趋化因子是一组可溶性, 大小为8~14 kDa的小分子蛋白, 是天然免疫系统的重要组成部分[1]。其通过与细胞表面的G蛋白偶联受体结合发挥生物学作用, 如刺激各种白细胞的定向和非定向迁移, 另外, 多种非白细胞可通过分泌趋化因子或表达其受体影响自身的生物学功能[2]。近年来研究表明, 趋化因子或其同源受体表达的异常与多种炎症性疾病、实体瘤和血液系统恶性肿瘤有关[3-6]。因此, 它们即可以作为这些疾病的潜在生物标志物, 也可以作为药物干预靶点, 针对其表达与功能研究, 对开发抗肿瘤药物具有十分重要的意义。
1.1 趋化因子分类趋化因子的分子量相对较小, 由多种细胞产生和分泌。趋化因子配体家族有48个特征性成员, 根据N端保守半胱氨酸残基的不同, 趋化因子可分为4类: CC、CXC、CX3C和C。CC型又称臼趋化因子亚家族, 结构特征为第1、2两个半胱氨酸紧密相连, CC趋化因子主要作用于单核细胞和淋巴细胞, 也能够促进其他类型细胞的迁移, 如树突状细胞(dendritic cell, DC)、自然杀伤(natural killer, NK) 细胞、嗜酸粒细胞和嗜碱粒细胞等。CXC型趋化因子, 特征为第1、2两个半胱氨酸之间隔有一个其他氨基酸, CXC趋化因子由一种黏蛋白茎状结构支持而表达于细胞表面, 能够促进中性粒细胞的趋化。CX3C型趋化因子, 第1、2两个半胱氨酸之间隔着3个其他氨基酸, 主要成员为神经趋化蛋白, 也称为fractalkine (FKN)。该蛋白有两种存在形式: 膜结合型以及游离型, 膜结合型的FKN可被转换酶(TNF-α converting enzyme, TACE) 切割, 得到游离型的FKN, 游离型FKN行使趋化细胞的功能。C型趋化因子是由两个半胱氨酸残基和一条二硫键组成, 淋巴细胞趋化因子属于此类趋化因子, 由胸腺细胞和活化的CD8+ T细胞产生, 可诱导T细胞和骨髓细胞趋化, 但对单核细胞无作用。
1.2 趋化因子受体迄今为止, 已发现的趋化因子受体有20多种, 趋化因子受体属于G蛋白偶联受体(G protein-coupled receptors, GPCRs), 其具有7个跨膜α-螺旋结构域。如图 1所示, 跨膜结构可将受体分割为膜外N端、3个膜外环、3个膜内环和膜内C端, 受体分子的其中一个膜内环和G蛋白偶联, 介导配体与受体结合后的胞内一系列信号级联反应, 进而发挥生物学作用。G蛋白是由α、β和γ三种亚基组成的三聚体, 静息状态时与鸟嘌呤核苷酸二磷酸(duanosine diphosphate, GDP) 结合。激活状态时异源三聚体G蛋白(GDP-αβγ) 从受体释放, 并水解成鸟嘌呤核苷酸三磷酸(guanosine triphosphate, GTP) 结合的Gα亚基和Gβ/Gγ二聚体[7]。这两种活性成分都与不同的效应蛋白相互作用并启动独特的细胞内信号转导, 如Janus激酶(janus kinase, JAK)/信号转导和转录激活因子(signal transducer and activator of transcription, STAT)、磷酸肌醇3激酶(phosphatidylinositol 3 kinase, PI3K)/丝氨酸/苏氨酸激酶(protein kinase B, AKT) 和核因子κB (nuclear factor kappa-B, NF-κB)、磷脂酶C (phospholipase, PLC)、腺苷酸环化酶、G蛋白偶联受体激酶(G protein receptor kinase, GRK) 等[8]。当配体离开受体时, α亚基本身具有GTP酶活性, 可促使GTP水解为GDP, 再重新和Gβ/Gγ二聚体结合, 形成非活性G蛋白三聚体, 恢复原来的静息状态[7]。
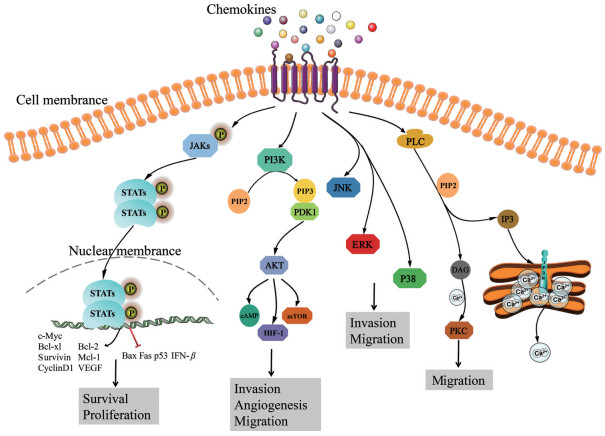
|
Figure 1 A summary of chemokines signaling pathways. Chemokines regulate cell function through GPCRs, activated GPCRs sensitize the downstream signaling pathways to promote cell proliferation, survival, invasion, angiogenesis and migration, such as JAK/STATs, PI3K/AKT, MAPK and PLC. GPCRs: G protein-coupled receptors; JAK/STATs: Janus kinase/signal transducer and activator of transcription; PI3K/AKT: Phosphatidylinositol 3 kinase/protein kinase B; MAPK: Mitogen-activated protein kinases; PLC: Phospholipase; Bcl-xl: B-cell lymphoma xl; Bcl-2: B-cell lymphoma 2; Mcl-1: Myeloid cell leukemia 1; VEGF: Vascular endothelial growth factor; Bax: Bcl-2-associated x; Fas: TNF receptor superfamily member 6; IFN-β: Interferon β; PIP2: Phosphatidylinositol biphosphate; PIP3: Phosphatidylinositol triphosphate; PDK1: 3-Phosphoinositide-dependent protein kinase-1; cAMP: Cyclic adenosine monophosphate; mTOR: Mammalian target of rapamycin; HIF-1: Hypoxia inducible factor-1; JNK: c-Jun N-terminal kinases; ERK: Extracellular signal-regulated kinases; IP3: Inositol-1, 4, 5-triphosphosate; DAG: Diacylglycerol; PKC: Protein kinase C |
另外一些趋化因子可能与受体结合而不诱导跨膜信号, 被称之为非典型受体(atypical chemokine receptors, ACKRs), 本文不对此部分进行讨论。
1.3 趋化因子相关信号通路 1.3.1 趋化因子介导JAK/STAT信号通路JAK/STAT信号通路参与多种细胞因子和生长因子的信号转导, 对细胞的生长、分化、增殖和凋亡等生物学过程具有重要调控作用。细胞因子、生长因子和趋化因子等与细胞膜表面受体结合后, 可活化JAK, 诱导STAT磷酸化, 磷酸化的STAT以二聚体形式进入细胞核激活靶基因的转录, 如髓细胞白血病因子1 (myeloid cell leukemia 1, Mcl-1)、B淋巴细胞瘤-2 (B-cell lymphoma 2, Bcl-2)、B淋巴细胞瘤-xl (B-cell lymphoma xl, Bcl-xl)、存活蛋白(survivin)、细胞周期蛋白D1 (cyclin D1)、血管内皮生长因子(vascular endothelial growth factor, VEGF) 和c-Myc[9-12]。此外, STAT被激活后也能抑制基因的转录, 如促凋亡Bcl-2相关x蛋白(Bcl-2-associated x, Bax)[13]、TNF受体超家族成员6 (TNF receptor superfamily member 6, Fas)、干扰素β (interferon β, IFN-β)[14]和抑癌基因p53[15]。
多种趋化因子通过JAK/STAT信号通路发挥生物学作用。研究发现, CCL5与CCR5的结合诱导CCR5酪氨酸的磷酸化, 活化JAK1, 进而促进STAT5b的转录激活作用[16]。CXCL12与CXCR4的结合能够快速激活JAK1和JAK2, 促进STAT1、2、3和5b的活化, 从而发挥其对下游靶基因的转录调控作用[17]。研究数据[18]显示, CCL25/CCR9和CXCL12/CXCR4通过JAK3/STAT信号通路调控骨髓T细胞的募集和向胸腺的定向迁移。JAK1和JAK2表达下调抑制了CXCL12和CCL21介导的naïve T细胞迁移的发生, 提示CXCL12和CCL21也通过JAK途径发挥生物学作用[19]。此外, FKN与CX3CR1的结合也可激活JAK/STAT信号途径, 促进胰腺癌细胞增殖和迁移[20]。
1.3.2 趋化因子介导的PI3K/AKT信号通路PI3K/AKT信号通路参与调节多种细胞功能, 包括增殖、黏附、迁移、侵袭、代谢和存活等[21]。部分趋化因子与其受体结合可激活PI3K, PI3K催化脂质磷脂酰肌醇4, 5二磷酸(phosphatidylinositol biphosphate, PIP2) 磷酸化为磷脂酰肌醇3, 4, 5三磷酸(phosphatidylinositol triphosphate, PIP3), PIP3作为第二信使与3-磷酸肌醇依赖性蛋白激酶1 (3-phosphoinositide-dependent protein kinase-1, PDK1) 结合激活AKT, 活化的AKT进一步调控下游分子, 如环磷酸腺苷(cyclic adenosine monophosphate, cAMP) 反应元件结合蛋白、叉头家族蛋白O (forkhead box protein, FOXO)、磷脂酰肌醇3-磷酸(phosphatidylinositol 3-phosphate, PI3P)、缺氧诱导因子1 (hypoxia inducible factor-1, HIF-1) 和哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin, mTOR) 等, 进而发挥多种生物学功能[22-24]。
部分趋化因子通过PI3K/AKT途径发挥生物学功能, 在多种癌症中扮演着重要角色, 如乳腺癌、胶质母细胞瘤、结肠癌、头颈部癌、非小细胞肺癌、肝癌和软骨肉瘤等。CCL5通过激活PI3K/AKT信号通路下调miR-200b表达水平, 增加VEGF的产生和体内血管生成, 进而促进肿瘤发生和转移[25]。在结肠癌中, CCR7的高表达可活化PI3K/AKT通路诱导肿瘤的发生和转移[26]。CXCL13/CXCR5轴也通过PI3K/AKT途径促进结肠癌细胞的生长、迁移及侵袭[27]。另外, CXCL12通过PI3K/AKT/mTOR途径促进结肠癌细胞分泌CXCL6, 产生的CXCL6与CXCL12协同调节结肠癌的转移[28]。在人结肠癌细胞LoVo中过表达CXCL8可激活PI3K/AKT/NF-κB通路, 诱导结肠癌细胞发生上皮-间充质转化(epithelial-mesenchymal transition, EMT)[29]。Li等[30]发现CCL25/CCR9通过活化PI3K/AKT上调下游抗凋亡蛋白Bcl-2和Bcl-xl, 下调促凋亡蛋白Bax, 进而抑制非小细胞肺癌细胞的凋亡。Zhang等[31]发现胃癌组织中CXCL9、CXCL10和CXCL11/CXCR3通过激活胃癌细胞中PI3K/AKT信号通路, 上调程序性死亡受体-1 (programmed cell death-1, PD-1), 从而促进胃癌细胞发生免疫逃避。在乳腺癌中, 肿瘤相关巨噬细胞(tumor-associated macrophages, TAMs) 分泌的CCL2通过激活PI3K/AKT/mTOR通路, 促进内分泌抵抗[32]。
