2. 呼吸疾病中医药防治省部共建协同创新中心, 河南 郑州 450046
2. Co-construction of Collaborative Innovation Center for Chinese Medicine and Respiratory Diseases by Henan and Education Ministry of P. R. China, Zhengzhou 450046, China
姜皮为姜科植物姜(Zingiber officinale Rosc.) 的干燥根茎外皮[1], 晒干后呈卷缩不整齐的碎片, 灰黄色, 有细皱纹, 有的具线状的环节痕迹, 内表面常具黄色油点, 有特殊香气, 姜皮味辛, 性凉; 归脾、肺经; 具有行水消肿的功效[2], 主治水肿初起, 小便不利[3]。姜皮中主要含有姜辣素、二苯庚烷、挥发油[4]以及多糖[5]等化学成分, 现代研究表明, 姜皮具有多种药理活性, 包括抗氧化、抗肿瘤、抗菌等[6-10]。国内外学者对于姜的研究多集中于生姜、干姜, 而对于姜皮化学成分研究却鲜有报道, 为了进一步明确姜皮的药效物质基础, 本实验运用多种色谱技术从姜皮中鉴定出3个二苯庚烷类化合物, 分别是: (2S, 2'S, 3R, 3'R, 4R, 4'R, 6R, 6'R)-6, 6'-bis((S)-1-hydroxy-2-(4-hydroxyphenyl)ethyl)-2, 2'-bis(4-hydroxy-3-meth-oxyphenyl)octahydro-2H, 2'H-[3, 3'-bipyran]-4, 4'-diol (1)、(E)-7-(4-hydroxy-3-methoxyphenyl)-1-(4-hydroxyphenyl)hept-4-en-3-one (2) 和alpinin B (3), 其中化合物1为新化合物, 化合物2、3为首次从姜皮中分离得到, 结构见图 1。
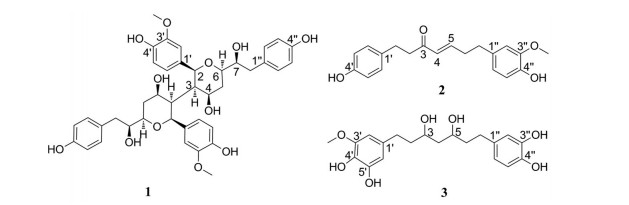
|
Figure 1 Structures of compounds 1-3 |
化合物1为黄色无定形粉末, 易溶于甲醇, [α]D20 -19.1 (c 0.1, MeOH); IR显示结构中含有羟基(3 495 cm-1)、苯环(1 515cm-1); UV (MeOH) λmax (log ε): 205 (4.80) nm、225 (4.38) nm、280 (3.92) nm; HR-ESI-MS [M+Na]+ m/z 741.288 1 (Calcd. 741.288 1), 提示化合物分子式为C40H46O12。遇FeCl3显色剂显蓝色, 提示化合物中存在酚羟基。分析其1H NMR (500 MHz, CD3OD) 数据(表 1), 在芳香区存在7个氢质子信号[δH 6.95 (1H, d, J = 1.8 Hz, H-2')、6.94 (2H, d, J = 8.5 Hz, H-2'', 6'')、6.83 (1H, dd, J = 8.1, 1.8 Hz, H-6')、6.79 (1H, d, J = 8.1 Hz, H-5')、6.65 (2H, d, J = 8.5 Hz, H-3'', 5'')], 在13C NMR (125 MHz, CD3OD) 中, 芳香区存在12个碳信号, 提示结构中含有两个苯环, 一个为ABX耦合系统, 另一个为AA'BB'耦合系统, 结合碳谱中δC 81.1 (C-2)、58.4 (C-3)、79.8 (C-4)、36.5 (C-5)、72.4 (C-6)、76.2 (C-7)、40.0 (C-8) 这7个碳信号, 提示其结构为二苯庚烷类化合物; 在HMBC谱(图 2) 中, δH 5.07 (1H, d, J = 6.0 Hz, H-2) 与δC 58.4 (C-3)、79.8 (C-4) 有相关, δH 2.02 (1H, m, H-5b) 与δC 58.4 (C-3)、79.8 (C-4)、72.4 (C-6) 有相关, δH 3.27 (1H, m, H-6) 与δC 81.1 (C-2) 有相关, 提示结构中有一个四氢吡喃环, δH 5.07 (1H, d, J = 6.0 Hz, H-2) 与δC 111.6 (C-2')、120.8 (C-6') 有相关, δH 2.50 (1H, dd, J = 13.8, 8.5 Hz, H-8a)、2.56 (1H, dd, J = 13.8, 4.9 Hz, H-8b) 与δC 131.3 (C-2'', 6'') 有相关, 说明ABX耦合系统的苯环与C-2相连, 而AA'BB'耦合系统的苯环与C-8相连, δH 3.83 (3H, s, 3'-OCH3) 与δC 149.2 (C-3') 有相关, 提示δC 56.5 (3'-OCH3) 与C-3'相连; 该平面结构与文献中化合物(2S, 3R, 4S, 6R)-6-[(R)-1-hydroxy-2-(4-hydroxyphenyl)ethyl]-2-(4-hydroxy-3-methoxyphenyl)-tetrahydro-2H-pyran-3, 4-diol[11]类似, 仅在C-3位取代基团不同, 但对比该化合物质谱数据HR-ESI-MS [M+Na]+ m/z 741.288 1 (Calcd. 