2. 昆明理工大学医学院衰老与肿瘤分子遗传学实验室, 云南 昆明 650500
2. Laboratory of Molecular Genetics of Aging and Tumor, Medical School, Kunming University of Science and Technology, Kunming 650500, China
全球人口正在进入老龄化阶段, 到2050年, 全世界1/6人口将达到65岁或65岁以上, 而2000年这一比例仅为1/14[1]。65岁以上的老年人肿瘤发生率升高, 证明衰老是许多肿瘤发生的重要风险因素。初期研究认为, 衰老和肿瘤是两个相反的病理过程: 衰老会导致细胞生长停滞, 而肿瘤则导致失去正常检查点控制的细胞增殖[2]。但越来越多的研究表明, 长寿小鼠模型中肿瘤的发生时间会有所延迟, 证明衰老和肿瘤并不是简单的相反关系[3]。在细胞水平上, 衰老细胞的特征包括细胞体积增加、β-半乳糖苷酶活性升高、细胞凋亡水平降低和衰老相关分泌表型(senescence-associated secretory phenotype, SASPs) 的产生[4, 5]。有趣的是, 在肿瘤细胞中也观察到了上述抗凋亡等衰老细胞的特征[6]。且衰老细胞分泌的细胞因子、趋化因子和蛋白酶等不同类型的SASPs可促进癌细胞的发生和发展[7]。这些证据表明衰老和肿瘤相互影响并可能具有共同的机制。
随着对衰老细胞和肿瘤细胞的不断研究, 目前已发现多种保守细胞信号通路同时参与了衰老和肿瘤的过程, 如哺乳动物雷帕霉素分子靶标(mammalian target of rapamycin, mTOR)、腺苷单磷酸激活依赖蛋白激酶[adenosine 5'-monophosphate (AMP)-activated protein kinase, AMPK] 和组蛋白去乙酰化酶sirtuins。从线虫到人类, 这些保守的细胞信号通路与寿命和健康衰老息息相关[8]。
通过抑制mTOR通路, 雷帕霉素和双胍类药物可使线虫、果蝇和小鼠的寿命延长, 而激活mTOR通路会促使细胞增殖并诱导衰老和肿瘤发生[9]。热量限制(caloric restriction, CR) 能激活人骨骼肌中sirtuins和AMPK通路, 与线粒体过氧化物酶体增殖物激活受体γ共同激活因子α (proliferator-activated receptor gamma co-activator alpha, PGC1α)一起构成控制线粒体生物合成能量感应的细胞网络, 进而影响生命质量[10]。抗衰老研究表明: 雷帕霉素、二甲双胍和热量限制可以调节上述营养感应的细胞信号通路, 对肿瘤细胞的能量代谢进行重编程, 并通过抑制正常的有丝分裂, 系统地调节机体代谢和细胞衰老。这些药物不同于紫杉醇和多柔比星等化疗药物会引起脱氧核糖核酸(deoxyribonucleic acid, DNA) 和蛋白质等分子损伤, 因此不会产生选择性抗性[11, 12]。因此作用于这些细胞信号通路的药物可能在延缓衰老和抑制肿瘤方面均能发挥作用。
几十年来, 多项研究已经发现中药(traditional Chinese medicine, TCM) 的活性成分可降低化疗、放疗等肿瘤治疗的毒性, TCM还可治疗多种衰老相关疾病动物模型和肿瘤动物模型[13, 14]。本文回顾了中药活性成分介导的衰老减缓和/或肿瘤抑制。由于中药成分众多且其中许多成分是混合物, 其药理机制尚不明确, 因此本综述仅总结了目前具有抗衰老和抗肿瘤特性的中药有效成分, 以及这些成分在上述细胞信号通路中的潜在机制, 旨在为中药有效成分治疗衰老和肿瘤提供新的研究策略和视野。
1 衰老与肿瘤中共同的细胞信号和通路: 中药的潜在靶点许多研究从信号通路中的蛋白到通路网络的角度探究了衰老与肿瘤之间的关系, 因此关注这些在衰老和肿瘤中共存的细胞信号通路来筛选无毒或低毒且具有抗衰老功效的中药可能适用于抑制老年人的肿瘤, 符合健康老龄化的观点。
1.1 衰老相关分泌表型(SASPs)DNA损伤和细胞衰老均可诱导细胞释放生长因子、趋化因子和细胞因子等不同的SASPs[15]。细胞因子类型的SASPs通过增加成肌纤维细胞分化来促进伤口愈合[16]; 趋化因子和细胞因子类型的SASPs可通过刺激血管生成, 激活肿瘤干细胞来诱导肿瘤的发生和发展[17]; 炎性细胞因子和趋化因子类型的SASPs可募集免疫细胞从而影响机体免疫状态[15]。通过旁分泌或内分泌的方式, SASPs诱导局部和全身炎症, 活化免疫系统, 诱导衰老扩散到局部和全身其他细胞[15-17]。有研究发现消除衰老小鼠体内的衰老细胞后, 衰老小鼠体内SASPs显著下降, 小鼠寿命延长, 且其肿瘤发展被抑制[18]。这些实验结果表明SASPs在衰老和肿瘤发生中扮演重要角色, 基于SASPs的抗衰老机制研究及相关药物新靶点也逐渐成为研究热点[19]。
大部分SASPs受DNA损伤应激通路(DNA damage response, DDR) 调控[20]。一些SASPs也可由转录因子核因子κB (nuclear factor kappa-B, NF-κB) 激活[21]。因此DDR通路和NF-κB被称为SASPs的“主要调控因子”, 对DDR通路、NF-κB和SASPs的靶向调控可能成为延缓衰老和抑制肿瘤的新目标。
1.2 mTOR通路mTOR包括两种蛋白质复合物: mTOR复合物1 (mTORC1) 和mTOR复合物2 (mTORC2)[22], 这两个靶点受磷脂酰肌醇3激酶(phosphatidylinositol-3 kinases, PI3K) 调控。mTOR是一种激酶, 可整合细胞内外信号(如营养, 生长因子、氧气水平和能量状况), 使细胞和机体做出即时反应, 以平衡分解代谢和合成代谢[23]。由于mTOR受到许多致癌通路(如PI3K/MAPK通路) 调节, 因此mTOR信号通路与线虫、酵母和哺乳动物等不同生物的衰老和肿瘤发展密切相关。mTOR的失调会诱导人转移性膀胱癌的发生[24]。抑制mTORC1基因可延长小鼠寿命[25], mTORC1底物S6K1的缺失也可延长哺乳动物的寿命[26]。
目前的研究已经发现, 以雷帕霉素为代表的mTOR抑制剂被广泛用于肿瘤治疗, 可提高HER-2/neu基因型肿瘤易感小鼠的寿命[27]。雷帕霉素的一项临床试验表明: 与对照组相比, 雷帕霉素治疗组可提高老年人的步行能力[28]。这些实验结果表明下调mTOR通路可以减缓衰老并抑制肿瘤。
1.3 AMPK通路体内增加的腺苷单磷酸(adenosine 5'-monophosphate, AMP) 可激活AMPK, 进而调节能量代谢。最初AMPK被发现是2型糖尿病的靶点[29], 随着研究的进展, AMPK的肿瘤抑制和介导健康衰老的作用逐渐被揭开[30, 31]。
AMPK通路被发现与肺癌、直肠癌、肝癌和乳腺癌等疾病相关, 在这些疾病中, AMPK通路通过调节能量和抑制细胞增殖进而抑制肿瘤发生[32-35]。在小鼠中敲除AMPK的调控子丝氨酸-苏氨酸肝激酶B1 (serine-threonine liver kinase B1, LKB1) 会增加肿瘤的发生率[36]。AMPK在维持能量平衡方面具有重要作用[如体内过低的三磷酸腺苷(adenosine-triphosphate, ATP)能激活AMPK], 激活AMPK可有效延长蠕虫和果蝇等模型动物的寿命[37]。研究发现, 在小鼠和线虫中使用AMPK的激活剂、过表达AMPK的催化亚基等方法可使这些模型动物的寿命延长[36, 37]。这些研究结果表明AMPK是调节寿命和治疗肿瘤的潜在靶标。