1.3.3 趋化因子介导的MAPK信号通路丝裂原活化蛋白激酶(mitogen-activated protein kinases, MAPK) 信号途径包括MAPK激酶激酶(MAP kinase kinase kinase, MKKK)、MAPK激酶(MAP kinase kinase, MKK) 和MAPK, 这3种激酶能依次激活且共同调节细胞的生长、分化和炎症反应等生理病理过程[33]。MAPK下游有4种不同的信号分支, 包括细胞外信号调节激酶1和2 (extracellular signal-regulated kinases 1/2, ERK1/2)、Jun氨基末端激酶1/2/3 (c-Jun N-terminal kinases 1/2/3, JNK1/2/3)、p38/MAPK和细胞外信号调节激酶5 (extracellular signal-regulated kinases 5, ERK5)[34]。
研究发现, CXCR3-B的激活诱导ERK1/2失活并促进p38/MAPK磷酸化, 调控转录因子BTB和CNC同源体1 (BTB and CNC homology 1, Bach 1) 核移位和NFE2相关因子2 (nuclear factor erythroid-2-related factor 2, Nrf2) 核输出, 诱导抗凋亡血红素加氧酶(heme oxygenase, HO-1) 表达减少, 最终促进乳腺癌细胞的生长抑制和凋亡[35]。CCL2通过ERK/MAPK途径激活Smad3信号途径, 促进乳腺癌的侵袭和转移[36]。CCL28通过MAPK信号通路上调抗凋亡蛋白Bcl-2和下调细胞黏附蛋白β-连环蛋白(β-catenin) 的表达, 促进乳腺癌细胞增殖和转移[37]。CXCR4通过MAPK信号途径调控人卵巢上皮癌细胞(SW626) 增殖和凋亡[38]。此外, CCL19或CCL21与CCR7结合能够激活p38/ERK信号通路, 促进前列腺癌发生转移[39]。
1.3.4 趋化因子介导的PLC信号通路PLC是磷脂酰肌醇信号通路的关键酶, 在机体内分布极为广泛。部分趋化因子与其相应的G蛋白偶联受体结合后激活PLC, 活化的PLC将膜上PIP2分解为细胞内的两种第二信使, 包括二脂酰甘油(diacylglycerol, DAG) 和1, 4, 5-三磷酸肌醇(inositol-1, 4, 5-triphosphosate, IP3)[40, 41]。IP3信使是水溶性的, 它可以从质膜扩散到胞质, 与内质网膜或液泡膜上的IP3/Ca2+通道结合, 使通道打开, Ca2+顺着浓度梯度由液泡迅速地释放出来, 增加胞质Ca2+浓度, 从而引起生理效应[42]。另一方面, DAG信使在Ca2+的协同下激活蛋白激酶C (protein kinase C, PKC), 引起级联反应, 进行细胞应答[43]。研究显示, CCL5通过与CCR5结合, 激活PLC/PKC, 促进口腔癌细胞迁移和金属蛋白酶-9 (matrix metallopeptidase 9, MMP-9) 的产生[44]。人类巨细胞病毒编码的US28是一种罕见的多种趋化因子结合受体, 活化状态的US28可通过PLC和NF-κB途径增强癌细胞的迁移能力[45]。
2 趋化因子在乳腺癌中的作用乳腺癌是全世界第二常见的癌症, 是女性最常见的恶性肿瘤之一[46]。越来越多的证据表明, 肿瘤微环境(tumor microenvironment, TME) 与肿瘤细胞之间的相互作用是影响肿瘤免疫逃逸和生长转移的重要因素。TME是一个复杂而动态的细胞群, 包括肿瘤上皮细胞、各种肿瘤支持细胞(免疫细胞、成纤维细胞、免疫抑制细胞、脂肪细胞、内皮细胞) 和一些非细胞组分(细胞因子和生长因子)。在TME中, 多种趋化因子可以由肿瘤细胞、免疫细胞和基质细胞分泌, 分泌的趋化因子和细胞表面受体结合可激活多种信号途径, 并募集免疫细胞亚群进入TME, 如图 2, T细胞、巨噬细胞和肿瘤相关成纤维细胞等进而调节肿瘤免疫反应。此外, 趋化因子也可直接靶向TME中的肿瘤细胞, 从而调控肿瘤细胞的增殖、肿瘤干细胞的特性、肿瘤的侵袭和转移。因此, 趋化因子在乳腺癌的进展中发挥着重要作用。

|
Figure 2 The distribution of chemokines and their receptors in immunocytes and breast cancer cells in tumor microenvironment. MDSCs: Myeloid-derived suppressor cells |
研究表明, 趋化因子及其受体参与调控肿瘤细胞的生长。Yang等[37]利用裸鼠移植瘤模型研究发现, CCL28促进乳腺癌细胞增殖和乳腺肿瘤生长。另外, 在MCF-7细胞中过表达CXCR7可加快细胞的基础生长速率[47]。CCL2能够通过上调乙醛脱氢酶1A1 (aldehyde dehydrogenase 1 A1, ALDH1A1) 表达和抑制线粒体丝氨酸蛋白酶2 (high temperature requirement protein A2, HTRA2) 来调节乳腺癌细胞的生长[48]。相反, Lv等[49]发现MDA-MB-231细胞中过表达CXCL12对乳腺癌细胞生长有负调控作用。
2.1.2 肿瘤细胞代谢代谢活动增强是肿瘤细胞的特征之一, 癌细胞快速增殖依赖糖代谢, 特别是糖酵解, 以产生足够能量与营养。值得注意的是, 趋化因子可通过激活相关信号通路加快葡萄糖摄取以及糖酵解催化。例如, CCL5能够激活磷酸果糖激酶2 (phosphofructokinase-2, PFK2), PFK2催化6-磷酸果糖转化为2, 6-二磷酸果糖, 进而促进糖酵解[50]。此外, 在乳腺癌细胞中CCL5和CCR5的相互作用通过快速诱导mTOR、AKT、真核细胞翻译起始因子4E结合蛋白(eukaryotic initiation factor 4E-binding protein 1, 4E-BP1) 和糖原合成酶激酶3 (glycogen synthase kinase 3β, GSK3β) 的磷酸化, 上调膜表面葡萄糖转运蛋白, 增加葡萄糖摄取, 增强糖酵解能力[51]。此外, CCL5与CCR5结合增加细胞代谢活性, 导致中间产物的积累, 包括丙酮酸、乳酸、葡萄糖6-磷酸和核糖5-磷酸等, 这些产物是诱导乳腺癌细胞增殖的关键物质[51]。
2.1.3 肿瘤细胞干性有证据表明[52], 包括乳腺癌在内的许多癌症是由一群具有干细胞特性的细胞所驱动的。趋化因子在诱导和促进乳腺肿瘤细胞干性中起着重要作用。如CXCL11和CXCL10与CXCR3B结合可通过上调STAT3、ERK、cAMP效应元件结合因子(cAMP response element binding, CREB) 磷酸化水平和Notch1的mRNA水平, 促进MDA-MB-231细胞成球能力[53]。IL-8与其同源受体CXCR1和CXCR2结合, 通过活化酪氨酸激酶Src和表皮生长因子受体(epithelial growth factor receptor, EGFR)/人表皮生长因子受体-2 (human epithelial growth factor receptor 2, HER-2) 途径直接调节患者来源的乳腺球的形成与自我更新能力[54]。Hu等[48]发现CCL2/CCR2可通过上调乙醛脱氢酶1 (aldehyde dehydrogenase 1, ALDH1) 的表达, 增强乳腺癌细胞的干性, 促进乳腺癌恶性进展。在MCF-7细胞中转染CXCL12质粒后, 细胞的ALDH活性明显升高, CD44+/CD24-干细胞亚群数量显著增加[55]。
2.1.4 肿瘤细胞凋亡细胞凋亡是生物体内普遍存在的现象, 它在肿瘤发生发展中起负调控的作用。正常情况下, 机体内细胞的增殖和凋亡处于平衡状态。如果增殖和凋亡失去平衡, 例如细胞增殖能力加强且细胞凋亡受到抑制, 或细胞增殖没有明显改变而细胞凋亡受到明显的抑制, 机体就会出现癌前病变。
趋化因子参与调控乳腺肿瘤细胞的凋亡。Yang等[37]研究发现, CCL28通过活化细胞内MAPK信号通路, 上调抗凋亡蛋白Bcl-2, 下调促凋亡蛋白Bcl-2拮抗/杀伤因子(Bcl-2 homologus antagonist/killer, Bak) 和细胞黏附蛋白β-catenin的表达, 进而抑制乳腺癌细胞的凋亡。MDA-MB-231细胞中过表达CXCL16可促进含半胖氨酸天冬氨酸蛋白酶3 (cysteine-containing aspartate-specific proteases 3, caspase-3) 依赖性肿瘤细胞凋亡[56]。研究显示[57], CXCL13/CXCR5可上调细胞周期调节因子cyclin D1和下调凋亡标志物含半胖氨酸天冬氨酸蛋白酶9 (cysteine-containing aspartate-specific proteases 9, caspase-9), 从而抑制MDA-MB-231细胞凋亡。Xu等[58]发现CXCR2可通过抑制p53介导的凋亡途径, 降低紫杉醇诱导的乳腺癌细胞凋亡, 促进乳腺癌化疗耐药。天然产物丹皮酚可通过激活CXCL4/CXCR3-B生长抑制信号途径, 调节Bach1和核转录因子Nrf2的表达, 进而下调HO-1, 最终促进乳腺肿瘤细胞的凋亡[59]。此外, 乳腺癌细胞能够通过自分泌产生CCL25, 活化AKT信号通路从而抑制细胞凋亡[60]。
2.1.5 肿瘤细胞侵袭在肿瘤发生侵袭和转移的前期阶段, 肿瘤细胞通常会失去原有的上皮细胞特性, 转化为间充质样细胞, 提高了侵袭和转移的能力, 这一过程称为EMT。多种趋化因子通过调控EMT过程, 增强肿瘤细胞运动、侵袭和转移的能力。研究显示, 成纤维细胞通过CXCL14/ACKR2/NOS1自分泌信号途径, 分泌产生促血管生成因子[成纤维细胞生长因子2 (fibroblast growth factor 2, FGF-2)、血管生成素和VEGF-A] 和基质重塑分子[血小板反应蛋白解整合素金属肽酶1 (a disintegrin and metalloproteinase with thrombospondin 1, ADAMTS1)、人基质金属蛋白酶8 (matrix metalloproteinase 8, MMP8) 和基质金属蛋白酶组织抑制剂(tissue inhibitors of metalloproteinase 1, TIMP-1)], 从而刺激乳腺癌细胞发生EMT[61]。CCL18通过激活PI3K/AKT/GSK3β/Snail信号通路, 诱导乳腺癌细胞EMT, 促进肿瘤进展与转移[62]。CCL21刺激MDA-MB-231和MCF-7细胞后, Slug、波形蛋白(vimentin) 和神经型钙黏附蛋白(N-cadherin) 在mRNA与蛋白水平表达均增加, 明显促进了细胞的EMT发生, 加快其迁移与侵袭[63]。同样, CCL20可通过PKC、Src、AKT、NF-κB和Snail信号途径诱导EMT相关分子表达, 促进乳腺癌细胞迁移[64]。CCL19/CCR7通过激活AKT途径促进乳腺癌细胞的EMT进程[65]。在乳腺癌发生淋巴结转移过程中, CXCL13/CXCR5通过NF-κB受体活化因子配体(receptor activator of NF-κB ligand, RANKL)/Src途径调控了癌细胞的EMT发生[66]。在MCF-7细胞中过表达CXCL12, 可通过激活NF-κB途径促进EMT[55]。CCL5可调控乳腺癌细胞发生EMT, 进而诱导多柔比星治疗耐药[67]。
