋白表达, 从而实现了其血管生成活性[28]。
此外, miR-23a家族与表皮生长因子受体(epidermal growth factor receptor, EGFR)、基因功能磷脂酶和张力蛋白同源物(phosphatase and tensin homolog deleted on chromosome ten, PTEN)、沉默信息调节因子2 (silent information regulator 2, SIRT2)、p21活化激酶4 (p21-activated protein kinase 4, PAK4) 和天冬氨酸特异性半胱氨酸蛋白酶-7 (cysteinyl aspartate specific proteinase-7, caspase-7) 等[29-33]心血管疾病相关的特异性靶标密切相关。通常miR-23a过表达会使内皮血管形成减少, 而内皮细胞在Rg1的作用下, 能够抑制miR-23a的表达, 从而达到促进血管生成作用。
1.2.3 miR-214miR-214也是一个对内皮细胞功能和血管生成影响显著的miRNA, 在外泌体介导的内皮细胞之间的信号传导中起主导作用[34], 在许多病理条件下表达异常, 它推动了包括癌症和心血管疾病[35]等多种疾病的发病进程, miR-214和eNOS关系密切, 而Rg1可调控miR-214的表达进而靶向eNOS促进血管生成的体外细胞迁移和管形成过程[36]。
1.3 抗氧化应激以及抑制炎症通路活性氧(reactive oxygen species, ROS) 和心血管疾病息息相关, ROS强大的氧化活性容易使细胞结构出现损伤, 所造成的氧化应激广泛参与到细胞病理变化过程, 如增加血管内皮细胞的通透性, 影响细胞的增殖活性, 干扰细胞内信号转化等, 在血管性疾病病理过程中起着重要作用[37]。在细颗粒物质诱导的内皮细胞损伤中, Rg1能够降低ROS和丙二醛(malondialdehyde, MDA) 含量, 通过上调细胞内抗氧化状态保护内皮细胞免受PM2.5的毒性[38]。Rg1还能够减少TNF-α刺激的内皮细胞损伤, 抑制炎症通路核转录因子-кB (nuclear factor kappa B, NF-кB), 降低心脏组织核中p65的表达, 减轻腹主动脉缩窄术(abdominal aorta coarctation, AAC) 引起的心肌肥大[39]。在冠状动脉粥样硬化性心脏病模型中, 同样发现Rg1通过提高机体抗氧化酶活性, 平衡血管舒张功能, 达到了改善心肌及冠状动脉病理损伤的效果[40]。
1.4 抗黏附作用保护细胞骨架并改善内皮屏障细胞骨架是由多种结构蛋白及收缩蛋白按照特定模式有机组装而形成的一种立体网格结构, 几乎参与细胞所有重要的生命活动[41]。Rg1可能上调与细胞黏附、迁移[RAS同源基因家族成员A (Ras homolog family, member A, RhoA)、RhoB、含有GTPase活化蛋白1的IQ基序(IQ motif containing GTPase activating protein 1, IQGAP1)、钙调蛋白2 (calmodulin gene 2, CALM2)、层黏蛋白4亚基(laminin alpha4, LAMA4)、鸟嘌呤核苷酸交换因子Vav2] 等和细胞骨架有关的基因, 在调节细胞结构动力学中以分层级联模式彼此相互作用[42], 诱发肌动蛋白细胞骨架重排, 改善血管内皮通透性。内皮糖萼位于血液流和内皮之间, 可提供某些分子的屏障, 并在内皮通透性和内皮功能中起到重要作用, 主要影响机械传导、血管通透性、流变性、血栓形成和白细胞黏附[43], 是具有一定潜力的血管治疗靶点。Rg1通过保护内皮糖萼, 减弱乙酰肝素酶相关表达, 对高糖诱导的内皮屏障功能障碍具有一定保护作用[44]。
2 miRNA介导的Rg1治疗其他相关疾病的作用机制血管生成已成为神经血管再生研究领域的重要方向, miRNA可能还介导了Rg1在脑缺血再灌注损伤后的神经保护作用。