741.288 1) 发现其相对分子质量与现有平面结构相差较大, 由于δH 3.27 (1H, m, H-3) 与δC 58.4 (C-3) 有相关, 推测其C-3位被另外一个相同的结构取代, 即化合物1为具有对称结构的二苯庚烷二聚体。化合物1的相对构型是通过分析其耦合常数和NOESY光谱确定的, JH (eq)-5/H-6 (3.7 Hz)、JH-4/H (eq)-5 (3.5 Hz) 表明H-4、H-6均为平伏键, JH-3/H-4 (6.8 Hz)、JH-2/H-3 (6.0 Hz) 表明H-3为直立键而H-2为平伏键, 上述分析得到了NOESY光谱中H-3与H-2、H-4有相关信号的证明(图 2), 由JH-6/H-7 (3.0 Hz) 确定了C-6和C-7两个邻位次甲基片段相对构型为赤式(erythro) 构型[12, 13], 这一点也由H-6与H-7存在的NOESY相关信号证明(图 2), 由此可推断其相对构型为2S, 2'S, 3R, 3'R, 4R, 4'R, 6R, 6'R, 7S, 7'S (1a) 或2R, 2'R, 3S, 3'S, 4S, 4'S, 6S, 6'S, 7R, 7'R (1b), 为了确定化合物1的绝对构型, 采取量子化学计算的方法[B3LYP/6-31G (d, p), MeOH] 计算了1a和1b的ECD谱, 结果表明1a的ECD谱与化合物1的实验值吻合较好(图 3), 确定化合物1的构型为2S, 2'S, 3R, 3'R, 4R, 4'R, 6R, 6'R, 7S, 7'S, 故确定其结构为: (2S, 2'S, 3R, 3'R, 4R, 4'R, 6R, 6'R)-6, 6'-bis((S)-1-hydroxy-2-(4-hydroxyphenyl)ethyl)-2, 2'-bis(4-hydroxy-3-meth-oxyphenyl)octahydro-2H, 2'H-[3, 3'-bipyran]-4, 4'-diol。
| Table 1 1H NMR (500 MHz in CD3OD) and 13C NMR (125 MHz in CD3OD) spectral data of compound 1 |
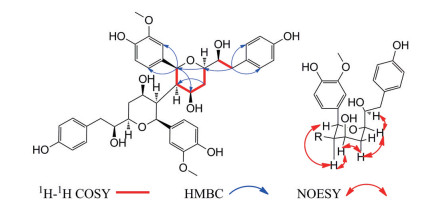
|
Figure 2 Key 1H-1H COSY, HMBC and NOESY of compound 1 |
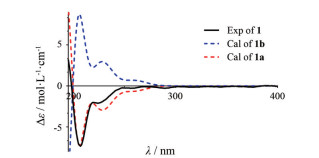
|
Figure 3 Experimental and calculated ECD spectra of 1 |
TripleTOF 6600型高效液相色谱-四极杆飞行时间高分辨质谱联用仪(AB SCIEX); Bruker AM-500 MHz核磁共振谱用超导核磁共振仪(TMS内标); RE-5210A型旋转蒸发仪(上海亚荣生化仪器厂); 赛谱锐思LC50型高压制备液相色谱仪(赛谱锐思北京科技有限公司); Rudolph AP-IV型旋光仪(Rudolph, USA); Thermo Nicolet IS10红外光谱仪(Thermo Scientific, USA); Thermo EVO300紫外分光光度计(Thermo Scientific, USA); Sephadex LH-20 (Parmacia Biotech公司); GF254硅胶薄层板(青岛海洋化工厂); 分析纯和色谱纯试剂(天津四友精细化学品有限公司)。
姜皮于2020年7月购自于云南省罗平县, 经河南中医药大学董诚明教授鉴定姜皮为姜科多年生草本植物姜(Z. offccinale Rosc.) 的干燥皮质部分, 保存于河南中医药大学中药化学提取分离实验室, 标本号: BS631006。
1 提取分离姜皮40 kg, 50%含水乙醇加热回流提取3次, 合并提取液, 减压浓缩得到总浸膏(5.40 kg)。浸膏加水混悬后, 分别用石油醚、乙酸乙酯、正丁醇萃取, 减压浓缩后依次得到石油醚部位(0.22 kg)、乙酸乙酯部位(1.20 kg)、正丁醇部位(0.51 kg) 和水部位(3.30 kg)。正丁醇部位上MCI柱, 乙醇-水(0∶100~95∶5) 梯度洗脱, 依次得到水、10%、30%、50%、60%、70%、95%乙醇部位, 30%乙醇部位(20 g) 上ODS柱, 甲醇-水(10∶90~100∶0) 梯度洗脱, 得到Fr.1~Fr.5, Fr.2 (2.8 g) 经Sephadex LH-20, 甲醇洗脱得到Fr.2.1~Fr.2.3, Fr.2.2 (564.5 mg) 干法上硅胶柱(200~300目) 二氯甲烷-甲醇(100∶0~1∶1) 梯度洗脱得到Fr.2.2.1~Fr.2.2.8, Fr.2.2.2 (24.0 mg) 经半制备高效液相色谱(甲醇-水= 50∶50) 得到化合物1 (7.2 mg), Fr.2.2.4 (137.8 mg) 经半制备高效液相色谱(甲醇-水= 45∶55) 得到化合物3 (6.1 mg)。50%乙醇部位(33.5 g) 干法上硅胶柱(200~300目) 二氯甲烷-甲醇(100∶0~1∶1) 梯度洗脱得到Fr.A~Fr.E, Fr.B (1.2 g) 经Sephadex LH-20, 甲醇洗脱得到Fr.B-1~Fr.B-5, Fr.B-4 (37.4 mg) 经半制备高效液相色谱(甲醇-水= 62∶38) 得到化合物2 (2.0 mg)。