2.2 趋化因子及其受体对肿瘤相关免疫细胞的调节作用 2.2.1 巨噬细胞巨噬细胞是TME中最丰富的免疫细胞, 与各种癌症的不良预后相关。巨噬细胞具有两种不同的分型, M1型和M2型。TAMs通常与M2型巨噬细胞相似, 促进乳腺癌的侵袭、转移和内分泌抵抗。
巨噬细胞可通过分泌趋化因子发挥促肿瘤作用。Su等[68]发现, TAMs可分泌产生CCL18, 促进乳腺癌细胞EMT过程, 发生EMT的细胞分泌产生巨噬细胞集落刺激因子(granulocyte-macrophage colony stimulating factor, GM-CSF), 活化TAMs, 进而形成一个正反馈循环, 加速乳腺癌的肺转移。Svensson等[69]发现, CCL2/CCL5可刺激巨噬细胞浸润, 诱导巨噬细胞活化, 增强癌细胞向周围扩散的能力。TAMs也可通过自身分泌产生CXCL1, 活化NF-κB/SRY (sex determination region of Ychromosome) 相关高迁移率蛋白B4 (SRY-related high mobolity group box 4, SOX4) 信号途径, 促进乳腺癌转移[70, 71]。另一方面, 趋化因子可募集巨噬细胞到肿瘤部位, 发挥促肿瘤作用。Kitamura等[72]证明CCL3可诱导转移相关巨噬细胞在转移部位滞留, 促进癌细胞外渗和乳腺癌的肺转移。Walens等[73]表明, 治疗后乳腺癌患者体内残余肿瘤中的CCL5能够募集CCR5+的巨噬细胞到残留肿瘤部位, 促进胶原蛋白沉积, 诱导乳腺癌复发。研究显示[74], CBP/p300结合转化激活因子2 (CBP/p300-interacting transactivator with Glu/Asp-rich C-terminal domain 2, CITED2) 可通过调控CCL20表达, 募集巨噬细胞, 影响肿瘤的生长。
2.2.2 T淋巴细胞根据T淋巴细胞表面CD分子表达的不同将其分为两类: CD4+ T细胞和CD8+ T细胞。T淋巴细胞可通过分泌肿瘤相关的趋化因子或表达趋化因子受体, 参与乳腺癌免疫微环境调控, 发挥其抗肿瘤或促肿瘤作用。Olkhanud等[75]发现CCR4+调节性T细胞(T regulatory cells, Tregs) 在肺组织中能够抑制NK细胞的活性或NK细胞的成熟, 促进乳腺癌肺部转移。Tregs细胞在自身免疫部位分泌CCL1, 激活自身受体CCR8, 增强Tregs细胞的抑制活性, 进而发挥免疫抑制作用[76]。CCL5和CCL22能够分别募集CCR5+辅助型T细胞1 (T helper 1 cell, Th1) 和CCR4+辅助型T细胞2 (T helper 2 cell, Th2) 到TME, 加快免疫抑制微环境的形成[77, 78]。Zhang等[79]发现CCL5通过作用于CD4+CCR3+ T细胞, 上调自主生长因子(growth factor independent 1, Gfi1) 表达, Gfi1活化IL4-STAT6信号途径, 诱导CD4+ T细胞极化成为Th2型, 进而促进乳腺癌肺转移。CXCL10和CXCL9可激活CXCR3+的CD4+ T细胞、CD8+ T细胞和NK细胞, 发挥抑制肿瘤生长和增强抗肿瘤免疫的作用[80]。乳腺癌细胞分泌产生的CXCL16与Th1细胞表面的CXCR6结合, 激活并招募CD8+ T细胞到炎症部位发挥抗肿瘤作用[81]。
2.2.3 骨髓来源的免疫抑制细胞(myeloid-derived suppressor cells, MDSCs)MDSCs在骨髓中产生, 是DCs、巨噬细胞和粒细胞的前体, 具有显著抑制免疫细胞应答的能力。MDSCs可分为多核(polymorphonuclear, PMN) MDSCs (PMN-MDSCs) 和单核(monocytic, M) MDSCs (M-MDSCs) 两种亚型。
有研究报道[82], 浸润性乳腺癌中M-MDSCs细胞表面CXCR2和CXCR4的激活能够趋化MDSCs迁移到TME, 从而影响患者的预后。Chen等[83]发现, 淋巴管内皮细胞中IL-8表达上调能够激活CXCR2, 促进MDSCs在荷瘤小鼠的肿瘤引流和远端淋巴结的募集。IL-8单克隆抗体治疗可以减少乳腺肿瘤转移组织中MDSCs的数量。造血干细胞中CCL5缺失可促进MDSCs产生异常积累, 从而抑制CD8+ T细胞杀伤毒性, 促进乳腺癌进展[84]。CCL1和CCL2能促进MDSCs在乳腺癌小鼠体内的积聚[85]。乳腺癌细胞分泌的CXCL17, 可增加MDSCs在肺部的积聚, 诱导肺血管生成, 促进肿瘤浸润和生存, 最终促进肺转移[86]。
2.2.4 树突状细胞(DCs)DCs是功能最强的抗原递呈细胞, 能够激活特异性T细胞应答, 促进免疫耐受形成。DCs分布于淋巴及非淋巴组织, 其在机体不同部位的定位是发挥免疫功能的基础, 而DCs的迁移过程受到多种趋化信号的严格调控。
研究发现, CCL19或CCL21可趋化携带抗原的DCs迁移到次级淋巴组织的T细胞富集区, 诱导T细胞激活和分化[87]。转染CCL21的MCF-7可促进DCs迁移、抗原摄取和呈递功能, 且刺激后的DCs诱导Th1型细胞因子产生, 激活CD8+ T细胞, 最终清除MCF-7[88]。高表达CX3CL1的乳腺癌细胞可促进DCs在瘤内募集, 从而改善乳腺癌患者的预后[89]。最近发现[90], 乳腺和肾脏表达的一种新型CXCL14趋化因子可通过NF-κB信号途径激活DCs, 调节其在非淋巴组织中的归巢和激活, 促进了T细胞增殖, 这为肿瘤免疫疗法提供了新思路。目前, 趋化因子介导的DCs细胞迁移在乳腺癌中的研究报道较少, 有待进一步探究。
3 趋化因子在乳腺癌治疗中的作用 3.1 乳腺癌的治疗现状乳腺癌根据分子分型的不同可分为3类: 激素受体阳性(hormone receptor, HR+) 乳腺癌、人表皮生长因子受体2阳性(HER-2+) 乳腺癌和雌激素受体(estrogen receptor, ER)、孕激素受体(progesterone receptor, PR)、HER-2均为阴性的三阴性乳腺癌(triple negative breast cancer, TNBC)。HR+乳腺癌是乳腺癌最常见的亚型, 内分泌治疗是其主要治疗手段, 如他莫昔芬[91]可以阻断雌激素与受体的结合; 氟维司群(fulvestrant) 直接干扰ER合成; 来曲唑(femara)、阿那曲唑(arimidex) 和依西美坦(aromasin) 等抑制雌激素的合成, 但目前多数治疗药物不可避免地会产生耐药性, 最终导致治疗失败。HER-2+型乳腺癌中, 癌细胞可通过细胞表面过度表达的生长因子受体结合更多生长因子, 进而加速肿瘤恶性的进展[92, 93]。临床应用药物为单克隆抗体(如曲妥珠单抗) 和酪氨酸激酶受体抑制剂(如拉帕蒂尼), 但患者最终也会产生耐药[94]。TNBC是乳腺癌中侵袭性最强的亚型, 由于缺乏靶点和高的复发率, 其预后最差。一线治疗仅限于传统的化疗, 最常用的化疗药物是紫杉烷类和蒽环类药物[95, 96]。尽管多种化疗药物已经研发, 但肿瘤耐药性的产生、严重的不良反应和高复发率仍然是乳腺癌治疗中的难题。因此, 不断探究新的、高效低不良反应的靶向药物在乳腺癌治疗中有着十分重要的意义。
3.2 靶向趋化因子及其受体药物在乳腺癌中的研究 3.2.1 靶向CXCR4的药物CXCR4拮抗剂T140多肽临床上首次用于HIV的治疗, 研究发现, T140及其类似物能有效抑制CXCL12诱导的MDA-MB-231细胞迁移、增殖和转移[97]。如表 1[97-111], TN14003、WZ811、MSX-122等T140类似物能够显著减少乳腺癌转移[98-100]。此外, 临床上用于HIV患者的CXCR4拮抗剂AMD3100也可通过阻断CXCL12/CXCR4信号通路发挥抗乳腺癌肺部转移作用[101, 102]。Yang等[103]研究数据显示, GST-NT21MP多肽可降低CXCL12诱导的乳腺癌细胞生长、黏附和迁移能力。CXCR4小分子拮抗剂AMD3465能抑制乳腺癌发生和向肺、肝部位转移, 且能减少免疫细胞在转移部位的浸润[104]。研究发现, AMD3465拮抗剂能够减少肿瘤内Tregs和MDSCs数量。由于吲哚胺2, 3-双加氧酶1抑制剂D1MT能够增强肿瘤内CD8+ T细胞的抗肿瘤作用, 联合应用AMD3465和D1MT, 可延缓乳腺癌骨转移, 此研究为治疗难治性转移性乳腺癌提供了临床前证据[105]。CXCL12的肽类似物CTCE-9908与多西紫杉醇、抗血管内皮生长因子受体2 (vascular endothelial growth factor receptor 2, VEGFR2) 单克隆抗体DC101或抗血管生成剂联合使用, 抗肿瘤作用明显增强[106]。CXCR4拮抗剂balixafortide与艾瑞布林联合应用, 在治疗重症复发转移性乳腺癌患者中呈现抗肿瘤活性[107]。
| Table 1 Chemokine therapeutic targets on breast cancer. Tregs: T regulatory cells |
CCR5拮抗剂马拉韦洛克(maraviroc, MVC)、vicriviroc (SCH 417690) 和CCL5人源化单克隆抗体(leronlimab) 目前正在临床试验阶段, 其适应症包括乳腺癌[108]。Velasco-Velazquez等[109]发现, maraviroc和vicriviroc均能刺激细胞内钙离子的升高, 减少基底乳腺癌细胞的侵袭。且临床前研究中发现, maraviroc可减少小鼠静脉注射MDA-MB-231细胞产生的肺转移。将MDA-MB-231-LN细胞植入乳腺脂肪垫, 给予maraviroc和cMR16-1 (抗IL-6R抗体的小鼠替代物) 联合应用可显著降低TNBC肿瘤的生长, 可消除胸部转移[110]。因此, 开发靶向CCR5的新药物或调控CCL5表达分泌型药物, 可能会为乳腺癌患者提供新的辅助治疗方法。
3.2.3 靶向CXCR3药物在多种肿瘤模型中, 增强癌细胞旁分泌CXCL9、CXCL10和CXCL11, 或抑制其受体CXCR3表达的药物显示出很好的抗肿瘤效果。CXCR3拮抗剂AMG487可以在体内抑制乳腺癌的肺转移[111]。由于旁分泌CXCL9、CXCL10、CXCL11/CXCR3轴具有支持宿主抗肿瘤作用, 那么联合应用免疫激活配体和CXCR3拮抗剂预防转移可能是一种更加有效的新途径。此外, 近年来靶向CXCR3信号通路的药物研发也有较大进展, 例如二肽基肽酶4 (dipeptidyl peptidase-4, DPP4) 抑制剂可通过CXCL10/CXCR3通路增强肿瘤排斥提高免疫治疗的效果[112]。但DPP4抑制剂还可通过CXCL12/CXCR4/mTOR/TGF-β信号通路增强乳腺癌患者的化疗抵抗[113]。研究显示, 环氧化酶(cyclooxygenase, COX) 抑制剂可通过增加CXCL9和CXCL10从癌细胞中的释放发挥抗肿瘤的作用[114]。此外, 研究发现抗PD-1抗体在CXCR3敲除小鼠中未能缩小肿瘤, 提示程序性死亡受体配体1 (programmed cell death-ligand 1, PD-L1)/PD-1轴可能通过CXCL9、CXCL10和CXCL11/CXCR3轴发挥抗肿瘤作用[115]。因此, 临床上进行有效的药物组合, 如PD-1抗体和抑制T细胞活化药物细胞毒性T淋巴细胞相关抗原4 (cytotoxic T lymphocyte antigen 4, CTLA4)、PD1抗体和COX抑制剂, 或免疫激活配体和CXCR3拮抗剂联用, 可能显示出更好的抗肿瘤疗效。