2.1 miR-26amiR-26家族在促进内皮细胞生长、血管生成过程中同样发挥了重要的作用, 其通过控制关键的信号通路作用于血管平滑肌细胞、心脏成纤维细胞和心肌细胞, 在心血管修复中发挥核心作用[45]。而miR-26家族与心血管疾病相关的已知细胞类型特异性靶标有: 转化生长因子β信号蛋白1 (mothers against decapentaplegic homolog 1, SMAD1)、GSK3β、内向整流钾通道亚家族J成员2 (potassium inwardly-rectifying channel, subfamily J, member 2, KCNJ2)、磷脂酶Cβ1 (磷酸肌醇特异) [phospholipase C, beta 1 (phosphoinositide-specific) PLCβ1]、结缔组织生长因子(connective tissue growth factor, CTGF) 和胶原蛋白I等[46-49]。在高糖诱导的糖尿病性视网膜病变模型中已经证明Rg1能够介导上调miR-26a表达, 抑制细胞外调节蛋白激酶(extracelluar regulated protein kinase, ERK) 和Wnt/β-catenin途径, 抑制AMP依赖蛋白激酶[adenosine 5'-monophosphate (AMP)-activated protein kinase, AMPK] 激活, 保护人视网膜色素上皮细胞免受高糖诱导的损伤[50]。结合Rg1具有的心血管修复功能, Rg1促血管生成的作用可能是通过调控miR-26a表达实现的, 有必要进一步探讨Rg1对miR-26a调控的机制, 以明确其对相关疾病治疗的作用机制。
2.2 miR-153miR-153是一种肿瘤抑制性miRNA[51], 也参与了脑缺血/再灌注中的神经元损伤过程。有研究[52]发现, 20 mg·kg-1·day-1 Rg1能够减缓肌萎缩侧索硬化症(amyotrophic lateral sclerosis, ALS) 大鼠的病程, 改善其运动症状, 减少脊髓运动神经元丢失并抑制小胶质细胞激活, 并通过抑制ALS小鼠脊髓miR-153表达, 解除对核因子E2相关因子2 (nuclear factor E2-related factor 2, Nrf2) 转录后的抑制作用, 从而使血红素氧合酶1 (heme oxygenase-1, HO-1) 抗氧化信号通路被激活, 同时miR-153也能通过该信号通路调节心肌细胞凋亡[53], 由此miR-153可能参与介导了Rg1的心血管保护作用。
2.3 miR-144miR-144能直接参与缺血性损伤相关的疾病并具有神经保护作用, Rg1可浓度依赖地激活抗氧化反应元件(antioxidant response element, ARE) 活性, 抑制miR-144与神经元中的Nrf2-3'-UTR结合, 激活体内Nrf2/ARE途径而非常规的Nrf2/胞浆样蛋白1 (kelch-like ECH-associated protein-1, Keap1) 途径, 进而调节HO-1、醌氧化还原酶1 [NAD(P)H: quinone oxidoreductase 1, NQO-1]、谷氨酸半胱氨酸连接酶催化亚基(glutamate-cysteine ligase catalytic subunit, GCLC)、谷氨酸半胱氨酸连接酶修饰因子亚基(glutamate cysteine ligase, modifier subunit, GCLM) 等抗氧化因子, 从而在抗缺血/再灌注作用中发挥重要作用[54]。
目前, 已知Rg1调控了HUVECs中的22个miRNA, 其中17个(miR-363、miR-550、miR-515-5p、miR-212、miR-335、miR-496、miR-214、miR-15b、miR-380-5p、miR-106a、miR-489、miR-23b、miR-510、miR-182、miR-603、miR-23a和miR-377) 被显著下调, 5个(miR-299-5p、miR-551a、miR-521、miR-487b和miR-197) 被显著上调。虽然, 已经证实介导了Rg1促进血管生成的体外细胞迁移和管形成的miRNA仅有miR-15b、miR-23a和miR-214三个, 但miR-26、miR-144和miR-153也与心血管疾病关系密切, 它们是否也介导了Rg1的血管保护作用?是否也能对内皮血管发挥作用?厘清这些问题将有力推动促血管生成剂Rg1治疗心脑血管疾病机制的阐明。Rg1的血管保护作用信号通路如图 1所示。