1.4 Sirtuins通路Sirtuins酶是一种烟酰胺腺嘌呤二核苷酸(nicotinamide adenine dinucleotide, NAD) 依赖的脱乙酰酶, 与组蛋白和非组蛋白等多种蛋白质靶向结合, 介导蛋白质的翻译后调节, 参与多种细胞过程[38]。由于赖氨酸的乙酰化可调节多种蛋白质功能, 因此sirtuins蛋白也是细胞增殖、细胞凋亡、DNA修复、细胞周期和氧化应激反应的关键调控子[39]。Sirtuins家族具有7个成员(SIRT1~7), 均可延长寿命又能抑制肿瘤发生[40]。SIRT2的同源物可延长线虫、果蝇和小鼠等模型动物的寿命并预防年龄相关的疾病[41]。在神经胶质瘤、乳腺癌和结直肠癌等疾病中, SIRT1的表达水平升高[42-44]; 在神经胶质瘤和乳腺癌中, SIRT2的表达降低[45, 46], SIRT2敲除的小鼠会发展成肝癌和乳腺癌[47]。这些证据表明, sirtuins的作用依赖于细胞和肿瘤类型, 因而sirtuins在衰老和肿瘤中具有更复杂的作用。进一步研究sirtuins蛋白的作用机制将有助于了解衰老与肿瘤之间的关系, 并开发出抗衰老和抗肿瘤药物。
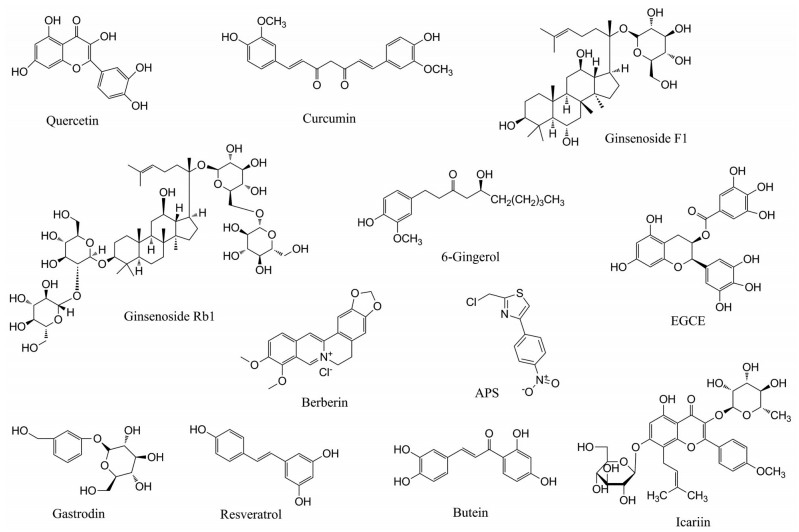
2 具有抗衰老和抗肿瘤功效的中药成分许多基础研究和临床研究都在寻找和鉴定能延长健康期和/或延缓衰老的抗肿瘤药物, 中药因其可调节衰老和肿瘤相关的多种信号通路和分子靶标而具有优势, 还具有不良反应较小的优点。当前使用的中药通常分为单方药和复方制剂。本文通过上述通路为切入点, 介绍具有抗衰老和抗肿瘤能力的中药成分, 其名称和化学结构如图 1所示。
|
图 1 Chemical structures of traditional Chinese medicine components with anti-aging and anti-tumor effects. EGCG: Epigallocatechin-3-gallate |
槲皮素(quercetin) 是一种类黄酮类的抗氧化剂。槲皮素能抑制由博来霉素诱导的人衰老成纤维细胞分泌的SASPs[48]。槲皮素可以延长酵母和线虫的寿命[49, 50]。最近的研究表明, 槲皮素可通过诱导细胞周期停滞和自噬, 进而抑制肿瘤的生长[49]。槲皮素还可通过抑制NF-κB的核转位(SASPs的“主要调控因子”) 来抑制人肝癌细胞的增殖和迁移[51]。近年来的研究发现, 达沙替尼(dasatinib) 和槲皮素的组合可清除衰老细胞移植小鼠中的衰老情况, 降低衰老移植组织中SASPs的水平, 并延长了衰老细胞移植小鼠的寿命[52], 在患有特发性肺纤维化的老年人群中应用达沙替尼和槲皮素的组合治疗, 可显著改善老年患者的活动能力[53]。槲皮素与化疗药物的联合使用, 改善槲皮素结构以增加药物吸收等方向是将来的研究热点。
2.1.2 姜黄素姜黄素(curcumin) 是姜黄的主要成分, 是一种具有抗氧化和免疫调节等多种药理作用的安全药物。姜黄素通过调节表观遗传酶(如HDACs和p30047) 进而减轻线虫和果蝇等模型动物体内的氧化应激, 姜黄素还可与转录因子NF-κB结合从而调节SASPs, 并与细胞周期蛋白作用, 抑制乳腺癌、结肠癌和肺癌细胞的增殖和转移[54, 55]。然而姜黄素由于其吸收较差且代谢快, 在体内生物利用度较低, 因此基于姜黄素的结构改造和优化成为近年来相关研究领域的热点, 有研究对姜黄素不稳定的β-二酮结构进行了改造, 构建出含有稳定且具有抗肿瘤活性的查尔酮(chalcone) 结构的姜黄素类似物[56]。
2.1.3 人参皂苷F1人参是著名的中药。人参根部有多种化合物被发现具有抗衰老的作用, 人参皂苷(ginsenoside F1) 是其中的一种成分。在人星形胶质细胞中, 人参皂苷F1通过抑制p38MAPK依赖的NF-κB活性, 进而抑制D-半乳糖在该细胞中诱导产生SASPs, 人参皂苷F1还能抑制胶质母细胞瘤的迁移[57]。因此, 人参皂苷F1在衰老相关神经疾病中有潜在的治疗潜力。
2.2 可调节mTOR的中药成分 2.2.1 人参皂苷Rb1和Rg3人参皂苷Rb1是人参中另一种具有代表性的成分。人参皂苷Rb1可降低衰老小鼠脑中mTOR蛋白的磷酸化水平, 这表明人参皂苷Rb1的抗衰老活性可能与mTOR通路的抑制有关[58]。一项大样本的临床试验研究表明, 人参皂苷Rg3和化疗结合的组合疗法有利于延长非小细胞肺癌(nonsmall-cell lung cancer, NSCLC) 患者的生存期[59]。近期有研究团队利用人参皂苷Rb1良好的亲水性质, 将其包裹疏水性的化疗药物多柔比星、光化学试剂和热激蛋白形成新型人参皂苷Rb1纳米颗粒, 此颗粒能将化疗药物快速递送到肿瘤细胞, 在近红外光的激发下杀死小鼠和人乳腺癌细胞, 是一种先进的光热化学联合疗法[60]。
2.2.2 6-姜油酚从生姜中提取的6-姜油酚(6-gingerol) 是一种多酚复合物。细胞和动物实验证明6-姜油酚具有抗氧化、抗炎和降血脂降血压等心血管保护作用[61]。6-姜油酚处理大鼠血管平滑肌细胞时, 可通过促进细胞周期停滞并抑制mTOR表达而显著降低该细胞的衰老程度[62], 该结果表明6-姜油酚可通过抑制mTOR通路进而延缓细胞衰老。该研究还进一步发现6-姜油酚可通过抑制mTOR磷酸化、降低P70S6K表达来抑制人宫颈癌HeLa细胞的生长并诱导癌细胞的周期停滞和凋亡[62]。
2.2.3 表没食子儿茶素没食子酸酯表没食子儿茶素没食子酸酯(epigallocatechin-3-gallate, EGCG) 是从绿茶中提取的多酚类物质, 绿茶多酚具有很强的抗自由基活性, 可调节免疫并能抑制细胞增殖[63]。EGCG可通过抑制血管内皮生长因子(vascular endothelial growth factor, VEGF) 及其受体功能进而抑制血管生成, 也可通过抑制基质金属蛋白酶(matrix metalloproteinase, MMP) 活性抑制血管生成, 达到抗肿瘤的作用[64, 65], 而MMP正是SASPs的一种。EGCG还可通过抑制NF-κB的活性, 诱导细胞凋亡[66]。有研究还发现EGCG可通过抑制mTOR/HIF1α(hypoxia inducible factor 1α) 途径来抑制子宫内膜癌异种移植模型中的肿瘤生长, 这表明EGCG有子宫内膜癌的应用潜力[67]。因此, EGCG可作为SASPs、NF-κB和mTOR的抑制剂, 发挥抗肿瘤的作用。然而, EGCG的稳定性较差, 且进入血液后的生物利用度差, 成为治疗应用的主要障碍, 对其进行化学修饰进行稳定性优化是未来热点的研究方向。
2.3 可调节AMPK的中药成分 2.3.1 小檗碱黄连(Rhizoma Coptidis, RC)被用作止泻剂和抗菌剂的历史悠久[68]。小檗碱(berberine) 是黄连中提取的一类异喹啉生物碱。小檗碱被发现可激活AMPK, 还可同时调节ROS和mTOR/pS6途径[69]。在小鼠心肌细胞、平滑肌细胞和癌细胞中, 小檗碱可激活AMPK通路[70]。而AMPK的激活可通过增加自噬和NAD+进而保护氧化应激诱导的细胞衰老[71]。小檗碱的抗肿瘤活性也被广泛报道, 可以抑制乳腺癌、胃癌和肝癌中的细胞生长、转移和血管生成[72-74]。此外, 小檗碱可以抑制上皮间质转化(epithelial mesenchymal transition, EMT) 过程, 而EMT是肿瘤不良预后的原因[75]。近期研究还发现, 小檗碱能通过调节过氧化物酶体增殖物激活受体γ(peroxisome proliferator-activated receptor γ, PPARγ)-促进AMPK磷酸化进而减轻地塞米松引起的高脂血症和高血糖, 减少小鼠内脏脂肪堆积和代谢紊乱, 证明小檗碱也有治疗老年性代谢疾病的潜力[76]。
2.3.2 黄芪多糖黄芪多糖(astragalus polysaccharide, APS) 是从黄芪中提取的主要成分。目前已发现APS具有多种药理作用, 如增加端粒酶活性、抗氧化、抗炎、免疫调节和抗肿瘤作用[77]。APS的作用与AMPK的激活一致, 具有抗衰老作用[78]。APS可以延长秀丽隐杆线虫的健康寿命[79]。在D-半乳糖诱发的衰老小鼠中, APS可增加其抗氧化能力[80]。在临床上, APS可与化疗剂一起组合使用, 增强抗肿瘤活性并减轻化疗的不良反应[81]。根据现有研究, APS在衰老和肿瘤中具有广泛的应用潜力。