4 未来展望大量研究表明, 趋化因子网络能够通过影响肿瘤细胞或肿瘤相关免疫细胞发挥其促进或抑制乳腺肿瘤细胞生长、侵袭和转移的作用。因此, 靶向趋化因子及其受体是治疗或辅助治疗乳腺癌等恶性肿瘤的重要手段。目前, 临床上已将部分趋化因子作为肿瘤诊断标志物, 并且部分靶向趋化因子的药物已进入各期临床, 但单一靶向趋化因子治疗药物多以失败告终, 研究多趋化因子靶向药物, 从多个途径出发, 联合发挥抗肿瘤作用方向仍有很多问题有待解决, 如不同免疫细胞产生趋化因子分子机制是否一致?趋化因子之间是否存在相互调节作用?在肿瘤进展不同阶段, 它们促进或者抑制肿瘤作用的变化?随着对趋化因子的深入研究, 对其参与重要信号通路及作用机制的不断阐明以及多个医疗数据库的建立与完善, 将更好地帮助研究人员发现基于靶向多趋化因子的高效和特异性乳腺癌治疗的新途径。
作者贡献: 王婉玉、刘姗姗为综述撰写思路; 吕晓希、胡卓伟进行综述内容评估; 王婉玉执笔; 刘姗姗审校。
利益冲突: 所有作者均声明不存在利益冲突。
| [1] |
Lata S, Raghava GP. Prediction and classification of chemokines and their receptors[J]. Protein Eng Des Sel, 2009, 22: 441-444. DOI:10.1093/protein/gzp016 |
| [2] |
Kabashima R, Sugita K, Sawada Y, et al. Increased circulating Th17 frequencies and serum IL-22 levels in patients with acute generalized exanthematous pustulosis[J]. J Eur Acad Dermatol Venereol, 2011, 25: 485-488. DOI:10.1111/j.1468-3083.2010.03771.x |
| [3] |
Giri J, Das R, Nylen E, et al. CCL2 and CXCL12 derived from mesenchymal stromal cells cooperatively polarize IL-10+ tissue macrophages to mitigate gut injury[J]. Cell Rep, 2020, 30: 1923-1934.e1924. DOI:10.1016/j.celrep.2020.01.047 |
| [4] |
Liu ZC, Wang ZL, Huang CY, et al. Duhuo Jisheng Decoction inhibits SDF-1-induced inflammation and matrix degradation in human degenerative nucleus pulposus cells in vitro through the CXCR4/NF-κB pathway[J]. Acta Pharmacol Sin, 2018, 39: 912-922. DOI:10.1038/aps.2018.36 |
| [5] |
Zhao XP, Huang YY, Huang Y, et al. Transforming growth factor-beta1 upregulates the expression of CXC chemokine receptor 4(CXCR4) in human breast cancer MCF-7 cells[J]. Acta Pharmacol Sin, 2010, 31: 347-354. DOI:10.1038/aps.2009.204 |
| [6] |
Benson C E, Southgate L. The DOCK protein family in vascular development and disease[J]. Angiogenesis, 2021. DOI:10.1007/s10456-021-09768-8 |
| [7] |
Gilman AG. G proteins: transducers of receptor-generated signals[J]. Annu Rev Biochem, 1987, 56: 615-649. DOI:10.1146/annurev.bi.56.070187.003151 |
| [8] |
Cojoc M, Peitzsch C, Trautmann F, et al. Emerging targets in cancer management: role of the CXCL12/CXCR4 axis[J]. Onco Targets Ther, 2013, 6: 1347-1361. |
| [9] |
Gao LF, Xu DQ, Wen LJ, et al. Inhibition of STAT3 expression by siRNA suppresses growth and induces apoptosis in laryngeal cancer cells[J]. Acta Pharmacol Sin, 2005, 26: 377-383. DOI:10.1111/j.1745-7254.2005.00053.x |
| [10] |
Verma NK, Davies AM, Long A, et al. STAT3 knockdown by siRNA induces apoptosis in human cutaneous T-cell lymphoma line Hut78via downregulation of Bcl-xL[J]. Cell Mol Biol Lett, 2010, 15: 342-355. DOI:10.2478/s11658-010-0008-2 |
| [11] |
Yokogami K, Yamashita S, Takeshima H. Hypoxia-induced decreases in SOCS3 increase STAT3 activation and upregulate VEGF gene expression[J]. Brain Tumor Pathol, 2013, 30: 135-143. DOI:10.1007/s10014-012-0122-0 |
| [12] |
Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment[J]. Nat Rev Immunol, 2007, 7: 41-51. DOI:10.1038/nri1995 |
| [13] |
Kunigal S, Lakka SS, Sodadasu PK, et al. Stat3-siRNA induces Fas-mediated apoptosis in vitro and in vivo in breast cancer[J]. Int J Oncol, 2009, 34: 1209-1220. |
| [14] |
Wang T, Niu G, Kortylewski M, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells[J]. Nat Med, 2004, 10: 48-54. DOI:10.1038/nm976 |
| [15] |
Niu G, Wright KL, Ma Y, et al. Role of Stat3 in regulating p53 expression and function[J]. Mol Cell Biol, 2005, 25: 7432-7440. DOI:10.1128/MCB.25.17.7432-7440.2005 |
| [16] |
Mellado M, Rodríguez-Frade JM, Mañes S, et al. Chemokine signaling and functional responses: the role of receptor dimerization and TK pathway activation[J]. Annu Rev Immunol, 2001, 19: 397-421. DOI:10.1146/annurev.immunol.19.1.397 |
| [17] |
Vila-Coro AJ, Rodríguez-Frade JM, Martín De Ana A, et al. The chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway[J]. FASEB J, 1999, 13: 1699-1710. DOI:10.1096/fasebj.13.13.1699 |
| [18] |
Soldevila G, Licona I, Salgado A, et al. Impaired chemokine-induced migration during T-cell development in the absence of Jak 3[J]. Immunology, 2004, 112: 191-200. DOI:10.1111/j.1365-2567.2004.01863.x |
| [19] |
Pérez-Rivero G, Cascio G, Soriano SF, et al. Janus kinases 1 and 2 regulate chemokine-mediated integrin activation and naïve T-cell homing[J]. Eur J Immunol, 2013, 43: 1745-1757. DOI:10.1002/eji.201243178 |
| [20] |
Huang L, Ma B, Ma J, et al. Fractalkine/CX3CR1 axis modulated the development of pancreatic ductal adenocarcinoma via JAK/STAT signaling pathway[J]. Biochem Biophys Res Commun, 2017, 493: 1510-1517. DOI:10.1016/j.bbrc.2017.10.006 |
| [21] |
Fruman DA, Chiu H, Hopkins BD, et al. The PI3K pathway in human disease[J]. Cell, 2017, 170: 605-635. DOI:10.1016/j.cell.2017.07.029 |