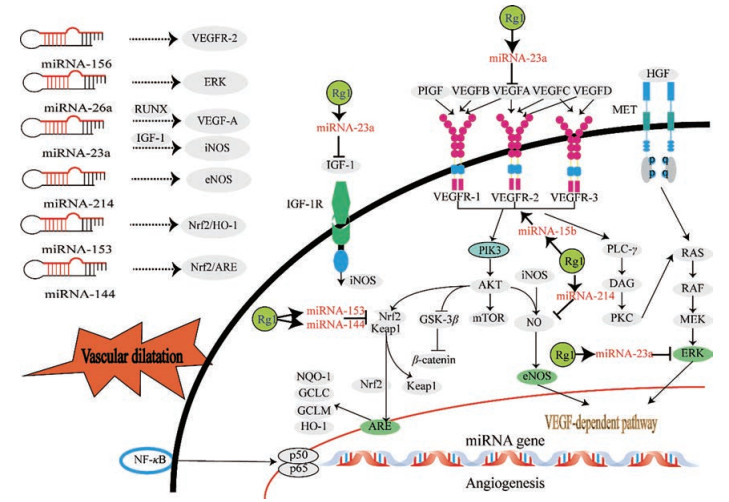
|
Figure 1 Ginsenoside Rg1 participates in angiogenesis and its vascular protection by regulating miRNA |
Rg1基于多种作用机制发挥了促血管生成及血管保护作用, 并进一步实现了对如脑缺血再灌注等疾病的治疗, 但Rg1本身透血脑屏障能力较弱, 造成了其生物利用度较低, 现代制剂技术的发展及新型制剂材料的出现为丰富Rg1的临床应用提供了有力支撑。
3.1 基于Rg1血管生成作用结合生物纳米材料突破血脑屏障治疗脑梗死长久以来, 血脑屏障是将治疗药物运送到大脑的巨大障碍[55], 针对Rg1无法有效穿透血脑屏障富集于大脑而发挥药效的问题, 如能研发新型纳米材料携带Rg1穿透血脑屏障将有力推动Rg1的临床应用。在糖尿病性脑梗死中, 靶向转铁蛋白受体的Rg1纳米颗粒[PHRO, 以γ-聚谷氨酸(γ-polyglutamic acid, γ-PGA)、L-苯丙氨酸乙酯(H) [L-phenylalanine ethylester, L-PAE (H)]、Rg1和OX26抗体制备] 具有缓释效应, 可促进脑血管内皮细胞(cerebrovascular endothelial cells, CECs) 迁移和管形成, 并可能穿过血脑屏障, 治疗脑梗死效果较好[56]。而在最新的研究中, 认为OX26抗体是从异源物种制备的, 免疫原性具有很高的临床风险[57], 因此, 在PHRO基础上制造出靶向转铁蛋白受体(transferrin receptor, TfR) 肽的纳米载体PATRC, 即马来酰亚胺固定的壳聚糖-γ-PGA复合物通过自组装策略装载大量Rg1, 与抗体相比, 对TfR具有高亲和力的肽的分子量要小得多, 并可实现更深的组织渗透, 且易于合成, 成本较低。动态光散射分析显示, PHRO平均粒径为(79 ± 18) nm, 多分散指数= 0.18, Zeta电位38 mV。PATRC平均粒径为(132 ± 12) nm, 多分散指数= 0.29和Zeta电位-38 mV, 二者表征参数均合格, 均能有较好的脑血管保护和再生功能, 显然PATRC较PHRO具有更好的生物活性, 对脑梗死的保护更显著[58]。
3.2 基于Rg1血管生成作用结合生物纳米释放材料应用于骨损伤骨再生是一个血管生成的依赖过程, 而Rg1对血管生成和骨骼形成有很好的促进作用, 结合生物纳米材料的研究发现, Rg1可以和众多新型材料结合形成具有集血管生成和一定机械性能于一体的血管生成骨水泥复合材料。如Rg1-Sr2-TiO2-PPF骨水泥复合材料[59], 其中富马酸聚丙烯(PPF) 作为一种有前途的可降解、可注射且无毒的水泥增强材料, 在骨坏死修复中具有十分广阔的应用前景。而Sr是一种主要积累在骨骼中的天然骨微量元素, 将Rg1整合到骨水泥配方中具有了高机械性能、射线不透性和血管生成活性, 适用于坏死骨的骨水泥化。
3.3 基于Rg1血管生成作用体内释放的控缓释材料Rg1具有类似雌激素的活性, 可通过对内皮细胞的作用直接调节血管生成, 在皮肤组织工程的血管生成和再生中具有潜在应用价值。如何将Rg1更好地应用于人体并充分发挥其作用, 是目前亟需解决的技术难题。将Rg1封装在Genipin交联明胶微球复合载体中, 能成功地促进大鼠心肌血管生成, 实现缓释控释以长效改善内皮细胞增殖和血管发芽的作用[60]。而将Rg1封装在胶原蛋白/壳聚糖-明胶微球(CC-GMS) 支架中发挥血管生成作用以增强伤口血管生成和愈合, 多孔CC-GMS支架成为了调节Rg1释放和增加细胞生长潜力的皮肤组织替代品[61]。