2.3.3 天麻素天麻素(gastrodin) 是天麻中的主要化合物, 在中医临床上有很悠久的使用历史。据报道, 天麻素在体外和体内水平均能激活AMPK通路, 减轻非酒精性脂肪肝病大鼠的氧化应激和炎症水平[82]。在阿尔茨海默病小鼠模型中, 天麻素能减轻模型的记忆障碍; 在帕金森病小鼠模型中, 天麻素通过下调连接蛋白来减轻疾病症状[83, 84]。天麻素可抑制移植肿瘤鼠肝腹水中癌细胞生长, 且毒性较低。这表明天麻素可能是抗衰老和抗肿瘤的潜在佐剂。
2.4 可调节sirtuins的中药成分 2.4.1 白藜芦醇白藜芦醇(resveratrol) 的医学潜力正日渐受到关注。白藜芦醇被鉴定为SIRT1的活化剂, 广泛存在于红葡萄、红酒和桑葚中[85]。白藜芦醇可以通过激活卡路里限制效应来调节SIRT1, 进而调节生物的平均寿命。据报道, 白藜芦醇通过直接刺激SIRT2的活性使酵母的寿命延长了70%[86]。在成年秀丽线虫和果蝇的成年早期, 白藜芦醇的使用可以延长其平均寿命[87]。除具有抗衰老特性外, 白藜芦醇还对癌细胞表现出抑制作用。白藜芦醇通过线粒体介导的凋亡机制, 使小鼠前列腺癌细胞凋亡[88]。一项临床研究表明, 白藜芦醇可通过调节细胞色素P450来影响咖啡因、氯沙坦和丁螺环酮等致癌物质的代谢, 进而抑制肿瘤[89]。
2.4.2 紫铆因紫铆因(butein) 是黄檀和拟南芥等植物中的主要黄酮类化合物, 具有抗氧化、抗炎、抗菌和抗肿瘤的功效[90]。研究发现, 啤酒酵母中加入浓度为10 μmol·L-1的紫铆因时, 啤酒酵母的SIRT1被激活使其寿命延长约31%[87]。在白介素(interleukin, IL)-10基因缺失的肠炎小鼠模型体内, 紫铆因可通过抑制IL-6、IL-1β、干扰素γ和MMP9的表达, 将该小鼠模型的炎症分数降低50%[91]。紫铆因还可以诱导细胞凋亡, 抑制细胞生长和微转移, 从而抵抗结直肠癌、肝癌和肺癌[92, 93]。另外, 对于饮食诱导肥胖的小鼠, 紫铆因显著减少小鼠体重增加和脂肪累积, 这种有益的作用是通过对NF-κB的抑制、激活胰岛素信号以及改善葡萄糖耐量而发挥作用的[94]。紫铆因还可通过降低糖基化产物(glycation end products, AGEs) 和醛糖还原酶, 从而预防糖尿病并发症[95]。因此, 紫铆因在抗衰老和抗肿瘤方面具有巨大的发展潜力。
2.4.3 淫羊藿苷淫羊藿苷(icariin) 是淫羊藿中的主要活性化合物, 具有广泛的药理和生物学作用, 如抗氧化、免疫调节和抗肿瘤活性[96]。淫羊藿苷可通过增加SIRT6抑制老龄小鼠的免疫水平[97]。淫羊藿苷可通过诱导不同类型癌细胞(如肺癌、胃癌和肾癌细胞) 的细胞周期停滞和凋亡而具有抗肿瘤活性[98-100]。此外, 淫羊藿苷可通过PI3K/AKT通路, 对人上皮细胞起到抗血管生成和免疫调节等作用[101]。该化合物还可通过SIRT6A介导的NF-κB通路进而改善范可尼贫血小鼠模型的造血干细胞功能[102], 诱导骨髓间充质干细胞分化[103], 而干细胞功能的降低与衰老息息相关。因此, 淫羊藿苷作为抗衰老和抗肿瘤成分, 具有巨大的发展潜力。
3 总结和展望衰老与肿瘤涉及众多靶点和信号通路间复杂的调控。随着研究的深入和技术的发展, 科学家们发现了共同调控衰老和肿瘤的mTOR、AMPK和sirtuins等常见的信号通路, 均是衰老和肿瘤研究中重要的里程碑。这些通路与一系列如肥胖、糖尿病、高血糖症和心血管疾病等年龄相关疾病有关[104]; 衰老组织中衰老细胞的积累可通过分泌释放SASPs等炎症反应促进肿瘤的发生[7]。对长寿人群和小鼠的研究证实延缓衰老可减少肿瘤的发生率[3, 8]。近年来, 清除衰老细胞的“senolytics”研究成为热点, 该研究选择性地清除衰老细胞并调控SASPs, 可以延缓衰老和调控衰老相关疾病, 预测也会在老年肿瘤预防方面有作用[105]。这些证据均表明调节衰老与肿瘤的共同通路靶标可延缓衰老, 并预防肿瘤和其他年龄相关疾病的发生。因此, 鉴定具有抗衰老和抗肿瘤作用的中药成分是非常必要的。
目前对具有抗衰老和抗肿瘤作用化合物的研究工作已鉴定出一系列药物, 如二甲双胍、雷帕霉素、阿司匹林和L-茶氨酸[11]。但这些药物由于耐药性和不良反应而使有效性受到了限制。中药的来源及其广泛, 且在中国和其他国家/地区已广泛使用了数千年, 形成了其独特的理论、诊断和治疗系统, 因此中药是抗衰老和抗肿瘤药物筛选的一片蓝海。尽管许多研究致力于鉴定中药的作用机制, 但中药的复杂成分和配方限制了对其作用机制的深入研究, 且由于中药的复杂性而导致研究结果的可重复性差, 因此目前仍然难以得出确定的作用机制, 因此, 对于中药活性成分在复杂细胞信号通路中的潜在机制的深入和清晰的理解至关重要。但目前的中药研究缺乏一致性模型和评价标准, 尤其缺乏高质量、大样本的临床数据, 这限制了中药作用机制的研究。
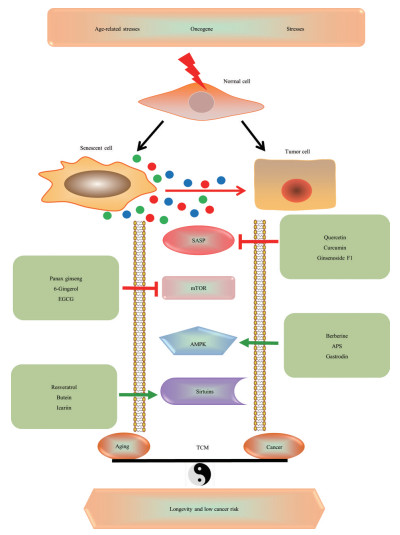
本文总结了基于几种共同通路的中药抗衰老和抗肿瘤作用的最新研究(图 2), 可作为鉴定筛选中药单一成分在抗衰老和抗肿瘤中作用的方法框架。但需要注意的是, 临床上使用的中药由多种活性成分组成并发挥协同作用, 因此需要在细胞水平、器官水平、动物水平和临床水平上进行多层次的渐进研究, 以探索中药成分的抗衰老和抗肿瘤作用。另外, 以生物信息学和数据挖掘等方法为代表的新兴学科网络药理学[106], 也是未来研究中药抗衰老和抗肿瘤的方向, 其创新点在于将经典的抗衰老中药复方和衰老相关疾病数据库筛选药物分子作用靶点联合使用, 预测其信号通路和作用机制, 并进行中药活性单体化合物-靶标-通路可视化, 经过拓扑参数分析预测药物作用的关键节点, 为抗衰老中药复方的机制研究提供依据, 同时解决传统中药研究中“单一成分-单一靶点-单一途径”的局限性, 有利于推进中药研究的现代化进程。
|
图 2 The role of some effective components of traditional Chinese medicine in common signaling pathways of aging and tumors |
作者贡献: 刘静和罗瑛构思了这篇文章的整体思路; 孙曼婷负责文献和数据的收集; 周若宇负责文献收集和文章的撰写。所有作者均参与文章的撰写工作。
利益冲突: 全体作者声明本文不存在任何利益冲突。
| [1] |
Hsu T. Educational initiatives in geriatric oncology - who, why, and how?[J]. J Geriatr Oncol, 2016, 7: 390-396. DOI:10.1016/j.jgo.2016.07.013 |
| [2] |
Liu J, Peng L, Huang W, et al. Balancing between aging and cancer: molecular genetics meets traditional Chinese medicine[J]. J Cell Biochem, 2017, 118: 2581-2586. DOI:10.1002/jcb.25898 |
| [3] |
Ikeno Y, Bronson RT, Hubbard GB, et al. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity[J]. J Gerontol A Biol Sci Med Sci, 2003, 58: 291-296. DOI:10.1093/gerona/58.4.B291 |
| [4] |
Rudzinska M, Parodi A, Balakireva AV, et al. Cellular aging characteristics and their association with age-related disorders[J]. Antioxidants, 2020, 9: 94. DOI:10.3390/antiox9020094 |