2 结构鉴定化合物1 黄色无定形粉末, 易溶于甲醇, UV (MeOH) λmax (logε)/nm: 205 (4.80)、225 (4.38)、280 (3.92); IR (MeOH) vmax: 3 495、1 676、1 515 cm-1; [α]D20 -19.1 (c 0.1, MeOH); HR-ESI-MS [M+Na]+ m/z 741.288 1 (Calcd. 741.288 1), 分子式为C40H46O12。1H NMR (500 MHz, CD3OD) 和13C NMR (125 MHz, CD3OD) 数据见表 1。
化合物2 黄色油状物, 易溶于甲醇, ESI-MS [M+Na]+ m/z 349, 分子式为C20H22O4。1H NMR (500 MHz, acetone-d6) δH 7.05 (2H, d, J = 8.2 Hz, H-2', 6'), 6.88 (1H, m, H-5), 6.84 (1H, s, H-2''), 6.75 (2H, d, J = 8.2 Hz, H-3', 5'), 6.72 (1H, d, J = 8.0 Hz, H-6''), 6.65 (1H, d, J = 8.0 Hz, H-5''), 6.10 (1H, d, J = 15.9 Hz, H-4), 3.82 (3H, s, 3''-OCH3), 2.77 (4H, m, H-1, H-2), 2.69 (2H, t, J = 7.6 Hz, H-7), 2.49 (2H, dd, J = 14.9, 7.3 Hz, H-7); 13C NMR (125 MHz, acetone-d6) δC 199.4 (C-3), 147.0 (C-5), 147.0 (C-3''), 147.0 (C-4'), 145.7 (C-4''), 133.8 (C-1'), 132.7 (C-1''), 131.5 (C-4), 130.2 (C-2', 6'), 121.5 (C-6''), 116.0 (C-3', 5'), 115.6 (C-5''), 112.9 (C-2''), 56.2 (3''-OCH3), 42.4 (C-2), 35.2 (C-6), 34.3 (C-7), 30.5 (C-1)。以上数据与文献[14]报道的1-(4-hydroxy -phenyl)-7-(4-hydroxy-3-methoxyphenyl)-4-hepten-3-one基本一致。
化合物3 黄色油状物, 易溶于甲醇, ESI-MS [M+Na]+ m/z 401, 分子式为C20H26O7。1H NMR (500 MHz, CD3OD) δH 6.64 (1H, d, J = 2.0 Hz, H-6'), 6.62 (1H, d, J = 2.0 Hz, H-2''), 6.50 (1H, dd, J = 8.0, 2.0 Hz, H-6''), 6.32 (1H, d, J = 8.0 Hz, H-5''), 6.32 (1H, d, J = 2.0 Hz, H-2'), 3.79 (3H, s, 3'-OCH3), 3.79 (2H, m, H-3, 5), 2.59 (2H, m, H-7), 2.48 (2H, m, H-1), 1.67 (4H, m, H-2, 6), 1.53 (2H, m, H-4); 13C NMR (125 MHz, CD3OD) δC 149.6 (C-3'), 146.4 (C-3''), 146.1 (C-4''), 144.2 (C-5'), 135.3 (C-4'), 134.6 (C-1'), 133.1 (C-1''), 120.6 (C-6''), 116.6 (C-2''), 116.3 (C-5''), 109.9 (C-6'), 104.8 (C-2'), 68.8 (C-5), 68.7 (C-3), 56.5 (3'-OCH3), 45.7 (C-4), 41.4 (C-6), 41.3 (C-2), 32.9 (C-7), 32.4 (C-1)。以上数据与文献[15]报道的alpinin B基本一致。
作者贡献: 王彦志、宋志敏、冯卫生设计研究; 宋志敏、张晓娟、李曼倩、刘煜飞和胡雪雨进行了研究并分析了数据; 王彦志给出了关于论文写作的建议, 宋志敏写了论文。所有作者都阅读并批准了最后的手稿。
利益冲突: 作者声明不存在利益冲突。
| [1] |
Pharmaceutical Administration of the People's Republic of China. National Chinese Medicine Processing Regulations (全国中药炮制规范)[M]. Beijing: People's Medical Publishing House, 1988: 32.