4 小结和展望血管生成是一个复杂的过程, 包括内皮细胞分裂、基底膜和周围细胞外基质的选择性降解及随后的内皮细胞迁移和新血管的形成[62], 参与维持胚胎发育、伤口愈合、炎症和繁殖等生理过程[63]。血管内皮功能在调节血管生物学和体内平衡中起着关键作用, 如果这种稳态失衡就会导致多种心脑血管疾病的产生。Rg1作为人参的主要活性成分, 其血管保护作用的发挥可能通过抗氧化应激、抗炎、抑制细胞增殖衰老和抑制细胞黏附实现, 同时外泌体及其携带的miRNA的细胞间通讯功能可能是充分发挥Rg1药理活性的重要途径。Rg1在心脑血管疾病的治疗过程中, 通过多种信号途径发挥保护心肌缺血再灌注、保护脑缺血再灌注、抗动脉粥样硬化、抗心律失常、抑制心肌细胞肥大、修复心脏功能障碍和抑制心室重构等作用[64, 65]。如前所述, 多种miRNA介导了Rg1血管生成和保护作用, 因此如果能在Rg1治疗心脑血管疾病过程中, 观察外泌体中的miRNA表达变化, 将有望推动Rg1血管生成保护机制的阐明。此外, 研究发现p-p38MAPK、CD31、HIF-1α、p-ERK2、ERK2、MKP-1等mRNA可能也参与血管生成作用[66-68]。在此基础上, 再随着新型制剂材料的开发与应用, 将Rg1开发成血管生成保护剂从而更好地应用于临床, 更好地服务于人类健康将具有重要意义。
作者贡献: 杨岩涛、杨岩是本文的主要完成者; 肖佳妹协助查询相关文献并进行图片的整理; 陈乃宏对本文撰写进行指导并提出修改意见。
利益冲突: 所有作者均声明不存在利益冲突。
| [1] |
Wang AY, Tao L, Lu Y, et al. Research progress on intervention by Salviae Miltiorrhizae Radix et Rhizoma on angiogenesis of tumor and ischemic disease[J]. Chin Tradit Herb Drugs (中草药), 2015, 46: 1399-1404. |
| [2] |
Li HR, Wei G, Yin YJ, et al. Research progress of ginseng in protecting vascular endothelial cells[J]. Chin J Gerontol (中国老年学杂志), 2015, 35: 5957-5960. |
| [3] |
Ma ZC, Gao Y, Wang YG, et al. Ginsenoside Rg1 inhibits proliferation of vascular smooth muscle cells stimulated by tumor necrosis factor-alpha[J]. Acta Pharmacol Sin, 2006, 27: 1000-1006. DOI:10.1111/j.1745-7254.2006.00331.x |
| [4] |
Liu NH, Huang XF, Wang ZF, et al. Study on the effect of ginsenoside Rg1 and Rb1 for the anti-damage of Aβ on human vascular endothelial cells[J]. J Gannan Med Univ (赣南医学院学报), 2019, 39: 1197-1200, 1240. |
| [5] |
Tang L. Research on the Effect and Mechanism of Ginsenoside Rg1 on the Proliferation of Murine Bone Marrow Endothelial Progenitor Cells In Vitro (人参皂苷Rg1对小鼠骨髓内皮祖细胞体外增殖的影响及其作用机制的研究) [D]. Changsha: Hunan Normal University, 2020.