| [5] |
Momtaz S, Baeeri M. Manipulation of molecular pathways and senescence hallmarks by natural compounds in fibroblast cells[J]. Cell Biochem, 2019, 120: 6209-6222. DOI:10.1002/jcb.27909 |
| [6] |
Mohamed MS, Bishr MK, Almutairi FM, et al. Inhibitors of apoptosis: clinical implications in cancer[J]. Apoptosis, 2017, 22: 1487-1509. |
| [7] |
Valenzuela CA, Quintanilla R, Olate-Briones A, et al. SASP-dependent interactions between senescent cells and platelets modulate migration and invasion of cancer cells[J]. Int J Mol Sci, 2019, 20: E5292. DOI:10.3390/ijms20215292 |
| [8] |
Orozco-Solis R, Sassone-Corsi P. Circadian clock: linking epigenetics to aging[J]. Curr Opin Genet Dev, 2014, 26: 66-72. DOI:10.1016/j.gde.2014.06.003 |
| [9] |
Anisimov VN. Conservative growth hormone/IGF-1 and mTOR signaling pathways as a target for aging and cancer prevention: do we really have an antiaging drug?[J]. Interdiscip Top Gerontol, 2015, 40: 177-188. |
| [10] |
Draznin B, Wang C, Adochio R, et al. Effect of dietary macronutrient composition on AMPK and SIRT1 expression and activity in human skeletal muscle[J]. Horm Metab Res, 2012, 44: 650-655. DOI:10.1055/s-0032-1312656 |
| [11] |
Yokoyama NN, Denmon A, Uchio EM, et al. When anti-aging studies meet cancer chemoprevention: can anti-aging agent kill two birds with one blow?[J]. Curr Pharmacol Rep, 2015, 1: 420-433. DOI:10.1007/s40495-015-0039-5 |
| [12] |
Li J, Kim SG, Blenis J. Rapamycin: one drug, many effects[J]. Cell Metab, 2014, 19: 373-379. DOI:10.1016/j.cmet.2014.01.001 |
| [13] |
Wang Z, Qi F, Cui Y, et al. An update on Chinese herbal medicines as adjuvant treatment of anticancer therapeutics[J]. Biosci Trends, 2018, 12: 220-239. DOI:10.5582/bst.2018.01144 |
| [14] |
Ding AJ, Zheng SQ, Huang XB, et al. Current perspective in the discovery of anti-aging agents from natural products[J]. Nat Prod Bioprospect, 2017, 7: 335-404. |
| [15] |
Frey N, Venturelli S, Zender L, et al. Cellular senescence in gastrointestinal diseases: from pathogenesis to therapeutics[J]. Nat Rev Gastro Hepat, 2018, 15: 81-95. DOI:10.1038/nrgastro.2017.146 |
| [16] |
Demaria M, Desprez PY, Campisi J, et al. Cell autonomous and non-autonomous effects of senescent cells in the skin[J]. J Invest Dermatol, 2015, 135: 1722-1726. DOI:10.1038/jid.2015.108 |
| [17] |
Cahu J, Bustany S, Sola B. Senescence-associated secretory phenotype favors the emergence of cancer stem-like cells[J]. Cell Death Dis, 2012, 3: e446. DOI:10.1038/cddis.2012.183 |
| [18] |
Baker DJ, Childs BG, Durik M, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan[J]. Nature, 2016, 530: 184-189. DOI:10.1038/nature16932 |
| [19] |
Zheng WG, Qin XM, Gao L, et al. Research advances in understanding the senescence-associated secretory phenotype and relevant drugs[J]. Acta Pharm Sin (药学学报), 2020, 55: 8-14. |
| [20] |
Rodier F, Coppe JP, Patil CK, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion[J]. Nat Cell Biol, 2009, 11: 973-979. DOI:10.1038/ncb1909 |
| [21] |
Salminen A, Kauppinen A, Kaarniranta K. Emerging role of NF-kappa B signaling in the induction of senescence-associated secretory phenotype (SASP)[J]. Cell Signal, 2012, 24: 835-845. DOI:10.1016/j.cellsig.2011.12.006 |
| [22] |
Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease[J]. Cell, 2017, 169: 361-371. |
| [23] |