| [22] |
Vasan N, Toska E, Scaltriti M. Overview of the relevance of PI3K pathway in HR-positive breast cancer[J]. Ann Oncol, 2019, 30(Suppl 10): x3-x11. |
| [23] |
Karar J, Maity A. PI3K/AKT/mTOR pathway in angiogenesis[J]. Front Mol Neurosci, 2011, 4: 51. |
| [24] |
Pompura SL, Dominguez-Villar M. The PI3K/AKT signaling pathway in regulatory T-cell development, stability, and function[J]. J Leukoc Biol, 2018, 103: 1065-1076. DOI:10.1002/JLB.2MIR0817-349R |
| [25] |
Liu GT, Chen HT, Tsou HK, et al. CCL5 promotes VEGF-dependent angiogenesis by down-regulating miR-200b through PI3K/Akt signaling pathway in human chondrosarcoma cells[J]. Oncotarget, 2014, 5: 10718-10731. DOI:10.18632/oncotarget.2532 |
| [26] |
Wang J, Zhang X, Thomas SM, et al. Chemokine receptor 7 activates phosphoinositide-3 kinase-mediated invasive and prosurvival pathways in head and neck cancer cells independent of EGFR[J]. Oncogene, 2005, 24: 5897-5904. DOI:10.1038/sj.onc.1208740 |
| [27] |
Zhu Z, Zhang X, Guo H, et al. CXCL13-CXCR5 axis promotes the growth and invasion of colon cancer cells via PI3K/AKT pathway[J]. Mol Cell Biochem, 2015, 400: 287-295. DOI:10.1007/s11010-014-2285-y |
| [28] |
Ma JC, Sun XW, Su H, et al. Fibroblast-derived CXCL12/SDF-1α promotes CXCL6 secretion and co-operatively enhances metastatic potential through the PI3K/Akt/mTOR pathway in colon cancer[J]. World J Gastroenterol, 2017, 23: 5167-5178. DOI:10.3748/wjg.v23.i28.5167 |
| [29] |
Shen T, Yang Z, Cheng X, et al. CXCL8 induces epithelial-mesenchymal transition in colon cancer cells via the PI3K/Akt/NF-κB signaling pathway[J]. Oncol Rep, 2017, 37: 2095-2100. DOI:10.3892/or.2017.5453 |
| [30] |
Li B, Wang Z, Zhong Y, et al. CCR9-CCL25 interaction suppresses apoptosis of lung cancer cells by activating the PI3K/Akt pathway[J]. Med Oncol, 2015, 32: 66. DOI:10.1007/s12032-015-0531-0 |
| [31] |
Zhang C, Li Z, Xu L, et al. CXCL9/10/11, a regulator of PD-L1 expression in gastric cancer[J]. BMC Cancer, 2018, 18: 462. DOI:10.1186/s12885-018-4384-8 |
| [32] |
Li D, Ji H, Niu X, et al. Tumor-associated macrophages secrete CC-chemokine ligand 2 and induce tamoxifen resistance by activating PI3K/Akt/mTOR in breast cancer[J]. Cancer Sci, 2020, 111: 47-58. DOI:10.1111/cas.14230 |
| [33] |
Guo YJ, Pan WW, Liu SB, et al. ERK/MAPK signalling pathway and tumorigenesis[J]. Exp Ther Med, 2020, 19: 1997-2007. |
| [34] |
Sun Y, Liu WZ, Liu T, et al. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis[J]. J Recept Signal Transduct Res, 2015, 35: 600-604. DOI:10.3109/10799893.2015.1030412 |
| [35] |
Balan M, Pal S. A novel CXCR3-B chemokine receptor-induced growth-inhibitory signal in cancer cells is mediated through the regulation of Bach-1 protein and Nrf2 protein nuclear translocation[J]. J Biol Chem, 2014, 289: 3126-3137. DOI:10.1074/jbc.M113.508044 |
| [36] |
Fang WB, Jokar I, Zou A, et al. CCL2/CCR2 chemokine signaling coordinates survival and motility of breast cancer cells through Smad3 protein- and p42/44 mitogen-activated protein kinase (MAPK)-dependent mechanisms[J]. J Biol Chem, 2012, 287: 36593-36608. DOI:10.1074/jbc.M112.365999 |
| [37] |
Yang XL, Liu KY, Lin FJ, et al. CCL28 promotes breast cancer growth and metastasis through MAPK-mediated cellular anti-apoptosis and pro-metastasis[J]. Oncol Rep, 2017, 38: 1393-1401. DOI:10.3892/or.2017.5798 |
| [38] |
Wang X, Wang H, Wei X, et al. Effect of CXCR4 silencing with shRNA on MAPK signaling in ovarian cancer[J]. Oncol Lett, 2018, 15: 10026-10030. |
| [39] |
Tang G, Du R, Tang Z, et al. MiRNALet-7a mediates prostate cancer PC-3 cell invasion, migration by inducing epithelial-mesenchymal transition through CCR7/MAPK pathway[J]. J Cell Biochem, 2018, 119: 3725-3731. DOI:10.1002/jcb.26595 |
| [40] |
Manzoli L, Mongiorgi S, Clissa C, et al. Strategic role of nuclear inositide signalling in myelodysplastic syndromes therapy[J]. Mini Rev Med Chem, 2014, 14: 873-883. DOI:10.2174/1389557514666141013125936 |
| [41] |
Rohacs T. Regulation of transient receptor potential channels by the phospholipase C pathway[J]. Adv Biol Regul, 2013, 53: 341-355. DOI:10.1016/j.jbior.2013.07.004 |
| [42] |
Katan M. New insights into the families of PLC enzymes: looking back and going forward[J]. Biochem J, 2005, 391: e7-e9. DOI:10.1042/BJ20051506 |
| [43] |
Yuan M, Gao Y, Li L, et al. Phospholipase C (PLC)ε promotes androgen receptor antagonist resistance via the bone morphogenetic protein (BMP)-6/SMAD axis in a castration-resistant prostate cancer cell line[J]. Med Sci Monit, 2019, 25: 4438-4449. DOI:10.12659/MSM.915828 |
| [44] |
Chuang JY, Yang WH, Chen HT, et al. CCL5/CCR5 axis promotes the motility of human oral cancer cells[J]. J Cell Physiol, 2009, 220: 418-426. DOI:10.1002/jcp.21783 |
| [45] |
Puengel T, Krenkel O, Kohlhepp M, et al. Differential impact of the dual CCR2/CCR5 inhibitor cenicriviroc on migration of monocyte and lymphocyte subsets in acute liver injury[J]. PLoS One, 2017, 12: e0184694. DOI:10.1371/journal.pone.0184694 |