|
| [6] |
Yue PYK, Mak NK, Cheng YK, et al. Pharmacogenomics and the Yin/Yang actions of ginseng: anti-tumor, angiomodulating and steroid-like activities of ginsenosides[J]. Chin Med, 2007, 2-6. |
| [7] |
Ma ZC, Gao Y, Wang J, et al. Proteomic analysis effects of ginsenoside Rg1 on human umbilical vein endothelial cells stimulated by tumor necrosis factor-alpha[J]. Life Sci, 2006, 79: 175-181. DOI:10.1016/j.lfs.2005.12.050 |
| [8] |
Lü JP, Ma ZC, Yang J, et al. Ginsenoside Rg1-induced alterations in gene expression in TNF-α stimulated endothelial cells[J]. Chin Med J, 2004, 117: 871-876. |
| [9] |
Cheung LWT, Leung KW, Wong CKC, et al. Ginsenoside-Rg1 induces angiogenesis via non-genomic crosstalk of glucocorticoid receptor and fibroblast growth factor receptor-1[J]. Cardiovasc Res, 2011, 89: 419-425. DOI:10.1093/cvr/cvq300 |
| [10] |
Leung KW, Cheng YK, Mak NK, et al. Signaling pathway of ginsenoside-Rg1 leading to nitric oxide production in endothelial cells[J]. FEBS Lett, 2006, 580: 3211-3216. DOI:10.1016/j.febslet.2006.04.080 |
| [11] |
Leung KW, Pon YL, Wong RNS, et al. Ginsenoside-Rg1 induces vascular endothelial growth factor expression through the gluco-corticoid receptor-related phosphatidylinositol 3-kinase/Akt and beta-catenin/T-cell factor-dependent pathway in human endothelial cells[J]. J Biol Chem, 2006, 281: 36280-36288. DOI:10.1074/jbc.M606698200 |
| [12] |
Pan C, Huo Y, An X, et al. Panax notoginseng and its components decreased hypertension via stimulation of endothelial-dependent vessel dilatation[J]. Vascul Pharmacol, 2012, 56: 150-158. DOI:10.1016/j.vph.2011.12.006 |
| [13] |
Kim HY, Chen X, Gillis CN. Ginsenosides protect pulmonary vascular endothelium against free radical-induced injury[J]. Biochem Biophys Res Commun, 1992, 189: 670-676. DOI:10.1016/0006-291X(92)92253-T |
| [14] |
Kang SY, Schini-Kerth VB, Kim ND, et al. Ginsenosides of the protopanaxatriol group cause endothelium-dependent relaxation in the rat aorta[J]. Life Sci, 1995, 56: 1577-1586. DOI:10.1016/0024-3205(95)00124-O |
| [15] |
Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis[J]. Cardiovasc Res, 2008, 79: 581-588. DOI:10.1093/cvr/cvn156 |
| [16] |
Caporali A, Emanueli C. MicroRNA regulation in angiogenesis[J]. Vascul Pharmacol, 2011, 55: 79-86. DOI:10.1016/j.vph.2011.06.006 |
| [17] |
Zhu LP, Zhou JP, Zhang JX, et al. MiR-15b-5p regulates collateral artery formation by targeting AKT3(protein kinase B-3)[J]. Arterioscler Thromb Vasc Biol, 2017, 37: 957-968. DOI:10.1161/ATVBAHA.116.308905 |
| [18] |
Chu M, Zhao Y, Yu S, et al. miR-15b negatively correlates with lipid metabolism in mammary epithelial cells[J]. Am J Physiol Cell Physiol, 2018, 314: C43-C52. DOI:10.1152/ajpcell.00115.2017 |
| [19] |
Chan LS, Yue PYK, Wong YY, et al. MicroRNA-15b contributes to ginsenoside-Rg1-induced angiogenesis through increased expression of VEGFR-2[J]. Biochem Pharmacol, 2013, 86: 392-400. DOI:10.1016/j.bcp.2013.05.006 |
| [20] |
Chhabra R, Dubey R, Saini N. Cooperative and individualistic functions of the microRNAs in the miR-23a~27a~24-2 cluster and its implication in human diseases[J]. Mol Cancer, 2010, 9: 232. DOI:10.1186/1476-4598-9-232 |
| [21] |
Oikawa S, Wada S, Lee M, et al. Role of endothelial microRNA-23 clusters in angiogenesis in vivo[J]. Am J Physiol Heart Circ Physiol, 2018, 315: H838-H846. DOI:10.1152/ajpheart.00742.2017 |