Cornu M, Albert V, Hall MN. mTOR in aging, metabolism, and cancer[J]. Curr Opin Genet Dev, 2013, 23: 53-62. DOI:10.1016/j.gde.2012.12.005 |
| [24] |
Sathe A, Nawroth R. Targeting the PI3K/AKT/mTOR pathway in bladder cancer[J]. Methods Mol Biol, 2018, 1655: 335-350. |
| [25] |
Lamming DW, Ye L, Katajisto P, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity[J]. Science, 2012, 335: 1638-1643. DOI:10.1126/science.1215135 |
| [26] |
Selman C, Tullet JM, Wieser D, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span[J]. Science, 2009, 326: 140-144. DOI:10.1126/science.1177221 |
| [27] |
Anisimov VN, Zabezhinski MA, Popovich IG, et al. Rapamycin extends maximal lifespan in cancer-prone mice[J]. Am J Pathol, 2010, 176: 2092-2097. DOI:10.2353/ajpath.2010.091050 |
| [28] |
Leslie M. Biomedicine. A putative antiaging drug takes a step from mice to men[J]. Science, 2013, 342-789. |
| [29] |
Li YY, Yu LF, Zhang LN, et al. Novel small-molecule AMPK activator orally exerts beneficial effects on diabetic db/db mice[J]. Toxicol Appl Pharmacol, 2013, 273: 325-334. DOI:10.1016/j.taap.2013.09.006 |
| [30] |
Li W, Saud SM, Young MR, et al. Targeting AMPK for cancer prevention and treatment[J]. Oncotarget, 2015, 6: 7365-7378. DOI:10.18632/oncotarget.3629 |
| [31] |
Burkewitz K, Zhang Y, Mair WB. AMPK at the nexus of energetics and aging[J]. Cell Metab, 2014, 20: 10-25. DOI:10.1016/j.cmet.2014.03.002 |
| [32] |
Zheng L, Yang W, Wu F, et al. Prognostic significance of AMPK activation and therapeutic effects of metformin in hepatocellular carcinoma[J]. Clin Cancer Res, 2013, 19: 5372-5380. DOI:10.1158/1078-0432.CCR-13-0203 |
| [33] |
William WN, Kim JS, Liu DD, et al. The impact of phosphorylated AMP-activated protein kinase expression on lung cancer survival[J]. Ann Oncol, 2012, 23: 78-85. DOI:10.1093/annonc/mdr036 |
| [34] |
Baba Y, Nosho K, Shima K, et al. Prognostic significance of AMP-activated protein kinase expression and modifying effect of MAPK3/1 in colorectal cancer[J]. Br J Cancer, 2010, 103: 1025-1033. DOI:10.1038/sj.bjc.6605846 |
| [35] |
Taliaferro-Smith L, Nagalingam A, Zhong D, et al. LKB1 is required for adiponectin-mediated modulation of AMPK-S6K axis and inhibition of migration and invasion of breast cancer cells[J]. Oncogene, 2009, 28: 2621-2633. DOI:10.1038/onc.2009.129 |
| [36] |
Shackelford DB, Vasquez DS, Corbeil J, et al. mTOR and HIF-1alpha-mediated tumor metabolism in an LKB1 mouse model of Peutz-Jeghers syndrome[J]. Proc Natl Acad Sci U S A, 2009, 106: 11137-11142. DOI:10.1073/pnas.0900465106 |
| [37] |
Apfeld J, O'Connor G, McDonagh T, et al. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans[J]. Genes Dev, 2004, 18: 3004-3009. DOI:10.1101/gad.1255404 |
| [38] |
Greer EL, Dowlatshahi D, Banko MR, et al. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans[J]. Curr Biol, 2007, 17: 1646-1656. DOI:10.1016/j.cub.2007.08.047 |
| [39] |
de Oliveira MV, Andrade JM, Paraiso AF, et al. Sirtuins and cancer: new insights and cell signaling[J]. Cancer Invest, 2013, 31: 645-653. DOI:10.3109/07357907.2013.853076 |
| [40] |
Vassilopoulos A, Fritz KS, Petersen DR, et al. The human sirtuin family: evolutionary divergences and functions[J]. Hum Genomics, 2011, 5: 485-496. DOI:10.1186/1479-7364-5-5-485 |
| [41] |
Guarente L. Introduction: sirtuins in aging and diseases[J]. Methods Mol Biol, 2013, 1077: 3-10. |
| [42] |
Ma W, Xiao GG, Mao J, et al. Dysregulation of the miR-34a-SIRT1 axis inhibits breast cancer stemness[J]. Oncotarget, 2015, 6: 10432-10444. DOI:10.18632/oncotarget.3394 |