| [46] |
Grayson M. Breast cancer[J]. Nature, 2012, 485: S49. DOI:10.1038/485S49a |
| [47] |
Boudot A, Kerdivel G, Habauzit D, et al. Differential estrogen-regulation of CXCL12 chemokine receptors, CXCR4 and CXCR7, contributes to the growth effect of estrogens in breast cancer cells[J]. PLoS One, 2011, 6: e20898. DOI:10.1371/journal.pone.0020898 |
| [48] |
Hu Q, Myers M, Fang W, et al. Role of ALDH1A1 and HTRA2 expression in CCL2/CCR2-mediated breast cancer cell growth and invasion[J]. Biol Open, 2019, 8: bio040873. |
| [49] |
Lv ZD, Kong B, Liu XP, et al. CXCL12 chemokine expression suppresses human breast cancer growth and metastasis in vitro and in vivo[J]. Int J Clin Exp Pathol, 2014, 7: 6671-6678. |
| [50] |
Chan O, Burke JD, Gao DF, et al. The chemokine CCL5 regulates glucose uptake and AMP kinase signaling in activated T cells to facilitate chemotaxis[J]. J Biol Chem, 2012, 287: 29406-29416. DOI:10.1074/jbc.M112.348946 |
| [51] |
Gao D, Rahbar R, Fish EN. CCL5 activation of CCR5 regulates cell metabolism to enhance proliferation of breast cancer cells[J]. Open Biol, 2016, 6: 160122. DOI:10.1098/rsob.160122 |
| [52] |
Luo M, Clouthier SG, Deol Y, et al. Breast cancer stem cells: current advances and clinical implications[J]. Methods Mol Biol, 2015, 1293: 1-49. |
| [53] |
Kundu N, Ma X, Brox R, et al. The chemokine receptor CXCR3 isoform B drives breast cancer stem cells[J]. Breast Cancer (Auckl), 2019, 13: 1178223419873628. |
| [54] |
Singh JK, Farnie G, Bundred NJ, et al. Targeting CXCR1/2 significantly reduces breast cancer stem cell activity and increases the efficacy of inhibiting HER2via HER2-dependent and -independent mechanisms[J]. Clin Cancer Res, 2013, 19: 643-656. DOI:10.1158/1078-0432.CCR-12-1063 |
| [55] |
Kong L, Guo S, Liu C, et al. Overexpression of SDF-1 activates the NF-kappaB pathway to induce epithelial to mesenchymal transition and cancer stem cell-like phenotypes of breast cancer cells[J]. Int J Oncol, 2016, 48: 1085-1094. DOI:10.3892/ijo.2016.3343 |
| [56] |
Fang Y, Henderson FC, Yi Q, et al. Chemokine CXCL16 expression suppresses migration and invasiveness and induces apoptosis in breast cancer cells[J]. Mediators Inflamm, 2014, 2014: 478641. |
| [57] |
Ma JJ, Jiang L, Tong DY, et al. CXCL13 inhibition induce the apoptosis of MDA-MB-231 breast cancer cells through blocking CXCR5/ERK signaling pathway[J]. Eur Rev Med Pharmacol Sci, 2018, 22: 8755-8762. |
| [58] |
Xu H, Lin F, Wang Z, et al. CXCR2 promotes breast cancer metastasis and chemoresistance via suppression of AKT1 and activation of COX2[J]. Cancer Lett, 2018, 412: 69-80. DOI:10.1016/j.canlet.2017.09.030 |
| [59] |
Saahene RO, Wang J, Wang ML, et al. The antitumor mechanism of paeonol on CXCL4/CXCR3-B signals in breast cancer through induction of tumor cell apoptosis[J]. Cancer Biother Radiopharm, 2018, 33: 233-240. DOI:10.1089/cbr.2018.2450 |
| [60] |
Chen L, Zhang S, Shen Y, et al. Thymus-expressed chemokine secreted by breast cancer cells promotes metastasis and inhibits apoptosis[J]. Oncol Rep, 2020, 43: 1875-1884. |
| [61] |
Sjoberg E, Meyrath M, Milde L, et al. A novel ACKR2-dependent role of fibroblast-derived CXCL14 in epithelial-to-mesenchymal transition and metastasis of breast cancer[J]. Clin Cancer Res, 2019, 25: 3702-3717. DOI:10.1158/1078-0432.CCR-18-1294 |
| [62] |
Zhao C, Zheng S, Yan Z, et al. CCL18 promotes the invasion and metastasis of breast cancer through annexin A2[J]. Oncol Rep, 2020, 43: 571-580. |
| [63] |
Li F, Zou Z, Suo N, et al. CCL21/CCR7 axis activating chemotaxis accompanied with epithelial-mesenchymal transition in human breast carcinoma[J]. Med Oncol, 2014, 31: 180. DOI:10.1007/s12032-014-0180-8 |
| [64] |
Marsigliante S, Vetrugno C, Muscella A. Paracrine CCL20 loop induces epithelial-mesenchymal transition in breast epithelial cells[J]. Mol Carcinog, 2016, 55: 1175-1186. DOI:10.1002/mc.22360 |
| [65] |
Xu B, Zhou M, Qiu W, et al. CCR7 mediates human breast cancer cell invasion, migration by inducing epithelial-mesenchymal transition and suppressing apoptosis through AKT pathway[J]. Cancer Med, 2017, 6: 1062-1071. DOI:10.1002/cam4.1039 |
| [66] |
Biswas S, Sengupta S, Roy Chowdhury S, et al. CXCL13-CXCR5 co-expression regulates epithelial to mesenchymal transition of breast cancer cells during lymph node metastasis[J]. Breast Cancer Res Treat, 2014, 143: 265-276. DOI:10.1007/s10549-013-2811-8 |
| [67] |
Ma G, Huang H, Li M, et al. Plasma CCL5 promotes EMT-medicated epirubicin-resistance in locally advanced breast cancer[J]. Cancer Biomark, 2018, 22: 405-415. DOI:10.3233/CBM-170986 |
| [68] |
Su S, Liu Q, Chen J, et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis[J]. Cancer Cell, 2014, 25: 605-620. DOI:10.1016/j.ccr.2014.03.021 |
| [69] |