| [22] |
Bao LL, You B, Shi S, et al. Metastasis-associated miR-23a from nasopharyngeal carcinoma-derived exosomes mediates angiogenesis by repressing a novel target gene TSGA10[J]. Oncogene, 2018, 37: 2873-2889. DOI:10.1038/s41388-018-0183-6 |
| [23] |
Wu XD, Guo T, Liu L, et al. MiR-23a targets RUNX2 and suppresses ginsenoside Rg1-induced angiogenesis in endothelial cells[J]. Oncotarget, 2017, 8: 58072-58085. DOI:10.18632/oncotarget.19489 |
| [24] |
Yin LH, Cheng WX, Qin ZS, et al. Effects of ginsenoside Rg-1 on the proliferation and osteogenic differentiation of human periodontal ligament stem cells[J]. Chin J Integr Med, 2015, 21: 676-681. DOI:10.1007/s11655-014-1856-9 |
| [25] |
Cai HA, Huang L, Zheng LJ, et al. Ginsenoside (Rg-1) promoted the wound closure of diabetic foot ulcer through iNOS elevation via miR-23a/IRF-1 axis[J]. Life Sci, 2019, 233: 116525. DOI:10.1016/j.lfs.2019.05.081 |
| [26] |
Matsumoto K, Umitsu M, De Silva DM, et al. Hepatocyte growth factor/MET in cancer progression and biomarker discovery[J]. Cancer Sci, 2017, 108: 296-307. DOI:10.1111/cas.13156 |
| [27] |
Gallo S, Sala V, Gatti S, et al. Cellular and molecular mechanisms of HGF/Met in the cardiovascular system[J]. Clin Sci (Lond), 2015, 129: 1173-1193. DOI:10.1042/CS20150502 |
| [28] |
Kwok HH, Chan LS, Poon PY, et al. Ginsenoside-Rg1 induces angiogenesis by the inverse regulation of MET tyrosine kinase receptor expression through miR-23a[J]. Toxicol Appl Pharmacol, 2015, 287: 276-283. DOI:10.1016/j.taap.2015.06.014 |
| [29] |
Wang S, He W, Wang C. MiR-23a regulates the vasculogenesis of coronary artery disease by targeting epidermal growth factor receptor[J]. Cardiovasc Ther, 2016, 34: 199-208. DOI:10.1111/1755-5922.12187 |
| [30] |
Zheng YF, Liu L, Chen C, et al. The extracellular vesicles secreted by lung cancer cells in radiation therapy promote endothelial cell angiogenesis by transferring miR-23a[J]. PeerJ, 2017, 5: e3627. DOI:10.7717/peerj.3627 |
| [31] |
Du J, Liang Y, Li J, et al. Gastric cancer cell-derived exosomal microRNA-23a promotes angiogenesis by targeting PTEN[J]. Front Oncol, 2020, 10: 326. DOI:10.3389/fonc.2020.00326 |
| [32] |
Sruthi TV, Edatt L, Raji GR, et al. Horizontal transfer of miR23a from hypoxic tumor cell colonies can induce angiogenesis[J]. J Cell Physiol, 2018, 233: 3498-3514. DOI:10.1002/jcp.26202 |
| [33] |
Ruan W, Xu JM, Li SB, et al. Effects of down-regulation of microRNA-23a on TNF-α-induced endothelial cell apoptosis through caspase-dependent pathways[J]. Cardiovasc Res, 2012, 93: 623-632. DOI:10.1093/cvr/cvr290 |
| [34] |
van Balkom BW, de Jong OG, Smits M, et al. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells[J]. Blood, 2013, 121: 3997-4006. DOI:10.1182/blood-2013-02-478925 |
| [35] |
Zhao YF, Ponnusamy M, Zhang L, et al. The role of miR-214 in cardiovascular diseases[J]. Eur J Pharmacol, 2017, 816: 138-145. DOI:10.1016/j.ejphar.2017.08.009 |
| [36] |
Chan LS, Yue YK, Mak NK, et al. Role of microRNA-214 in ginsenoside-Rg1-induced angiogenesis[J]. Eur J Pharm Sci, 2009, 38: 370-377. DOI:10.1016/j.ejps.2009.08.008 |
| [37] |
Zhang XH, Xu XX, Wang NQ. Progress of studies on protective mechanism of Radix Astragali in vascular endothelial cells[J]. Chin Pharm J (中国药学杂志), 2013, 48: 1526-1530. |
| [38] |