| [43] |
Lee JS, Park JR, Kwon OS, et al. SIRT1 is required for oncogenic transformation of neural stem cells and for the survival of "cancer cells with neural stemness" in a p53-dependent manner[J]. Neuro Oncol, 2015, 17: 95-106. DOI:10.1093/neuonc/nou145 |
| [44] |
Chen X, Sun K, Jiao S, et al. High levels of SIRT1 expression enhance tumorigenesis and associate with a poor prognosis of colorectal carcinoma patients[J]. Sci Rep, 2014, 4: 7481. |
| [45] |
McGlynn LM, Zino S, MacDonald AI, et al. SIRT2:tumour suppressor or tumour promoter in operable breast cancer?[J]. Eur J Cancer, 2014, 50: 290-301. DOI:10.1016/j.ejca.2013.10.005 |
| [46] |
Hiratsuka M, Inoue T, Toda T, et al. Proteomics-based identification of differentially expressed genes in human gliomas: down-regulation of SIRT2 gene[J]. Biochem Biophys Res Commun, 2003, 309: 558-566. DOI:10.1016/j.bbrc.2003.08.029 |
| [47] |
Kim HS, Vassilopoulos A, Wang RH, et al. SIRT2 maintains genome integrity and suppresses tumorigenesis through regulating APC/C activity[J]. Cancer Cell, 2011, 20: 487-499. DOI:10.1016/j.ccr.2011.09.004 |
| [48] |
Lim H, Park H, Kim HP. Effects of flavonoids on senescence-associated secretory phenotype formation from bleomycin-induced senescence in BJ fibroblasts[J]. Biochem Pharmacol, 2015, 96: 337-348. DOI:10.1016/j.bcp.2015.06.013 |
| [49] |
Argyropoulou A, Aligiannis N, Trougakos IP, et al. Natural compounds with anti-ageing activity[J]. Nat Prod Rep, 2013, 30: 1412-1437. DOI:10.1039/c3np70031c |
| [50] |
Belinha I, Amorim MA, Rodrigues P, et al. Quercetin increases oxidative stress resistance and longevity in Saccharomyces cerevisiae[J]. J Agric Food Chem, 2007, 55: 2446-2451. DOI:10.1021/jf063302e |
| [51] |
Ren KW, Li YH, Wu G, et al. Quercetin nanoparticles display antitumor activity via proliferation inhibition and apoptosis induction in liver cancer cells[J]. Int J Oncol, 2017, 50: 1299-1311. DOI:10.3892/ijo.2017.3886 |
| [52] |
Xu M, Pirtskhalava T, Farr JN, et al. Senolytics improve physical function and increase lifespan in old age[J]. Nat Med, 2018, 24: 1246-1256. DOI:10.1038/s41591-018-0092-9 |
| [53] |
Justice JN, Nambiar AM, Tchkonia T, et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study[J]. EBioMedicine, 2019, 40: 554-563. DOI:10.1016/j.ebiom.2018.12.052 |
| [54] |
Aggarwal BB, Shishodia S, Takada Y, et al. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice[J]. Clin Cancer Res, 2005, 11: 7490-7498. DOI:10.1158/1078-0432.CCR-05-1192 |
| [55] |
Plummer SM, Holloway KA, Munks RJL, et al. The chemopreventive agent curcumin suppresses cyclooxygenase-2 expression in colon cells by inhibiting the NIK/I kappa B kinase activation of NF-kappa B[J]. Brit J Cancer, 1999, 81: 575-575. |
| [56] |
Wang YC, Li YS, Yang HZ, et al. Synthesis of novel curcumin mimics and preliminary evaluation for their antitumor activity[J]. Acta Pharm Sin (药学学报), 2014, 49: 1022-1028. |
| [57] |
Hou J, Cui C, Kim S, et al. Ginsenoside F1 suppresses astrocytic senescence-associated secretory phenotype[J]. Chem Biol Interact, 2018, 283: 75-83. DOI:10.1016/j.cbi.2018.02.002 |
| [58] |
Peng PS, Yu SJ, Liu Y, et al. Ginsenoside Rb1 against natural aging of brain in mice and its influence on mTOR/p70s6k signaling pathway[C]. Beijing: Monograph of the sixteenth international congress of cardiology south China, 2015.