Svensson S, Abrahamsson A, Rodriguez GV, et al. CCL2 and CCL5 are novel therapeutic targets for estrogen-dependent breast cancer[J]. Clin Cancer Res, 2015, 21: 3794-3805. DOI:10.1158/1078-0432.CCR-15-0204 |
| [70] |
Wang S, Liu X, Huang R, et al. XIAOPI Formula inhibits breast cancer stem cells via suppressing tumor-associated macrophages/C-X-C motif chemokine ligand 1 pathway[J]. Front Pharmacol, 2019, 10: 1371. DOI:10.3389/fphar.2019.01371 |
| [71] |
Wang N, Liu W, Zheng Y, et al. CXCL1 derived from tumor-associated macrophages promotes breast cancer metastasis via activating NF-κB/SOX4 signaling[J]. Cell Death Dis, 2018, 9: 880. DOI:10.1038/s41419-018-0876-3 |
| [72] |
Kitamura T, Qian BZ, Soong D, et al. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages[J]. J Exp Med, 2015, 212: 1043-1059. DOI:10.1084/jem.20141836 |
| [73] |
Walens A, DiMarco AV, Lupo R, et al. CCL5 promotes breast cancer recurrence through macrophage recruitment in residual tumors[J]. Elife, 2019, 8: e43653. DOI:10.7554/eLife.43653 |
| [74] |
Jayaraman S, Doucet M, Kominsky SL. CITED2 attenuates macrophage recruitment concordant with the downregulation of CCL20 in breast cancer cells[J]. Oncol Lett, 2018, 15: 871-878. |
| [75] |
Olkhanud PB, Baatar D, Bodogai M, et al. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells[J]. Cancer Res, 2009, 69: 5996-6004. DOI:10.1158/0008-5472.CAN-08-4619 |
| [76] |
Barsheshet Y, Wildbaum G, Levy E, et al. CCR8(+) FOXp3(+) T(reg) cells as master drivers of immune regulation[J]. Proc Natl Acad Sci U S A, 2017, 114: 6086-6091. DOI:10.1073/pnas.1621280114 |
| [77] |
Khalid A, Wolfram J, Ferrari I, et al. Recent advances in discovering the role of CCL5 in metastatic breast cancer[J]. Mini Rev Med Chem, 2015, 15: 1063-1072. DOI:10.2174/138955751513150923094709 |
| [78] |
Kutukculer N, Azarsiz E, Aksu G, et al. CD4+CD25+Foxp3+ T regulatory cells, Th1(CCR5, IL-2, IFN-γ) and Th2(CCR4, IL-4, IL-13) type chemokine receptors and intracellular cytokines in children with common variable immunodeficiency[J]. Int J Immunopathol Pharmacol, 2016, 29: 241-251. DOI:10.1177/0394632015617064 |
| [79] |
Zhang Q, Qin J, Zhong L, et al. CCL5-mediated Th2 immune polarization promotes metastasis in luminal breast cancer[J]. Cancer Res, 2015, 75: 4312-4321. DOI:10.1158/0008-5472.CAN-14-3590 |
| [80] |
Karin N. CXCR3 ligands in cancer and autoimmunity, chemoattraction of effector T cells, and beyond[J]. Front Immunol, 2020, 11: 976. DOI:10.3389/fimmu.2020.00976 |
| [81] |
Matsumura S, Wang B, Kawashima N, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells[J]. J Immunol, 2008, 181: 3099-3107. DOI:10.4049/jimmunol.181.5.3099 |
| [82] |
Seo EH, Namgung JH, Oh CS, et al. Association of chemokines and chemokine receptor expression with monocytic-myeloid-derived suppressor cells during tumor progression[J]. Immune Netw, 2018, 18: e23. DOI:10.4110/in.2018.18.e23 |
| [83] |
Chen JY, Lai YS, Chu PY, et al. Cancer-derived VEGF-C increases chemokine production in lymphatic endothelial cells to promote CXCR2-dependent cancer invasion and MDSC recruitment[J]. Cancers (Basel), 2019, 11: 1120. DOI:10.3390/cancers11081120 |
| [84] |
Zhang Y, Lv D, Kim HJ, et al. A novel role of hematopoietic CCL5 in promoting triple-negative mammary tumor progression by regulating generation of myeloid-derived suppressor cells[J]. Cell Res, 2013, 23: 394-408. DOI:10.1038/cr.2012.178 |
| [85] |
Sceneay J, Parker BS, Smyth MJ, et al. Hypoxia-driven immunosuppression contributes to the pre-metastatic niche[J]. Oncoimmunology, 2013, 2: e22355. DOI:10.4161/onci.22355 |
| [86] |
Hsu YL, Yen MC, Chang WA, et al. CXCL17-derived CD11b(+) Gr-1(+) myeloid-derived suppressor cells contribute to lung metastasis of breast cancer through platelet-derived growth factor-BB[J]. Breast Cancer Res, 2019, 21: 23. DOI:10.1186/s13058-019-1114-3 |
| [87] |
Gunn MD. Chemokine mediated control of dendritic cell migration and function[J]. Semin Immunol, 2003, 15: 271-276. DOI:10.1016/j.smim.2003.08.004 |
| [88] |
Wu S, Xing W, Peng J, et al. Tumor transfected with CCL21 enhanced reactivity and apoptosis resistance of human monocyte-derived dendritic cells[J]. Immunobiology, 2008, 213: 417-426. DOI:10.1016/j.imbio.2007.10.003 |
| [89] |
Park MH, Lee JS, Yoon JH. High expression of CX3CL1 by tumor cells correlates with a good prognosis and increased tumor-infiltrating CD8+ T cells, natural killer cells, and dendritic cells in breast carcinoma[J]. J Surg Oncol, 2012, 106: 386-392. DOI:10.1002/jso.23095 |
| [90] |
Shurin GV, Ferris RL, Tourkova IL, et al. Loss of new chemokine CXCL14 in tumor tissue is associated with low infiltration by dendritic cells (DC), while restoration of human CXCL14 expression in tumor cells causes attraction of DC both in vitro and in vivo[J]. J Immunol, 2005, 174: 5490-5498. DOI:10.4049/jimmunol.174.9.5490 |
| [91] |
Zhang JY, Chen ZY, Liu TT, et al. 3-Bromopyruvic acid increases the sensitivity of MCF-7/TR cells to tamoxifen[J]. Acta Pharm Sin (药学学报), 2020, 55: 164-169. |
| [92] |