Li CP, Qin G, Shi RZ, et al. Ginsenoside Rg1 reduces toxicity of PM(2.5) on human umbilical vein endothelial cells by upregu-lating intracellular antioxidative state[J]. Environ Toxicol Pharmacol, 2013, 35: 21-29. DOI:10.1016/j.etap.2012.11.006 |
| [39] |
Tang F, Lu M, Yu L, et al. Inhibition of TNF-α-mediated NF-κB activation by ginsenoside Rg1 contributes the attenuation of cardiac hypertrophy induced by abdominal aorta coarctation[J]. J Cardiovasc Pharmacol, 2016, 68: 257-264. DOI:10.1097/FJC.0000000000000410 |
| [40] |
Chen YX, Li SS, Zhang HF. Effects of ginsenoside Rg1 on cardiac function and vasomotor function in rats with coronary heart disease[J]. Acta Chin Med (中医学报), 2020, 35: 1491-1496. |
| [41] |
Cheng YH, Han M, Wen JK. Effect of SM22α on VSMC cytoskeleton and contraction[J]. Chin J Cell Biol (细胞生物学杂志), 2003, 25: 384-388. |
| [42] |
Yue PYK, Wong DYL, Ha WY, et al. Elucidation of the mechanisms underlying the angiogenic effects of ginsenoside Rg(1) in vivo and in vitro[J]. Angiogenesis, 2007, 10: 69. DOI:10.1007/s10456-006-9036-y |
| [43] |
Sieve I, Münster-Kühnel AK, Hilfiker-Kleiner D. Regulation and function of endothelial glycocalyx layer in vascular diseases[J]. Vascul Pharmacol, 2018, 100: 26-33. DOI:10.1016/j.vph.2017.09.002 |
| [44] |
Zhu T, Wang H, Wang L, et al. Ginsenoside Rg1 attenuates high glucose-induced endothelial barrier dysfunction in human umbilical vein endothelial cells by protecting the endothelial glycocalyx[J]. Exp Ther Med, 2019, 17: 3727-3733. |
| [45] |
Icli B, Dorbala P, Feinberg MW. An emerging role for the miR-26 family in cardiovascular disease[J]. Trends Cardiovasc Med, 2014, 24: 241-248. DOI:10.1016/j.tcm.2014.06.003 |
| [46] |
Icli B, Wara AK, Moslehi J, et al. MicroRNA-26a regulates pathological and physiological angiogenesis by targeting BMP/SMAD1 signaling[J]. Circ Res, 2013, 113: 1231-1241. DOI:10.1161/CIRCRESAHA.113.301780 |
| [47] |
Zhang ZH, Li J, Liu BR, et al. MicroRNA-26 was decreased in rat cardiac hypertrophy model and may be a promising therapeutic target[J]. J Cardiovasc Pharmacol, 2013, 62: 312-319. DOI:10.1097/FJC.0b013e31829b82e6 |
| [48] |
Luo XB, Pan ZW, Shan HL, et al. MicroRNA-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation[J]. J Clin Invest, 2013, 123: 1939-1951. DOI:10.1172/JCI62185 |
| [49] |
Wei CY, Kim IK, Kumar S, et al. NF-κB mediated miR-26a regulation in cardiac fibrosis[J]. J Cell Physiol, 2013, 228: 1433-1442. DOI:10.1002/jcp.24296 |
| [50] |
Shi QQ, Chen XY, Sun G, et al. Ginsenoside Rg1 protects human retinal pigment epithelial ARPE-19 cells from toxicity of high glucose by up-regulation of miR-26a[J]. Life Sci, 2019, 221: 152-158. DOI:10.1016/j.lfs.2019.02.021 |
| [51] |
Zhao XS, Wang YN, Lv M, et al. miR-153-3p, a new bio-target, is involved in the pathogenesis of acute graft-versus-host disease via inhibition of indoleamine-2, 3-dioxygenase[J]. Oncotarget, 2016, 7: 48321-48334. DOI:10.18632/oncotarget.10220 |
| [52] |
Zhang Z, Wang SS, Zhu TB, et al. Rg1 alleviates the damage in ALS model through regulation of miR153/Nrf2/HO-1[J]. Acta Pharm Sin (药学学报), 2018, 53: 546-552. |
| [53] |
Zhu XT, Zhao YL, Hou W, et al. MiR-153 regulates cardiomyo-cyte apoptosis by targeting Nrf2/HO-1 signaling[J]. Chromosome Res, 2019, 27: 167-178. DOI:10.1007/s10577-019-09608-y |
| [54] |