|
| [59] |
Zhang YWX, Liu H, Liu J, et al. A multicenter, large-sample, randomized clinical trial on improving the median survival time of advanced nonsmall cell lung cancer by combination of Ginseng Rg3 and chemotherapy[J]. Chin J Oncol (中华肿瘤杂志), 2018, 40: 295-299. |
| [60] |
Luo Z, An J, Shi W, et al. One step assembly of ginsenoside Rb1-based nanovehicles with fast cellular transport in photothermal-chemical combined cancer therapy[J]. Nanotechnology, 2021, 32: 195103. DOI:10.1088/1361-6528/abe1f0 |
| [61] |
Nicoll R, Henein MY. Ginger (Zingiber officinale Roscoe): a hot remedy for cardiovascular disease?[J]. Int J Cardiol, 2009, 131: 408-409. DOI:10.1016/j.ijcard.2007.07.107 |
| [62] |
Zhou YF Zhang GH, Wang H. A study on 6-gingerol attenuate vascular smooth muscle cells senescence through inhibition of mTOR pathway molecular[J]. Chongqing Med (重庆医学), 2014, 14: 1687-1689. |
| [63] |
Thawonsuwan J, Kiron V, Satoh S, et al. Epigallocatechin-3-gallate (EGCG) affects the antioxidant and immune defense of the rainbow trout, oncorhynchus mykiss[J]. Fish Physiol Biochem, 2010, 36: 687-697. DOI:10.1007/s10695-009-9344-4 |
| [64] |
Rashidi B, Malekzadeh M, Goodarzi M, et al. Green tea and its anti-angiogenesis effects[J]. Biomed Pharmacother, 2017, 89: 949-956. DOI:10.1016/j.biopha.2017.01.161 |
| [65] |
Garbisa S, Sartor L, Biggin S, et al. Tumor gelatinases and invasion inhibited by the green tea flavanol epigallocatechin-3-gallate[J]. Cancer, 2001, 91: 822-832. DOI:10.1002/1097-0142(20010215)91:4<822::AID-CNCR1070>3.0.CO;2-G |
| [66] |
Gupta S, Hastak K, Afaq F, et al. Essential role of caspases in epigallocatechin-3-gallate-mediated inhibition of nuclear factor kappaB and induction of apoptosis[J]. Oncogene, 2004, 23: 2507-2522. DOI:10.1038/sj.onc.1207353 |
| [67] |
Zhang F, Zhang JG, Qu J, et al. Assessment of anti-cancerous potential of 6-gingerol (Tongling White Ginger) and its synergy with drugs on human cervical adenocarcinoma cells[J]. Food Chem Toxicol, 2017, 109: 910-922. DOI:10.1016/j.fct.2017.02.038 |
| [68] |
Wang J, Man GCW, Chan TH, et al. A prodrug of green tea polyphenol (-)-epigallocatechin-3-gallate (Pro-EGCG) serves as a novel angiogenesis inhibitor in endometrial cancer[J]. Cancer Lett, 2018, 412: 10-20. DOI:10.1016/j.canlet.2017.09.054 |
| [69] |
Kumar A, Ekavali, Chopra K, et al. Current knowledge and pharmacological profile of berberine: an update[J]. Eur J Pharmacol, 2015, 761: 288-297. |
| [70] |
McCarty MF. AMPK activation--protean potential for boosting healthspan[J]. Age (Dordr), 2014, 36: 641-663. DOI:10.1007/s11357-013-9595-y |
| [71] |
Zhao L, Sun LN, Nie HB, et al. Berberine improves kidney function in diabetic mice via AMPK activation[J]. PLoS One, 2014, 9: e113398. DOI:10.1371/journal.pone.0113398 |
| [72] |
Wang J, Yang S, Cai X, et al. Berberine inhibits EGFR signaling and enhances the antitumor effects of EGFR inhibitors in gastric cancer[J]. Oncotarget, 2016, 7: 76076-76086. |
| [73] |
Su K, Hu P, Wang X, et al. Tumor suppressor berberine binds VASP to inhibit cell migration in basal-like breast cancer[J]. Oncotarget, 2016, 7: 45849-45862. |
| [74] |
Han X, Tai H, Wang X, et al. AMPK activation protects cells from oxidative stress-induced senescence via autophagic flux restoration and intracellular NAD(+) elevation[J]. Aging Cell, 2016, 15: 416-427. |
| [75] |
Yang X, Huang N. Berberine induces selective apoptosis through the AMPK-mediated mitochondrial/caspase pathway in hepatocellular carcinoma[J]. Mol Med Rep, 2013, 8: 505-510. |
| [76] |
Ma XL, Jiang W, Fan WM, et al. Berberine ameliorates dexamethasone-induced metabolic disorder in C57 mice[J]. Acta Pharm Sin(药学学报), 2020, 55: 2636-2641. |
| [77] |
Kou Y, Li L, Li H, et al. Berberine suppressed epithelial mesenchymal transition through cross-talk regulation of PI3K/AKT and RARalpha/RARbeta in melanoma cells[J]. Biochem Biophys Res Commun, 2016, 479: 290-296. |
| [78] |
Liu P, Zhao H, Luo Y. Anti-aging implications of astragalus membranaceus (huangqi): a well-known Chinese tonic[J]. Aging Dis, 2017, 8: 868-886. |
| [79] |
Zou F, Mao XQ, Wang N, et al. Astragalus polysaccharides alleviates glucose toxicity and restores glucose homeostasis in diabetic states via activation of AMPK[J]. Acta Pharmacol Sin, 2009, 30: 1607-1615. |
| [80] |
Wang N, Liu J, Xie F, et al. miR-124/ATF-6, a novel lifespan extension pathway of astragalus polysaccharide in Caenorhabditis elegans[J]. J Cell Biochem, 2015, 116: 242-251. |
| [81] |
Li XT, Zhang YK, Kuang HX, et al. Mitochondrial protection and anti-aging activity of astragalus polysaccharides and their potential mechanism[J]. Int J Mol Sci, 2012, 13: 1747-1761. |