Mitri Z, Constantine T, O'Regan R. The HER2 receptor in breast cancer: pathophysiology, clinical use, and new advances in therapy[J]. Chemother Res Pract, 2012, 2012: 743193. |
| [93] |
Rybarova S, Hodorova I, Hajdukova M, et al. Expression of MDR proteins in breast cancer and its correlation with some clinical and pathological parameters[J]. Neoplasma, 2006, 53: 128-135. |
| [94] |
Vu T, Claret FX. Trastuzumab: updated mechanisms of action and resistance in breast cancer[J]. Front Oncol, 2012, 2: 62. |
| [95] |
Hudis CA, Gianni L. Triple-negative breast cancer: an unmet medical need[J]. Oncologist, 2011, 16(Suppl 1): 1-11. |
| [96] |
Cheng FY, Chan CH, Wang BJ, et al. The oxygen-generating calcium peroxide-modified magnetic nanoparticles attenuate hypoxia-induced chemoresistance in triple-negative breast cancer[J]. Cancers (Basel), 2021, 13: 606. DOI:10.3390/cancers13040606 |
| [97] |
Tamamura H, Hori A, Kanzaki N, et al. T140 analogs as CXCR4 antagonists identified as anti-metastatic agents in the treatment of breast cancer[J]. FEBS Lett, 2003, 550: 79-83. DOI:10.1016/S0014-5793(03)00824-X |
| [98] |
Liang Z, Zhan W, Zhu A, et al. Development of a unique small molecule modulator of CXCR4[J]. PLoS One, 2012, 7: e34038. DOI:10.1371/journal.pone.0034038 |
| [99] |
Liang Z, Wu T, Lou H, et al. Inhibition of breast cancer metastasis by selective synthetic polypeptide against CXCR4[J]. Cancer Res, 2004, 64: 4302-4308. DOI:10.1158/0008-5472.CAN-03-3958 |
| [100] |
Zhan W, Liang Z, Zhu A, et al. Discovery of small molecule CXCR4 antagonists[J]. J Med Chem, 2007, 50: 5655-5664. DOI:10.1021/jm070679i |
| [101] |
Smith MC, Luker KE, Garbow JR, et al. CXCR4 regulates growth of both primary and metastatic breast cancer[J]. Cancer Res, 2004, 64: 8604-8612. DOI:10.1158/0008-5472.CAN-04-1844 |
| [102] |
Dong XC, Wen R. Advances in the new anti-HIV drugs acting on chemokine receptor[J]. Acta Pharm Sin (药学学报), 2001, 36: 796-800. |
| [103] |
Yang Q, Zhang F, Ding Y, et al. Antitumour activity of the recombination polypeptide GST-NT21MP is mediated by inhibition of CXCR4 pathway in breast cancer[J]. Br J Cancer, 2014, 110: 1288-1297. DOI:10.1038/bjc.2014.1 |
| [104] |
Ling X, Spaeth E, Chen Y, et al. The CXCR4 antagonist AMD3465 regulates oncogenic signaling and invasiveness in vitro and prevents breast cancer growth and metastasis in vivo[J]. PLoS One, 2013, 8: e58426. DOI:10.1371/journal.pone.0058426 |
| [105] |
Zhang J, Pang Y, Xie T, et al. CXCR4 antagonism in combination with IDO1 inhibition weakens immune suppression and inhibits tumor growth in mouse breast cancer bone metastases[J]. Onco Targets Ther, 2019, 12: 4985-4992. DOI:10.2147/OTT.S200643 |
| [106] |
Huang EH, Singh B, Cristofanilli M, et al. A CXCR4 antagonist CTCE-9908 inhibits primary tumor growth and metastasis of breast cancer[J]. J Surg Res, 2009, 155: 231-236. DOI:10.1016/j.jss.2008.06.044 |
| [107] |
Pernas S, Martin M, Kaufman PA, et al. Balixafortide plus eribulin in HER2-negative metastatic breast cancer: a phase 1, single-arm, dose-escalation trial[J]. Lancet Oncol, 2018, 19: 812-824. DOI:10.1016/S1470-2045(18)30147-5 |
| [108] |
Jiao X, Nawab O, Patel T, et al. Recent advances targeting CCR5 for cancer and its role in immuno-oncology[J]. Cancer Res, 2019, 79: 4801-4807. DOI:10.1158/0008-5472.CAN-19-1167 |
| [109] |
Velasco-Velazquez M, Jiao X, De La Fuente M, et al. CCR5 antagonist blocks metastasis of basal breast cancer cells[J]. Cancer Res, 2012, 72: 3839-3850. DOI:10.1158/0008-5472.CAN-11-3917 |
| [110] |
Jin K, Pandey NB, Popel AS. Simultaneous blockade of IL-6 and CCL5 signaling for synergistic inhibition of triple-negative breast cancer growth and metastasis[J]. Breast Cancer Res, 2018, 20: 54. DOI:10.1186/s13058-018-0981-3 |
| [111] |
Walser TC, Rifat S, Ma X, et al. Antagonism of CXCR3 inhibits lung metastasis in a murine model of metastatic breast cancer[J]. Cancer Res, 2006, 66: 7701-7707. DOI:10.1158/0008-5472.CAN-06-0709 |
| [112] |
Barreira da Silva R, Laird ME, Yatim N, et al. Dipeptidylpeptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally occurring tumor immunity and immunotherapy[J]. Nat Immunol, 2015, 16: 850-858. DOI:10.1038/ni.3201 |
| [113] |
Li S, Fan Y, Kumagai A, et al. Deficiency in dipeptidyl peptidase-4 promotes chemoresistance through the CXCL12/CXCR4/mTOR/TGFβ signaling pathway in breast cancer cells[J]. Int J Mol Sci, 2020, 21: 805. DOI:10.3390/ijms21030805 |
| [114] |
Bronger H, Kraeft S, Schwarz-Boeger U, et al. Modulation of CXCR3 ligand secretion by prostaglandin E2 and cyclooxygenase inhibitors in human breast cancer[J]. Breast Cancer Res, 2012, 14: R30. DOI:10.1186/bcr3115 |
| [115] |
Chheda ZS, Sharma RK, Jala VR, et al. Chemoattractant receptors BLT1 and CXCR3 regulate antitumor immunity by facilitating CD8+ T cell migration into tumors[J]. J Immunol, 2016, 197: 2016-2026. DOI:10.4049/jimmunol.1502376 |
 2021, Vol. 56
2021, Vol. 56