Chu SF, Zhang Z, Zhou X, et al. Ginsenoside Rg1 protects against ischemic/reperfusion-induced neuronal injury through miR-144/Nrf2/ARE pathway[J]. Acta Pharmacol Sin, 2019, 40: 13-25. DOI:10.1038/s41401-018-0154-z |
| [55] |
Haqqani AS, Thom G, Burrell M, et al. Intracellular sorting and transcytosis of the rat transferrin receptor antibody OX26 across the blood-brain barrier in vitro is dependent on its binding affinity[J]. J Neurochem, 2018, 146: 735-752. DOI:10.1111/jnc.14482 |
| [56] |
Shen JY, Zhao ZM, Shang W, et al. Ginsenoside Rg1 nanoparticle penetrating the blood-brain barrier to improve the cerebral function of diabetic rats complicated with cerebral infarction[J]. Int J Nanomedicine, 2017, 12: 6477-6486. DOI:10.2147/IJN.S139602 |
| [57] |
Knezevic I, Kang HN, Thorpe R. Immunogenicity assessment of monoclonal antibody products: a simulated case study correlating antibody induction with clinical outcomes[J]. Biologicals, 2015, 43: 307-317. DOI:10.1016/j.biologicals.2015.06.009 |
| [58] |
Shen JY, Zhao ZM, Shang W, et al. Fabrication and evaluation a transferrin receptor targeting nano-drug carrier for cerebral infarction treatment[J]. Artif Cells Nanomed Biotechnol, 2019, 4: 192-200. |
| [59] |
Salarian M, Xu WZ, Bohay R, et al. Angiogenic Rg1/Sr-doped TiO2 nanowire/poly(propylene fumarate) bone cement composites[J]. Macromol Biosci, 2017, 17: 10. |
| [60] |
Awada HK, Johnson NR, Wang Y. Sequential delivery of angiogenic growth factors improves revascularization and heart function after myocardial infarction[J]. J Control Release, 2015, 207: 7-17. DOI:10.1016/j.jconrel.2015.03.034 |
| [61] |
Zheng YR, Feng ZZ, You CG, et al. In vitro evaluation of Panax notoginseng Rg1 released from collagen/chitosan-gelatin micro-sphere scaffolds for angiogenesis[J]. Biomed Eng Online, 2013, 12: 134. DOI:10.1186/1475-925X-12-134 |
| [62] |
Santulli G. MicroRNAs and endothelial (Dys) function[J]. J Cell Physiol, 2016, 231: 1638-1644. DOI:10.1002/jcp.25276 |
| [63] |
Tiwari A, Mukherjee B, Dixit M. MicroRNA key to angiogenesis regulation: miRNA biology and therapy[J]. Curr Cancer Drug Targets, 2018, 18: 266-277. DOI:10.2174/1568009617666170630142725 |
| [64] |
Xie WJ, Zhou P, Sun YF, et al. Protective effects and target network analysis of ginsenoside Rg1 in cerebral ischemia and reperfusion injury: a comprehensive overview of experimental studies[J]. Cells, 2018, 7: 270. DOI:10.3390/cells7120270 |
| [65] |
Zhang YJ, Zhang XL, Li MH, et al. The ginsenoside Rg1 prevents transverse aortic constriction-induced left ventricular hypertrophy and cardiac dysfunction by inhibiting fibrosis and enhancing angiogenesis[J]. J Cardiovasc Pharmacol, 2013, 62: 50-57. DOI:10.1097/FJC.0b013e31828f8d45 |
| [66] |
Gao Y, Deng J, Yu XF, et al. Ginsenoside Rg1 inhibits vascular intimal hyperplasia in balloon-injured rat carotid artery by down-regulation of extracellular signal-regulated kinase 2[J]. J Ethnopharmacol, 2011, 138: 472-478. DOI:10.1016/j.jep.2011.09.029 |
| [67] |
Leung KW, Ng HM, Tang MK, et al. Ginsenoside-Rg1 mediates a hypoxia-independent upregulation of hypoxia-inducible factor-1α to promote angiogenesis[J]. Angiogenesis, 2011, 14: 515-522. DOI:10.1007/s10456-011-9235-z |
| [68] |
Tang LL, Hao ML, Li GL, et al. Effect of notoginsenoside Rg1 on p38MAPK expression of pulmonary arterial smooth muscle cells exposed to hypoxia hypercapnia[J]. Chin J Appl Physiol (中国应用生理学杂志), 2012, 28: 230-233. |
 2021, Vol. 56
2021, Vol. 56