| [82] |
Guo L, Bai SP, Zhao L, et al. Astragalus polysaccharide injection integrated with vinorelbine and cisplatin for patients with advanced non-small cell lung cancer: effects on quality of life and survival[J]. Med Oncol, 2012, 29: 1656-1662. |
| [83] |
Qu LL, Yu B, Li Z, et al. Gastrodin ameliorates oxidative stress and proinflammatory response in nonalcoholic fatty liver disease through the AMPK/Nrf2 pathway[J]. Phytother Res, 2016, 30: 402-411. |
| [84] |
Hu Y, Li C, Shen W. Gastrodin alleviates memory deficits and reduces neuropathology in a mouse model of Alzheimer's disease[J]. Neuropathology, 2014, 34: 370-377. |
| [85] |
Wang Y, Wu Z, Liu X, et al. Gastrodin ameliorates Parkinson's disease by downregulating connexin 43[J]. Mol Med Rep, 2013, 8: 585-590. |
| [86] |
Li X, Li J, Wang L, et al. The role of metformin and resveratrol in the prevention of hypoxia-inducible factor 1alpha accumulation and fibrosis in hypoxic adipose tissue[J]. Br J Pharmacol, 2016, 173: 2001-2015. |
| [87] |
Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan[J]. Nature, 2003, 425: 191-196. |
| [88] |
Bhullar KS, Hubbard BP. Lifespan and healthspan extension by resveratrol[J]. Biochim Biophys Acta, 2015, 1852: 1209-1218. |
| [89] |
Kumar S, Eroglu E, Stokes JA, et al. Resveratrol induces mitochondria-mediated, caspase-independent apoptosis in murine prostate cancer cells[J]. Oncotarget, 2017, 8: 20895-20908. |
| [90] |
Chow HH, Garland LL, Hsu CH, et al. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study[J]. Cancer Prev Res, 2010, 3: 1168-1175. |
| [91] |
Lee SD, Choe JW, Lee BJ, et al. Butein effects in colitis and interleukin-6/signal transducer and activator of transcription 3 expression[J]. World J Gastroenterol, 2015, 21: 465-474. |
| [92] |
Padmavathi G, Roy NK, Bordoloi D, et al. Butein in health and disease: a comprehensive review[J]. Phytomedicine, 2017, 25: 118-127. |
| [93] |
Padmavathi G, Rathnakaram SR, Monisha J, et al. Potential of butein, a tetrahydroxychalcone to obliterate cancer[J]. Phytomedicine, 2015, 22: 1163-1171. |
| [94] |
Benzler J, Ganjam GK, Pretz D, et al. Central inhibition of IKK beta/NF-kappa B signaling attenuates high-fat diet-induced obesity and glucose intolerance[J]. Diabetes, 2015, 64: 2015-2027. |
| [95] |
Lee EH, Song DG, Lee JY, et al. Inhibitory effect of the compounds isolated from rhus verniciflua on aldose reductase and advanced glycation endproducts[J]. Biol Pharm Bull, 2008, 31: 1626-1630. |
| [96] |
Wang L, Zhang L, Chen ZB, et al. Icariin enhances neuronal survival after oxygen and glucose deprivation by increasing SIRT1[J]. Eur J Pharmacol, 2009, 609: 40-44. |
| [97] |
Chen YPJ, Zhang J, Huang JH, et al. Study of icariin on raising SIRT6 activity and inhibiting NF-κB inflammation signal pathway of mouse[J]. Gerontol Health Care, 2012, 18: 338-341. |
| [98] |
Zheng Q, Liu WW, Li B, et al. Anticancer effect of icaritin on human lung cancer cells through inducing S phase cell cycle arrest and apoptosis[J]. J Huazhong Univ Sci Technol Med Sci, 2014, 34: 497-503. DOI:10.1007/s11596-014-1305-1 |
| [99] |
Li S, Priceman SJ, Xin H, et al. Icaritin inhibits JAK/STAT3 signaling and growth of renal cell carcinoma[J]. PLoS One, 2013, 8: e81657. |
| [100] |
Wang Y, Dong H, Zhu M, et al. Icariin exterts negative effects on human gastric cancer cell invasion and migration by vasodilator-stimulated phosphoprotein via Rac1 pathway[J]. Eur J Pharmacol, 2010, 635: 40-48. |
| [101] |
Chung BH, Kim JD, Kim CK, et al. Icariin stimulates angiogenesis by activating the MEK/ERK- and PI3K/Akt/eNOS-dependent signal pathways in human endothelial cells[J]. Biochem Bioph Res Co, 2008, 376: 404-408. |
| [102] |
Li YB, Li X, Cole A, et al. Icariin improves Fanconi anemia hematopoietic stem cell function through SIRT6-mediated NF-kappa B inhibition[J]. Cell Cycle, 2018, 17: 367-376. |
| [103] |
Bian Q. Different molecular targets of icariin on bone mesenchymal stem cells in cort-intervented and ovx rats revealed by stem cell microarray[J]. Osteoporosis Int, 2010, 21: S746-S746. |
| [104] |
Aiello A, Accardi G, Candore G, et al. Nutrient sensing pathways as therapeutic targets for healthy ageing[J]. Expert Opin Ther Targets, 2017, 21: 371-380. |
| [105] |
Kang C. Senolytics and senostatics. A two-pronged approach to target cellular senescence for delaying aging and age-related diseases[J]. Mol Cells, 2019, 42: 821-827. |
| [106] |
Hopkins AL. Network pharmacology[J]. Nat Biotechnol, 2007, 25: 1110-1111. |
 2021, Vol. 56
2021, Vol. 56