|
| [2] |
Xu QL, Zhou YQ, Zhan Y, et al. Research on extraction technology of flavonoid from ginger peel[J]. Mod Food Sci Technol (现代食品科技), 2012, 28: 998-1001. |
| [3] |
Luo XJ, Cheng S, Pan YN, et al. Quality evaluation of ginger skins in different regions[J]. China Cond (中国调味品), 2014, 39: 35-37. |
| [4] |
Guo Y, Wang YZ, Xu ZP, et al. Two new monoterpenoids of Zingiber officinale peel[J]. Acta Pharm Sin (药学学报), 2020, 55: 484-488. |
| [5] |
Feng X, Xia Y, Chen GT, et al. Purification and structural analysis of polysaccharides from ginger peels[J]. Food Sci (食品科学), 2017, 38: 185-190. |
| [6] |
Li TY, Liu WH, Liang N, et al. Quantitative assessment of gingerols and the antioxidant activity in ginger and its processed ginger[J]. Food Ind (食品工业), 2016, 37: 180-183. |
| [7] |
Das S. Evaluation of antimicrobial activities of various solvent extracts of ginger Rhizome peels and whole ginger Rhizome without peels[J]. World J Pharm Res, 2017, 6: 1450-1468. |
| [8] |
Mariangela M, Francesco M, Filomena C. A comparative study of Zingiber officinale Roscoe pulp and peel: phytochemical composition and evaluation of antitumour activity[J]. Nat Prod Res, 2015, 29: 2045-2054. DOI:10.1080/14786419.2015.1020491 |
| [9] |
Ko MJ, Nam HH, Chung MS. Conversion of 6-gingerol to 6-shogaol in ginger (Zingiber officinale) pulp and peel during subcritical water extraction[J]. Food Chem, 2019, 270: 149-155. DOI:10.1016/j.foodchem.2018.07.078 |
| [10] |
Xia Y. Research on Extraction, Purification, Structural Analysis and Antioxidai Activity of Polysaccharides from the Ginger Skin (生姜皮多糖的分离纯化、结构分析及其抗氧化活性研究)[D]. Nanjing: Nanjing Agricultural University, 2016.
|
| [11] |
Lin YS, Lin JH, Chang CC, et al. Tetrahydropyran- and tetrahydrofuran-containing diarylheptanoids from Hedychium coronarium Rhizomes[J]. J Nat Prod, 2015, 78: 181-188. DOI:10.1021/np500441r |
| [12] |
Li CW, Cui CB. Application of several physicochemical techniques in natural products to elucidate stereochemistry[J]. Int J Pharm Res, 2015, 42: 811-828. |
| [13] |
He XF, Zhang XK, Geng CA, et al. Tsaokopyranols A-M, 2, 6-epoxydiarylhe ptanoids from Amomum tsaoko and their α-glucosidase inhibitory activity[J]. Bioorg Chem, 2020, 96: 103638. DOI:10.1016/j.bioorg.2020.103638 |
| [14] |
Jin WY, Cai XF, Na MK, et al. Triterpenoids and diarylheptanoids from Alnus hirsuta inhibit HIF-1 in ags cells[J]. Arch Pharm Res, 2007, 30: 412-420. DOI:10.1007/BF02980213 |
| [15] |
Fu GM, Zhang W, Du DS, et al. Diarylheptanoids from Rhizomes of Alpinia officinarum inhibit aggregation of α-synuclein[J]. J Agric Food Chem, 2017, 65: 6608-6614. DOI:10.1021/acs.jafc.7b02021 |
 2021, Vol. 56
2021, Vol. 56


